Abstract
New echocardiographic modalities including pulmonary artery acceleration time (PAAT) and right ventricular ejection time (RVET) are evolving to facilitate an early non-invasive diagnosis for pulmonary hypertension (PH) in adults. In children, PAAT depends on age, body surface area (BSA) and heart rate (HR) and is used to predict PH. Normal values of RVET and their role to predict PH in children are still missing. PAAT/RVET-index correlates negatively with PH. We hypothesized that this index is a good predictor for PH in children and adolescents independent of age, BSA and HR and RVET is significantly reduced in PH. PAAT and RVET of 401 healthy children and 30 PH-patients were measured using pulsed-wave-Doppler. PH was diagnosed in PH-group invasively. PAAT/RVET-index for both groups was calculated. Sensitivity and specificity in prediction of PH of PAAT, PAAT z-score and PAAT/RVET-index were compared. We demonstrated normal values of RVET in children. In the healthy group, PAAT and RVET correlated significant positive to age (p < 0.001), and BSA (p < 0.001) and negative to HR (p < 0.001). PAAT/RVET-index correlated weakly to age, BSA and HR (p < 0.001). Mean pulmonary artery pressure (PAPM) ranged in the PH-group from 27 to 82 mmHg (mean 44 mmHg). In predicting PH, RVET is significantly reduced (p < 0.001). Comparing area under the curve (AUC), the difference between sensitivity and specificity of PAAT/RVET-index < 0.29 and calculated PAAT cut-off-point (87 ms) was significant (p < 0.001). Equally, AUC comparison between PAAT/RVET-index < 0.29 and PAAT z-score of − 1.33 was significant (p = 0.008). PAAT/RVET-index < 0.29 represents a good predictor of PH with a 100% sensitivity and a 95.8% specificity. PAAT/RVET-index is a simple tool and facilitates prediction of PH independent from z-scores.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
PH is a progressive disease with nonspecific symptoms and minor echocardiographic changes at the beginning, which make an early diagnosis challenging. An early diagnosis and treatment is important to improve the outcome, whereas novel therapeutic strategies improved mortality and morbidity of this disease [1]. The gold standard of the diagnosis of PH is still an invasive right-heart catheterization (RHC), with PH defined as a PAPM ≥ 25 mmHg, therefore it is important to establish echocardiographic criteria for children and adolescents to predict PH. Tricuspid valve regurgitation velocity (TRV) is a well-established measurement for the estimation of the pulmonary artery systolic pressure (PASP) in the absence of pulmonary stenosis, but it is not always sufficiently available [2], and it could be borderline. PAAT is now being used frequently with or without TRV to estimate PH in adults [2, 3], and it is measurable in nearly all patients [2]. PAAT in children correlates positively to age and BSA and negatively to HR, compared to adults [4, 5]. RVET is shortened in adults with PH [6], and the correlation between RVET and PH in children is not evaluated. To the best of our knowledge, data about the normal RVET values in children are missing. PAAT/RVET-index is reduced in adults with PH [6], and in neonates with bronchopulmonary dysplasia compared to controls [7]. We assumed that PAAT/RVET-index is less dependent on age, BSA and HR and hypothesized, that it is possible to define a cut-off-point for PAAT/RVET-index to predict PH with good sensitivity and specificity and independent of age, BSA and HR.
We measured PAAT and RVET in a healthy pediatric group of all ages to define normal values, and in a pediatric PH population. We diagnosed PH in our PH-group by RHC in accordance to the current guidelines by PAPM ≥ 25 mmHg. Then we calculated the PAAT/RVET-index, and compared sensitivity and specificity of a PAAT-cut-off point, a PAAT z-score and a PAAT/RVET-index-cut-off-point in the prediction of PH.
Methods
Study population
This multicenter retrospective study includes data from a healthy group (401 probands) and a PH group (30 patients).
Our healthy cohort included 401 children (187 healthy probands, who were evaluated in the Heart and Diabetes Centre in Bad Oeynhausen/Germany and 214 children and adolescents, who were evaluated in a pediatric cardiology practice in Nuernberg/Germany between January 01, 2012 and July 31, 2016 to rule out congenital heart diseases indicated by an innocent pediatric cardiac murmur). All heathy children and adolescents underwent physical examination (including age, weight, height and heart rate) and echocardiography. They had a normal echocardiogram including (morphology and function of the right heart, without any evidence of elevated right ventricular pressure), according to the American Society of Echocardiography guidelines and standards [8]. Neonates with a small Patent Foramen Ovale (PFO) were included. Probands older than 1 month with a PFO as well as all probands with a Patent Ductus Arteriosus (PDA) were excluded.
The PH-group included 30 patients with PH. Catheterization protocols dating from January 01, 2012 to September 30, 2017 at the Department of Pediatric Cardiology, University Hospital Erlangen/Germany were reviewed by a database search.
Inclusion criteria were (1) a biventricular heart, (2) a PAPM ≥ 25 mm Hg, (3) no pulmonary stenosis, (4) no severe pulmonary regurgitation, (5) no evidence of intracardiac shunting and (6) an adequate echocardiographic imaging quality obtained ≤ 2 days prior to RHC. The medication was not changed between the echocardiographic study and the RHC.
The underlying diagnoses were: restrictive cardiomyopathy (n = 9), dilated cardiomyopathy (n = 7), mitral valve stenosis or insufficiency after surgical repair of a complete atrioventricular septal defect (n = 9), surgical repair of a ventricular septal defect without residual defects (n = 1) and surgical repair of anomalous pulmonary vein return (n = 4).
Echocardiographic study
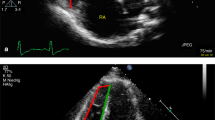
The echocardiographic equipment used was (GE health care, Vivid E9, Vivid 7, or Vivid S5) with an appropriate transducer. Echocardiographic images were acquired by four investigators according to the American Society of Echocardiography guidelines [8]. The pulmonary artery Doppler flow velocity envelope (PA-FVE) was obtained by the pulsed-wave Doppler (pw-Doppler) using parasternal short axis [9]. Pw- Doppler sample was placed on the pulmonary artery side of the pulmonary valve. The PAAT- and RVET- measurements were done offline by well-trained investigators blinded to age, BSA and results of RHC of the subjects (Fig. 1a, b). PAAT and RVET were measured in 3–5 cardiac cycles and mean values were taken.
Measurement of pulmonary artery acceleration time (PAAT) and right ventricular ejection time (RVET) in a healthy subject and a subject with pulmonary hypertension (PH). a Healthy subject. PAAT was measured from onset of systolic flow to peak velocity (1. Time 148 ms). RVET was measured from systolic flow onset to end of systolic flow (2. Time 366 ms).The pulmonary artery Doppler flow velocity envelope (PA-FVE) obtained from the parasternal short axis. b PH subject. PAAT was measured from onset of systolic flow to peak velocity (1. Time 83 ms). RVET was measured from systolic flow onset to end of systolic flow (2. Time 405 ms). PA-FVE obtained from the parasternal short axis of an 11-year old patient with PH and invasively measured mean pulmonary artery pressure of 65 mm Hg
Hemodynamic measurements
All 30 patients were breathing spontaneously during RHC. Systolic and end-diastolic right ventricular pressure as well as pulmonary artery mean (PAPM), systolic (PASP) and diastolic pressure were registered.
Statistical analysis
All statistical analyses were performed using R Version 3.2.2 (R core team (2016)) [10]. T-tests were performed for group comparisons purposes using a significance-level of 0.05. The cut-off-point determination was done with conditional inference trees [11], using the PAAT and RVET as response variables and age, BSA and HR as independent variables. Reported cut-off- points indicate largest feasible splits in the independent variables when modelling the response variables using the conditional inference trees.
A regression analysis was performed defining PAAT and RVET as responses and age as the independent variable. The normal values were reported giving the 95% prediction interval (PI). Correlated HR values were generated (modelled) accordingly and the results were reported as mean and 95% PI. z-scores were calculated using regression analysis data, as reported by Koestenberger et al. [4]. A Receiver Operating Characteristic (ROC) analysis was performed and the area under the curve (AUC) for PAAT, PAAT z-score and PAAT/RVET-index were calculated. The Youden-index (sensitivity + specificity−1) was used to define optimal cut-off-points for prediction of PH by using PAAT, RVET and PAAT/RVET-index. We used De Long-test to compare between AUCs of PAAT cut-off-point, PAAT z-score, and PAAT/RVET-index to test statistically significant differences between sensitivities and specificities of these parameters for prediction of PH by using a significance-level of 0.05. Interclass correlation coefficient (ICC) was used to determine interobserver and intraobserver variability.
Results
Demographic data
Demographic data of healthy group and PH group are summarized in Table 1.
Availability and reproducibility of PAAT- and RVET-measurements
PAAT and RVET could be measured for all probands and patients. The intraobserver variability for PAAT was (ICC: 0.92) and for RVET (ICC: 0.94). The interobserver variability for PAAT was (ICC: 0.85) and for RVET (ICC: 0.88).
PAAT- and RVET-values
Healthy group: We found a significant positive correlation between PAAT and age (p < 0.001, rho = 0.78) (Fig. 2a) as well as PAAT and BSA (p < 0.001, rho = 0.77) (Fig. 2b), and a significant negative correlation between PAAT and HR (p < 0.001, rho=-0.79) (Fig. 2c). RVET correlated significantly positive to age (p < 0.001, rho = 0.78) (Fig. 3a) and BSA (p < 0.001, rho = 0.78) (Fig. 3b), and significantly negative to HR (p < 0.001, rho = − 0.84) (Fig. 3c).
Correlation of pulmonary artery acceleration time (PAAT) to age, body surface area (BSA) and heart rate (HR). a Scatter plot shows a positive correlation between PAAT and age. The age of 11.79 years is determined as right-most node of a conditional inference tree, indicating that age dependency of PAAT trails off for values > 11.79 years. b Scatter plot shows a positive correlation between PAAT and BSA. The BSA of 1.31 m2 is determined as right-most node of a conditional inference tree, indicating that BSA dependency of PAAT trails off for values > 1.31 m2. c Scatter plot shows a negative correlation between PAAT and HR. The HR of 124/min is determined as right-most node of a conditional inference tree, indicating that HR dependency of PAAT trails off for values > 124/min. PAAT (ms), age (years), BSA (m2), HR (beat/min)
Correlation of right ventricular ejection time (RVET) to age, body surface area (BSA) and heart rate (HR). a Scatter plot shows a positive correlation between RVET and age. The age of 11.19 years is determined as right-most node of a conditional inference tree, indicating that age dependency of RVET trails off for values > 11.19 years. b Scatter plot shows a positive correlation between RVET and BSA. The BSA of 1.57 m2 is determined as right-most node of a conditional inference tree, indicating that BSA dependency of RVET trails off for values > 1.57 m2. c Scatter plot shows a negative correlation between RVET and HR. The HR of 142/min is determined as right-most node of a conditional inference tree, indicating that HR dependency of RVET trails off for values > 142/min. RVET (ms), age (years), BSA (m2), HR (beat/min)
The normal PAAT- and RVET-values did not vary significantly due to gender (p = 0.43 (PAAT/gender), p = 0.57 (RVET/gender)). With increasing age and BSA, the positive correlation between PAAT, age and BSA as well as between RVET, age and BSA becomes weaker. This applies to the course of the negative correlation between PAAT and HR, as well between RVET and HR. The calculated cut-off-points by means of conditional inference trees for the correlation of PAAT and RVET to age, BSA and HR are depicted in (Fig. 2a–c) and (Fig. 3a–c).
Age related normal values of PAAT and RVET are demonstrated in Table 2.
PH group: Both PAAT and RVET were reduced significantly in the PH group (p < 0.001) (Fig. 4a, b).
Comparison between pulmonary artery acceleration time (PAAT), right ventricular ejection time (RVET) and PAAT/RVET-index in the healthy group and the pulmonary hypertension (PH) group. a Box plot shows, that PAAT is significantly reduced in the PH group. b Box plot shows, that RVET is significantly reduced in the PH group. c Box plot shows PAAT/RVET-index is significantly reduced in the PH group. Significant level 0.05. PAAT (ms), RVET (ms)
PAAT/RVET-index
In the healthy group, PAAT/RVET-index correlated significantly positive to age (p < 0.001, rho = 0.42) and BSA (p < 0.001, rho = 0.40), and significantly negative to HR (p < 0.001, rho=-0.37). The calculated mean PAAT/RVET-index was in the healthy group 0.369 ms (range 0.276 to 0.494 ms) and in the PH-group 0.246 ms (range 0.180 to 0.291 ms).
The calculated PAAT/RVET-index was not gender dependent in the healthy group (p = 0.102) and PH group (p = 0.927).
PAAT/RVET-index was significantly reduced in PH group (p < 0.001) (Fig. 4c).
Comparing PAAT, RVET with PAAT/RVET-index in predicting PH
PAAT, RVET and PAAT/RVET-index were reduced significantly in the PH-group (p < 0.001). ROC curves were used to compare PAAT, RVET and PAAT/RVET-index in predicting PH. We calculated a PAAT z-score of − 1.33 as the best PAAT z-score with 90.3% sensitivity and 92.5% specificity to predict PH in children and adolescents (AUC = 0.967; Figs. 5a, 6). The best calculated PAAT-cut-off-point was 87 ms with a sensitivity of 90.3% and specificity of 79.3% to predict PH (AUC = 0.930; Figs. 5b, 6). A PAAT/RVET-index of less than 0.29 was the best calculated PAAT/RVET-index with a 100% sensitivity and a 95.8% specificity to predict PH (AUC = 0.997; Fig. 5c). Comparing the AUCs, we found that the difference between AUC (PAAT z-score; optimal cut-off point: -1.33) and AUC (PAAT/RVET-index; optimal cut-off point: 0.29) as well as between AUC (PAAT; optimal cut-off point: 87 ms) and AUC (PAAT/RVET-index; optimal cut-off point: 0.29) was significant (DeLong test: p = 0.008 and p < 0.001, respectively). Further, PAAT/RVET- index showed a significant negative correlation with PAPS (p = 0.38; rho = − 0.38), PAPM (p = 0.012; rho = − 0.452) and PAPD (p = 0.01; rho = − 0.464).
Receiver operating characteristic (ROC) curve for pulmonary artery acceleration time (PAAT) z-score of (− 1.33), PAAT cut-off-point (87 ms) and PAAT/RVET-index (0.29) in predicting pulmonary hypertension (PH). a ROC curve shows that a PAAT z-score of − 1.33 has a sensitivity of 90.3% and specificity of 92.5% to predict PH (AUC = 0.967). b ROC curve shows that a PAAT cut-off-point of 87 ms has a sensitivity of 90.3% and specificity of 79.3% to predict PH (AUC = 0.930). c ROC curve shows that a PAAT/RVET-index with a cut-off-point of 0.29 has a sensitivity of 100% and specificity of 95.8% to predict PH (AUC = 0.997). PAAT/RVET-index < 0.29 hast the best AUC in prediction PH
Comparison between pulmonary artery acceleration time (PAAT) cut-off-point (87 ms), PAAT z-score (− 1.33) and PAAT z-score (− 1.565) from Koestenberger et al. publication [4] in predicting pulmonary hypertension (PH). PAAT (87 ms) as a cut-off-point (solid line), PAAT z-score of − 1.33 related to our data (bold solid line) and PAAT z-score of − 1.565 related to the data of Koestenberger et al. [4] (bold broken line) calculated for our PH group. Our PAAT z-score has a better specificity than the best calculated PAAT z-score from the data of Koestenberger et al. [4] in predicting PH in our PH group. The specificity of the best PAAT cut-off-point of 87 ms is lower than PAAT z-score. PAAT (ms), age (years)
The best calculated RVET cut-off-point of 285 ms had a sensitivity of 77.4% and specificity of 62.8% to predict PH (Fig. 7b), and the best calculated RVET z-score of − 1.52 had a sensitivity of 32.3% and specificity of 95.0% to predict PH (Fig. 7a).
Receiver operating characteristic (ROC) curve for right ventricular ejection time (RVET) z-score (− 1.52) and RVET cut-off-point (285 ms) in predicting pulmonary hypertension (PH). a ROC curve shows that a RVET z- score of − 1.52 has a sensitivity of 32.3% and specificity of 95% in prediction of PH (AUC = 0.581). b ROC curve shows that a RVET cut-off-point of 285 ms has a sensitivity of 77.4% and specificity of 62.8% in predicting PH (AUC = 0.684). Youden-index was used to calculate optimal cut-off- point. AUC = area under the curve
Discussion
Findings
PAAT/RVET-index less than 0.29 had a100% sensitivity and a 95.8% specificity to predict PH with an AUC of 0.997. This index has statistically significant the best sensitivity and specificity in predicting PH comparing the AUCs of the calculated PAAT cut-off-point, PAAT z-score and PAAT/RVET-index. We measured the normal values of RVET in children and adolescents and determined the sensitivity and specificity of RVET to predict PH in pediatric population.
Characteristics of PAAT- and RVET-distribution in the healthy group
Our study confirms that PAAT correlates positively to age and BSA, and it correlates negatively to HR [4]. The normal PAAT values in our data were in line with the normal values published by Koestenberger et al. [4]. However, depending on the PAAT z-score published in the mentioned study, we expected a part of the healthy subjects in our study showing values suggesting PH, because the normal calculated values [4] are slightly higher as our normal values (Fig. 8). RVET is equally distributed. To the best of our knowledge, this data is the first presenting normal RVET-values in healthy children and adolescents.
Pulmonary artery acceleration time (PAAT)—normal values related to age with calculated z-scores. The normal PAAT values related to age with our z-scores (Z = 0 black solid line, Z = ± 2 black brocken line). Z-scores according to the data of Koestenberger et al. [4] are red solid line (Z = 0; female), blue solid line (Z = 0; male), red broken line (Z = ± 2; female) and blue broken line (Z = ± 2; male). There is a largely correlation between our normal PAAT values and those published by Koestenberger et al. according to the calculated z-scores and a part of normal values in our study are <(− 2) z-score due to the data of Koestenberger et al. [4]. PAAT (ms), age (years)
Many echocardiographic parameters in children and adolescents correlate positively to age and BSA [12,13,14]. We observed that the positive correlation between PAAT to age and BSA as well as between RVET to age and BSA becomes vague in adolescents, therefore we defined cut-off-points for this positive correlation (Figs. 2a, b, 3a, b). We suggest, this is due to physical maturity, which is reached between the age of 12 and 14 years, thus growth rate is reduced after this age [15]. Accordingly, the normal HR does not vary significantly between 12 and 18 years [16].
Despite shortening of systolic time with increasing HR, the systolic time/diastolic time ratio correlates positive with HR [17], and the negative correlation between PAAT/HR and RVET/HR in children and adolescents extenuates with high HR (Figs. 2c, 3c). A significant but weak negative correlation between PAAT and HR persists in adults [5].
PAAT, RVET and PAAT/RVET-index as predictor of PH
PH was diagnosed invasively; pulmonary pressure values are demonstrated in Table 1. Severity of PH was analyzed. 12 patients presented with mild PH (PAPM < 35 mmHg), 9 patients with moderate PH (PAPM 35–50 mmHg) and 9 patients showed severe PH (PAPM > 50 mmHg). Our novel index showed a significant negative correlation with the severity of PH. This should be discussed and validated as a follow-up parameter.
PAAT/RVET-index cut-off point of 0.29 is statistically significantly better than a PAAT (cut-off point of 87 ms) as well as a PAAT Z-score (cut-off point of − 1.33) in predicting PH.
Because of the weak correlation between PAAT/RVET-index to age, BSA and HR, there is no need for z-score-transformation and using a cut-off-point to predict PH is reliable and comprehensible. Our cut-off-point correlated largely with other studies [7, 18, 19].
PAAT/RVET-index was reduced in PH patients versus control group in adults [6]. Furthermore, the PAAT/RVET-index was reduced in subjects susceptible to high altitude pulmonary edema and the ratio correlated to invasively measured PAPM and PVR [20]. PAAT/RVET was lower in neonates with bronchopulmonary dysplasia than in healthy controls, and it was helpful to differentiate between vasoactive PH and non-vasoactive PH [7].
PAAT was significantly reduced in our PH-group (p < 0.001), as well in other studies [21, 22]. Different echocardiographic models are used to predict PAP [21,22,23,24,25,26,27], and PAAT is well described in adults as a predictive value for PH with different cut-off-points [25, 28, 29]. In children, only few studies about the role of PAAT in estimation of PH are available [4, 21]. PAAT is age, BSA and HR dependent, hence defining a PAAT-cut-off point yields in children to low sensitivity and specificity in prediction of pH [21].
The best PAAT z-score in our study to predict PH was − 1.33 and it was significantly inferior to PAAT/RVET-index cut-off point in prediction of PH. Recently, PAAT z-score was calculated to predict PH in pediatric population [4], the specificity of this PAAT z-score is lower than our PAAT z-score.
RVET is significant reduced in our PH-group. In a small group of children with PH (29 patients) and small group of healthy probands (40 probands) no significant difference was shown between cases and controls [21], which is probably due to the small number of the healthy probands. It is known, that RVET shortened in adults with PH [6].
Clinical implication
PAAT is easily measurable [3, 25] and the reproducibility of PAAT and PAAT/RVET is high [4, 6]. RVET-measurement is equally simple and reproducible. According to good sensitivity and specificity of PAAT/RVET-index in prediction of PH, it is absolutely reliable to use this index to predict PH especially in the absence of TVR or in the presence of borderline values of TRV.
Further investigations are warranted to understand the role of the PAAT/RVET-index in pediatric population and to evaluate the use of PAAT and PAAT/RVET-index in differentiating between pre- and post-capillary PH. PAAT/RVET-index in follow up of PH-patients and assessment of the therapeutic success should be thoroughly evaluated.
Conclusion
We demonstrate for the first time the normal values of RVET for children and adolescents. Equally to PAAT, the normal values of RVET correlate to age, BSA, and HR. RVET is significantly reduced in PH. Despite this significant reduction neither RVET cut-off-point nor RVET z-score showed a good sensitivity to predict PH. The PAAT/RVET-index is less dependent on age, BSA and HR and it is significantly reduced in PH. An index value of less than 0.29 is significantly better than a PAAT cut-off-point and a PAAT z-score in prediction of PH. The PAAT/RVET index facilitates prediction of PH independent from z-score calculation and represents a simple tool for all pediatric cardiologists.
Limitation
Our PH group includes children of all ages; due to the small number investigated detailed interpretation of smaller age groups was not possible lacking statistical power. The most our PH patients presented with post capillary PH.
References
Montani D, Gunther S, Dorfmuller P, Perros F, Girerd B, Garcia G, Jais X, Savale L, Artaud-Macari E, Price LC, Humbert M, Simonneau G, Sitbon O (2013) Pulmonary arterial hypertension. Orphanet J Rare Dis 8:97. https://doi.org/10.1186/1750-1172-8-97
Yared K, Noseworthy P, Weyman AE, McCabe E, Picard MH, Baggish AL (2011) Pulmonary artery acceleration time provides an accurate estimate of systolic pulmonary arterial pressure during transthoracic echocardiography. J Am Soc Echocardiogr 24(6):687–692. https://doi.org/10.1016/j.echo.2011.03.008
Roberts JD, Forfia PR (2011) Diagnosis and assessment of pulmonary vascular disease by Doppler echocardiography. Pulm Circ 1(2):160–181. https://doi.org/10.4103/2045-8932.83446
Koestenberger M, Grangl G, Avian A, Gamillscheg A, Grillitsch M, Cvirn G, Burmas A, Hansmann G (2017) Normal reference values and z scores of the pulmonary artery acceleration time in children and its importance for the assessment of pulmonary hypertension. Circ Cardiovasc Imaging. https://doi.org/10.1161/CIRCIMAGING.116.005336
Marra AM, Benjamin N, Ferrara F, Vriz O, D’Alto M, D’Andrea A, Stanziola AA, Gargani L, Cittadini A, Grunig E, Bossone E (2017) Reference ranges and determinants of right ventricle outflow tract acceleration time in healthy adults by two-dimensional echocardiography. Int J Cardiovasc Imaging 33(2):219–226. https://doi.org/10.1007/s10554-016-0991-0
Kitabatake A, Inoue M, Asao M, Masuyama T, Tanouchi J, Morita T, Mishima M, Uematsu M, Shimazu T, Hori M, Abe H (1983) Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation 68(2):302–309
Nagiub M, Lee S, Guglani L (2015) Echocardiographic assessment of pulmonary hypertension in infants with bronchopulmonary dysplasia: systematic review of literature and a proposed algorithm for assessment. Echocardiography 32(5):819–833. https://doi.org/10.1111/echo.12738
Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T (2010) Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the pediatric measurements writing group of the American Society of echocardiography pediatric and congenital heart disease council. J Am Soc Echocardiogr 23(5):465–495. https://doi.org/10.1016/j.echo.2010.03.019.
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23(7):685–713. https://doi.org/10.1016/j.echo.2010.05.010.
Nguefack-Tsague G, Zucchini W, Fotso S (2016) Frequentist model averaging and applications to bernoulli trials. Open J Stat 6(3):545–553. https://doi.org/10.4236/ojs.2016.63046
Hothorn T, Hornik K, Zeileis A (2006) Unbiased recursive partitioning: a conditional inference framework. J Comput Gr Stat 15(3):651–674. https://doi.org/10.1198/106186006X133933
Kampmann C, Wiethoff CM, Wenzel A, Stolz G, Betancor M, Wippermann CF, Huth RG, Habermehl P, Knuf M, Emschermann T, Stopfkuchen H (2000) Normal values of M mode echocardiographic measurements of more than 2000 healthy infants and children in central Europe. Heart 83(6):667–672
Pettersen MD, Du W, Skeens ME, Humes RA (2008) Regression equations for calculation of z-scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. J Am Soc Echocardiogr 21(8):922–934. https://doi.org/10.1016/j.echo.2008.02.006
Koestenberger M, Ravekes W, Everett AD, Stueger HP, Heinzl B, Gamillscheg A, Cvirn G, Boysen A, Fandl A, Nagel B (2009) Right ventricular function in infants, children and adolescents: reference values of the tricuspid annular plane systolic excursion (TAPSE) in 640 healthy patients and calculation of z score values. J Am Soc Echocardiogr 22(6):715–719. https://doi.org/10.1016/j.echo.2009.03.026
Sherar LB, Esliger DW, Baxter-Jones AD, Tremblay MS (2007) Age and gender differences in youth physical activity: does physical maturity matter? Med Sci Sports Exerc 39(5):830–835. https://doi.org/10.1249/mss.0b013e3180335c3c
Fleming S, Thompson M, Stevens R, Heneghan C, Pluddemann A, Maconochie I, Tarassenko L, Mant D (2011) Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet 377(9770):1011–1018. https://doi.org/10.1016/S0140-6736(10)62226-X
Sarnari R, Kamal RY, Friedberg MK, Silverman NH (2009) Doppler assessment of the ratio of the systolic to diastolic duration in normal children: relation to heart rate, age and body surface area. J Am Soc Echocardiogr 22(8):928–932. https://doi.org/10.1016/j.echo.2009.05.004
Jone PN, Ivy DD (2014) Echocardiography in pediatric pulmonary hypertension. Front Pediatr 2:124. https://doi.org/10.3389/fped.2014.00124
Schober KE, Baade H (2006) Doppler echocardiographic prediction of pulmonary hypertension in West Highland white terriers with chronic pulmonary disease. J Vet Intern Med 20(4):912–920
Yagi H, Yamada H, Kobayashi T, Sekiguchi M (1990) Doppler assessment of pulmonary hypertension induced by hypoxic breathing in subjects susceptible to high altitude pulmonary edema. Am Rev Respir Dis 142(4):796–801. https://doi.org/10.1164/ajrccm/142.4.796
Cevik A, Kula S, Olgunturk R, Tunaoglu FS, Oguz AD, Saylan B, Cilsal E, Sanli C (2013) Assessment of pulmonary arterial hypertension and vascular resistance by measurements of the pulmonary arterial flow velocity curve in the absence of a measurable tricuspid regurgitant velocity in childhood congenital heart disease. Pediatr Cardiol 34(3):646–655. https://doi.org/10.1007/s00246-012-0520-4
Scapellato F, Temporelli PL, Eleuteri E, Corra U, Imparato A, Giannuzzi P (2001) Accurate noninvasive estimation of pulmonary vascular resistance by Doppler echocardiography in patients with chronic failure heart failure. J Am Coll Cardiol 37(7):1813–1819
Tabib A, Khorgami MR, Meraji M, Omidi N, Mirmesdagh Y (2014) Accuracy of Doppler-derived indices in predicting pulmonary vascular resistance in children with pulmonary hypertension secondary to congenital heart disease with left-to-right shunting. Pediatr Cardiol 35(3):521–529. https://doi.org/10.1007/s00246-013-0818-x
Lanzarini L, Fontana A, Lucca E, Campana C, Klersy C (2002) Noninvasive estimation of both systolic and diastolic pulmonary artery pressure from Doppler analysis of tricuspid regurgitant velocity spectrum in patients with chronic heart failure. Am Heart J 144(6):1087–1094. https://doi.org/10.1067/mhj.2002.126350
Torbicki A, Skwarski K, Hawrylkiewicz I, Pasierski T, Miskiewicz Z, Zielinski J (1989) Attempts at measuring pulmonary arterial pressure by means of Doppler echocardiography in patients with chronic lung disease. Eur Respir J 2(9):856–860
Lanzarini L, Fontana A, Campana C, Klersy C (2005) Two simple echo-Doppler measurements can accurately identify pulmonary hypertension in the large majority of patients with chronic heart failure. J Heart Lung Transplant 24(6):745–754. https://doi.org/10.1016/j.healun.2004.03.026
Mo V, Bellucci B, Fisher J, Balekian A (2015) PAAT and RVET versus RVSP to predict pulmonary hypertension. Eur Respir J. https://doi.org/10.1183/13993003.congress-2015.PA3784
Bossone E, D’Andrea A, D’Alto M, Citro R, Argiento P, Ferrara F, Cittadini A, Rubenfire M, Naeije R (2013) Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J Am Soc Echocardiogr 26(1):1–14. https://doi.org/10.1016/j.echo.2012.10.009
Tossavainen E, Soderberg S, Gronlund C, Gonzalez M, Henein MY, Lindqvist P (2013) Pulmonary artery acceleration time in identifying pulmonary hypertension patients with raised pulmonary vascular resistance. Eur Heart J Cardiovasc Imaging 14(9):890–897. https://doi.org/10.1093/ehjci/jes309
Acknowledgements
The present work was performed in fulfillment of the requirements for obtaining the degree “Dr. med” at “Friedrich-Alexander-Universität, Erlangen-Nürnberg (FAU)” for Sheeraz Habash.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the ethics committee of the University of Erlangen-Nurnberg and University of Bochum. All procedures were based on standard of care, and established clinical guidelines were followed. The study was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Habash, S., Laser, K.T., Moosmann, J. et al. Normal values of the pulmonary artery acceleration time (PAAT) and the right ventricular ejection time (RVET) in children and adolescents and the impact of the PAAT/RVET-index in the assessment of pulmonary hypertension. Int J Cardiovasc Imaging 35, 295–306 (2019). https://doi.org/10.1007/s10554-019-01540-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-019-01540-w