Abstract
To describe the fetal regional myocardial strain rate in the membranous ventricular septum across gestation and to determine their predictive value for a complete membranous ventricular septum (without defect) after delivery. In 1150 fetuses, the peak systolic strain rate (SRs), peak early diastolic strain rate (SRe) and peak late diastolic strain rate (SRa) in the membranous ventricular septum were measured at four time points across gestation (18–20, 24–26, 30–32 and 36–38 weeks). The integrity of the interventricular septum was examined at 12 weeks’ postnatal age. The correlations between myocardial strain rates and gestational age as well as fetal left ventricular mass were analyzed, and the performance of myocardial strain rates in predicting a complete membranous ventricular septum was deducted. Strain rate absolute values in the membranous ventricular septum all increased across gestation. They all significantly correlated with gestational age and left ventricular mass. At 24 weeks during pregnancy, the areas under the receiver operating characteristics curve (AUC) for SRe and SRa were all > 0.72 (p < 0.05) in predicting a complete membranous ventricular septum, while the AUC for SRs was only 0.55. The sensitivity, specificity and accuracy of the cut off value (> 1.53 s−1) for SRe was 62.5, 85.7 and 73.3%, respectively, and the sensitivity, specificity and accuracy of the cut off value (> 1.51 s−1) for SRa was 75.2, 71.9 and 73.8%, respectively. The changes of myocardial strain rates in the membranous ventricular septum across gestation maybe can be used to predict a complete membranous ventricular septum after delivery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ventricular septal defect, especially perimembranous ventricular septal defect, is a common type of fetal and neonatal cardiac abnormalities. To date, it is still difficult for fetal echocardiography to detect a < 2 mm perimembranous ventricular septal defect during the whole pregnancy due to a low two or three-dimensional resolution, an unclear interventricular shunt and a low differential pressure between left and right ventricle. Sometimes, ones initially diagnosed with possible septal defects had no defect (0.4–0.8%), and ones initially diagnosed with no septal defect had defects (0.5–1%).
It is commonly known that loading conditions (left ventricular preload and right ventricular afterload) change throughout fetal life and with the transition to infancy [1]. Doppler-based echocardiography measures reflect not only inherent maturational changes in myocardial properties but also changes in loading conditions [2]. In contrast, strain rate values are thought to be relatively independent of loading conditions [2,3,4], and mainly reflect the inherent maturational changes in myocardial properties. They can now be applied to the fetus with adequate spatial and temporal resolution [3,4,5,6], as well as good inter- and intra reader reproducibility [7, 8].
In the present investigation, we assumed that myocardial strain rates reflected the changes of intrinsic myocardial properties during fetal heart development. Therefore, we described the fetal regional myocardial strain rate in the membranous ventricular septum across gestation using two-dimensional strain rate imaging and determined their predictive value for a complete membranous ventricular septum (without defect) after delivery.
Materials and methods
Study population
The study was approved by the local Institutional Review Board (IRB) committee of Yancheng and the Human Research Ethics Committee of Yancheng Institute of Clinical, Xuzhou Medical University. Signed informed consent was obtained from all the pregnant women. This is a prospective study. 1150 pregnant women (1150 fetuses), who without risk factors, such as diabetes mellitus, hypertension, congenital heart disease, cardiomyopathy, and endocrine diseases, were enrolled before 20 weeks’ gestation and received a dedicated fetal echocardiography at four time points across gestation (18–20, 24–26, 30–32 and 36–38 weeks) in our antenatal care center. The fetuses were evaluated by means of clinical and physical assessment, chromosome, ultrasonographic examinations. The pregnant women were evaluated by means of clinical and physical assessment, laboratory data, electrocardiogram, and ultrasonographic examinations.
Echocardiographic protocol
The echocardiographic data were acquired transabdominally with the following ultrasound systems: Vivid E9 (GE Healthcare, Horten, Norway) equipped with an M5S single-crystal matrix array transducer and quantitative analysis software of tissue velocity imaging for the analysis of strain rate. All acquisitions were performed independently by two experienced operators. Data of each fetus was obtained longitudinally at four time points across gestation (mentioned above). The integrity of the interventricular septum was examined at 12 weeks’ postnatal age via a complete echocardiography.
First of all, a dedicated fetal echocardiography was performed to detect various malformations through a series of views, such as a transverse section of the fetal abdomen, four-chamber view, left ventricular outflow tract view, right ventricular outflow tract view, three-vessel tracheoesophageal view, superior and inferior vena cava view, aortic arch view and ductal arch view. In addition, the left ventricular mass at end diastole (LVd mass) was measured, as previously described [9].
Secondly, the standard left ventricular outflow tract view was obtained. The tissue velocity imaging function was activated and raw data of five cardiac cycles in this view were stored digitally at a frame rate of 130 frames per second for offline analysis. In this process, width angles were kept at 30°–45°; gains were adjusted at the minimum optimal level to minimize noise; frequencies were adjusted to 2.3–4.6 MHz; and the filter settings were kept low (50 Hz).
Strain rate is the speed at which deformation (i.e. strain) occurs. The instantaneous natural strain rate can be calculated as:
with L′(t) the rate of deformation [e.g. (2.4 cm − 2 cm)/2 s = 0.2 cm s−1] and L(t) the instantaneous length of the object [10]. Strain rate is expressed in s−1.
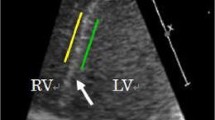
Finally, a region of interest was placed at the membranous ventricular septum. Adequate tracking was verified and, if necessary, adjusted. The peak systolic strain rate (SRs), peak early diastolic strain rate (SRe) and peak late diastolic strain rate (SRa) in the membranous ventricular septum were measured (Fig. 1). All values for each parameter were obtained by averaging measurements from three successive cardiac cycles.
Statistical analysis
Results in this study are expressed as mean ± standard deviation. The differences between the two groups were tested using an unpaired two tailed t test. An ANOVA test with adjustment was used for multiple comparison. The correlation between different parameters was performed using the Pearson test. A receiver operating characteristic curve (ROC) analysis of strain rates parameters at 24 weeks during pregnancy was used to predict a complete membranous ventricular septum (without defect) and to determine the optimal cut-off points and validity parameters. Bland and Altman was used to measure the inter/intra observer variability. A value of P < 0.05 was considered statistically significant. All statistical analysis was performed with SPSS version 16 software for Windows (SPSS Inc, Chicago, IL).
Results
The echocardiographic measurements were successfully completed in all fetuses. Among the 1150 fetuses, 7 suffered from < 1.5 mm perimembranous ventricular septal defect after delivery, the others were healthy confirmed by clinical and physical examinations, laboratory data, electrocardiogram, and ultrasonographic examinations.
As shown in Table 1, the absolute values of SRs, SRe and SRa in the membranous ventricular septum all steadily increased across gestation, and they were significantly less in fetuses suffered from VSD than in those without VSD after delivery (P < 0.05). Pearson correlation analysis showed that there was a significant positive correlation between SRs and gestational age (r = 0.62; P < 0.0001) and LVd mass (r = 0.63; P < 0.0001), and between SRe and gestational age (r = 0.48); and LVd mass (r = 0.57; P < 0.0001), as well as between SRa and gestational age (r = 0.32; P < 0.05) and LVd mass (r = 0.52; P < 0.005; Fig. 2).
The areas under the receiver operating characteristics curve (AUC) for SRe and SRa at 24 weeks during pregnancy were all > 0.72 (p < 0.05) in predicting a complete membranous ventricular septum (without defect), while the AUC for SRs was only 0.55. The sensitivity, specificity and accuracy of the cut off value (> 1.53 s−1) for SRe was 62.5, 85.7 and 73.3%, respectively, the sensitivity, specificity and accuracy of the cut off value (> 1.51 s−1) for SRa was 75.2, 71.9 and 73.8%, respectively, and the sensitivity, specificity and accuracy of the cut off value (> 1.69 s−1) for SRs was 0.50, 67.9 and 56.5%, respectively (Fig. 3).
Intraobserver and interobserver variability rates for SRs ranged from 5.4 to 6.7%. Intraobserver and interobserver variability rates for SRe ranged from 4.1 to 5.9%, and Intraobserver and interobserver variability rates for SRa ranged from 6.1 to 8.3%.
Discussion
In this study, we report the first longitudinally acquired cohort of fetuses in whom the regional myocardial systolic and diastolic strain rate parameters in the membranous ventricular septum were assessed across gestation. We found that these strain rate parameters all increased across gestation, and correlated with gestational age as well as fetal left ventricular mass. They maybe can be used to predict the integrity of membranous ventricular septum after delivery.
There are other methods to assess fetal heart development, such as computational fluid dynamics and 4D echocardiography. Lee et al. [11] reported that moving domain simulations computational fluid dynamics highlighted hemodynamic changes in relation to cardiac morphogenesis and could provide a 2-D quantitative approach to complement imaging analysis. Wiputra et al. [12] reported that peristaltic-like motion did not affect flow patterns significantly, but had significant influence on energy dynamics in human fetal right ventricle using 4D clinical ultrasound imaging and computational fluid dynamics. Miller [13] explored how spatial distributions of the normal forces acting on the heart wall change as the endocardial cushions grow and as the cardiac wall increases in stiffness using computational fluid dynamics. Lai et al. [14] explored fluid mechanics of blood flow in human fetal left ventricles using 4D STIC ultrasound scans. However, to the best of our knowledge, these methods have never been used to predict fetal development, especially ventricular septal defect.
The membranous interventricular septum develop from primitive jelly-like cushions lining the early myocardial heart tube. The endothelial cells covering the cushions invade the underlying matrix by epithelial to mesenchymal transformation, where they proliferate and reshape the primitive cushions into relatively thick valve-like cellular structures by mid-gestation. Subsequently, the cushions remodel to form the upper membranous part of the ventricular septum and the thin mature leaflets of the atrioventricular and semilunar valves [15, 16]. According to the embryology of the ventricular septum and echocardiographic findings, we think, in the processes of cardiac septation, the membranous ventricular septum, as a connective tissue, is getting denser and denser, stiffer and stiffer across gestation, and accordingly, the regional diastolic function in which is decreasing, while the strain rate in which is increasing.
Myocardial deformation analysis (measurement of strain and strain rate) is a direct assessment of conformational change of the myocardium and has been used in adults and in children to prognosticate subclinical disease [17] or to determine the severity of the diseases. Measurement of myocardial strain and strain rate in the normal fetus were first reported in the literature 9 years ago [18,19,20] and are now numerous [8, 17]. Maskatia et al. [8, 17] reported that global circumferential systolic strain rate, global longitudinal left ventricular systolic strain rate, and global longitudinal right ventricular systolic strain rate decreased from 20 to 21 weeks’ gestation to the remainder of gestation and then remained stable until delivery, and left ventricular longitudinal and circumferential diastolic strain rate values decreased across gestation, while right ventricular longitudinal values remained stable. Perles et al. [5] and Peng et al. [6] reported that strain rate are stable throughout gestation, and Kapusta et al. [21] also reported that global and regional strain rates of both ventricles decrease in a similar way during pregnancy. However, in our study, we found that regional myocardial systolic and diastolic strain rates in the membranous ventricular septum all increased across gestation, and were not stable throughout gestation. We speculate that the special development pattern (mentioned above) of membranous interventricular septum that is different from muscular interventricular septum mainly accounts for this phenomenon. In addition, the different methods used and the different cohorts recruited may also explain this phenomenon. Further studies are warranted.
Although the measurement and clinical significance of strain rate have not been thoroughly investigated in fetuses, measurement and analysis of strain rate changes across gestation might provide us a way to prejudge the occurrence of ventricular septal defect. Our study showed all the absolute values of strain rate (SRs, SRe and SRa) in the membranous ventricular septum decreased significantly in fetuses suffered from VSD after delivery. Zhang et al. [22] also reported that interventricular septal early diastolic strain rate in VSD patients decreased significantly compared with healthy children. In our study, because of a relatively poor repeatability at 18 weeks and relatively late examination time at 30 weeks, we chose the strain rate values at 24 weeks during pregnancy as a parameter to predict a complete membranous ventricular septum. We found that both SRe and SRa at this moment have good predictive value for a complete membranous ventricular septum, moreover, they were all better than SRs. We noticed that not SRs, but both SRe and SRa, at this moment, had greater differences between fetuses suffered from VSD and those without VSD after delivery (Table 1). VSD was more likely to affect the diastolic function of membranous ventricular septum. This was consistent with the development pattern (mentioned above) of membranous interventricular septum.
Our study had some limitations. First, because of inherent challenges to fetal ultrasonography (high fetal heart rate and small myocardial mass), the repeatability of the strain rate measurements was not very satisfactory. Although all images were assessed with high frame rates (130 frames s−1) to detect small changes more accurately, sometimes, fetal movement and the abdominal wall movement of pregnant women still have a serious impact on the acquisition of raw data, so that the strain rate imaging can not be measured more accurately. Another limitation is the lack of correlation of the strain values with other imaging modalities, such as MRI.
Conclusion
In this study, we described the changes of fetal regional myocardial strain rate in the membranous ventricular septum across gestation and evaluated the usefulness of these strain rates for predicting a perimembranous ventricular septal defect after delivery. Our results showed that these strain rate parameters all increased across gestation, and correlated with gestational age as well as fetal left ventricular mass. They can be used to predict the integrity of membranous ventricular septum after delivery. Notwithstanding some limitations mentioned above, measurement of strain rates still holds considerable clinical promise for the assessment of the development of membranous ventricular septum.
References
Gardiner HM (2005) Response of the fetal heart to changes in load: from hyperplasia to heart failure. Heart 91:871–873
Oh JK, Park SJ, Nagueh SF (2011) Established and novel clinical applications of diastolic function assessment by echocardiography. Circ Cardiovasc Imaging 4:444–455
Greenberg NL, Firstenberg MS, Castro PL, Main M, Travaglini A, Odabashian JA, Drinko JK, Rodriguez LL, Thomas JD, Garcia MJ (2002) Doppler-derived myocardial systolic strain rate is a strong index of left ventricular contractility. Circulation 105:99–105
Korinek J, Wang J, Sengupta PP, Miyazaki C, Kjaergaard J, McMahon E, Abraham TP, Belohlavek M (2005) Two-dimensional strain—a Doppler-independent ultrasound method for quantitation of regional deformation: validation in vitro and in vivo. J Am Soc Echocardiogr 18:1247–1253
Perles Z, Nir A, Gavri S, Rein AJ (2007) Assessment of fetal myocardial performance using myocardial deformation analysis. Am J Cardiol 99:993–996
Peng QH, Zhou QC, Zeng S, Tian LQ, Zhang M, Tan Y, Pu DR (2009) Evaluation of regional left ventricular longitudinal function in 151 normal fetuses using velocity vector imaging. Prenat Diagn 29:1149–1155
Crispi F, Sepulveda-Swatson E, Cruz-Lemini M, Rojas-Benavente J, Garcia-Posada R, Dominguez JM, Sitges M, Bijnens B, Grataco’s E (2012) Feasibility and reproducibility of a standard protocol for 2D speckle tracking and tissue Doppler-based strain and strain rate analysis of the fetal heart. Fetal Diagn Ther 32(1–2):96–108
Maskatia SA, Pignatelli RH, Ayres NA, Altman CA, Sangi-Haghpeykar H, Lee W (2016) Fetal and neonatal diastolic myocardial strain rate: normal reference ranges and reproducibility in a prospective, longitudinal cohort of pregnancies. J Am Soc Echocardiogr 29:663–669
Zheng XZ, Yang B, Wu J (2014) Fetal left ventricular mass determination on 2-dimensional echocardiography using area-length calculation methods. J Ultrasound Med 33:349–354
D’hooge J, Heimdal A, Jamal F, Kukulski T, Bijnens B, Rademakers F, Hatle L, Suetens P, Sutherland GR (2000) Regional strain and strain rate measurements by cardiac ultrasound: principles, implementation and limitations. Eur J Echocardiogr 1:154–170
Lee J, Moghadam ME, Kung E, Cao H, Beebe T, Miller Y, Roman BL, Lien CL, Chi NC, Marsden AL, Hsiai TK (2013) Moving domain computational fluid dynamics to interface with an embryonic model of cardiac morphogenesis. PLoS ONE 8:e72924. https://doi.org/10.1371/journal.pone.0072924
Wiputra H, Lim GL, Chua KC, Nivetha R, Soomar SM, Biwas A, Mattar CNZ, Leo HL, Yap CH (2017) Peristaltic-like motion of the human fetal right ventricle and its effects on fluid dynamics and energy dynamics. Ann Biomed Eng 45:2335–2347
Miller LA (2011) Fluid dynamics of ventricular filling in the embryonic heart. Cell Biochem Biophys 61:33–45
Lai CQ, Lim GL, Jamil M, Mattar CN, Biswas A, Yap CH (2016) Fluid mechanics of blood flow in human fetal left ventricles based on patient-specific 4D ultrasound scans. Biomech Model Mechanobiol 15:1159–1172
Rosenthal N, Harvey RP (2010) Heart development and regeneration. Academic Press, Amsterdam
Mommersteeg MT, Yeh ML, Parnavelas JG, Andrews WD (2015) Disrupted Slit-Robo signalling results in membranous ventricular septum defects and bicuspid aortic valves. Cardiovasc Res 106:55–66
Maskatia SA, Pignatelli RH, Ayres NA, Altman CA, Sangi-Haghpeykar H, Lee W (2016) Longitudinal changes and interobserver variability of systolic myocardial deformation values in a prospective cohort of healthy fetuses across gestation and after delivery. J Am Soc Echocardiogr 29:341–349
Ta-Shma A, Perles Z, Gavri S, Golender J, Tarshansky S, Shlichter C, Bar Tov H, Rein AJ (2008) Analysis of segmental and global function of the fetal heart using novel automatic functional imaging. J Am Soc Echocardiogr 21:146–150
Di Salvo G, Russo MG, Paladini D, Felicetti M, Castaldi B, Tartaglione A, di Pietto L, Ricci C, Morelli C, Pacileo G, Calabr R (2008) Two-dimensional strain to assess regional left and right ventricular longitudinal function in 100 normal foetuses. Eur J Echocardiogr 9:754–756
Younoszai AK, Saudek DE, Emery SP, Thomas JD (2008) Evaluation of myocardial mechanics in the fetus by velocity vector imaging. J Am Soc Echocardiogr 21:470–474
Kapusta L, Mainzer G, Weiner Z, Deutsch L, Khoury A, Haddad S, Lorber A (2013) Changes in fetal left and right ventricular strain mechanics during normal pregnancy. J Am Soc Echocardiogr 26:1193–1200
Zhang LM, Wang YQ, Wang WY, Li S, Ma CY, Yang J (2016) Assessing interventricular septal function after ventricular septal defect repair by using two-dimensional longitudinal strain and strain rate. Chin J Med Phys 33:805–809
Acknowledgements
The authors gratefully acknowledge the technical assistance of Xia EH, Huang XQ, Fan GX at the department of ultrasound, The First People’s Hospital of Yancheng, Jiangsu Province, P. R. China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Zhang, LJ., Chen, KQ., Shi, YY. et al. Fetal regional myocardial strain rate in the membranous ventricular septum: changes with gestational age and the left ventricular mass and predictive value for a complete membranous ventricular septum (without defect). Int J Cardiovasc Imaging 34, 1403–1408 (2018). https://doi.org/10.1007/s10554-018-1354-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-018-1354-9