Abstract
The new methods for diagnosing the ischemia with coronary computed tomographic angiography (CTA) as a noninvasive test have been investigated. To compare the relative plaque volume to quantitative CTA and quantitative coronary angiography (QCA) for detecting flow-limiting coronary artery stenoses. We studied 49 patients with 55 intermediate lesions (30–69% diameter stenosis) who underwent CTA, coronary angiography (CAG), and FFR. CTA and QCA measures included lesion length, percent diameter stenosis (%DS), minimal lumen diameter (MLD), target main vessel percent plaque volume (%PV), lesion %PV, target main vessel percent lumen volume (%LV), and lesion %LV. FFR ≤0.80 was considered diagnostic of a flow-limiting lesion. The area under the receiver-operating characteristic curve (AUC) was used to determine the accuracy of detecting flow-limiting lesions. We also investigated the AUC of discrimination of flow-limiting lesion according to calcium score. Eighteen of 55 lesions (32.7%) had an FFR ≤0.80. Only vessel %PV differentiated between lesions with and without flow obstruction (67.6 vs. 62.7%, p = 0.018). The AUC for vessel %PV was greatest (0.76; 95% CI 0.61–0.87). The AUC for the discrimination of the flow-limiting lesions according to low calcium score (≤400) improved to 0.82 (95% CI 0.57–0.94). In intermediate coronary artery stenoses, vessel %PV is more accurate than conventional stenosis assessment for detecting flow-limiting lesions. In low calcium score, vessel %PV is more useful for diagnosis of ischemic heart disease compared with conventional quantitative measures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary computed tomographic (CT) angiography (CTA) is a noninvasive test for identification of anatomic coronary artery disease (CAD) severity; however, it does not discriminate whether a stenosis causes ischemia. The fractional flow reserve vs. angiography for multivessel evaluation (FAME) and FAME 2 studies have suggested that it is important to prove the ischemia with fractional flow reserve (FFR) before percutaneous coronary intervention [1, 2]. The new methods for diagnosing ischemia with noninvasive tests have recently been investigated. For example, CT stress myocardial perfusion was reported to improve the diagnosis of ischemia [3, 4], but patients have to receive much radiation exposure with the test. Transluminal attenuation gradient (TAG) was reported to predict severe stenosis, but it did not certify the relation between TAG and ischemia [5]. FFRCT is a new method that applies computational fluid dynamics to determine the physiologic significance of CAD [6]; however, it has not spread as a clinical test until today because it is costly and needs proprietary software.
Quantitative plaque volume analysis does not need any special method when a patient undergoes CTA. Nakazato et al. reported that percent aggregate plaque volume (%APV) improved identification of ischemic lesions of intermediate stenosis [7]. On the other hand, Park HB et al. reported that plaque size, such as %APV and lesion length, did not predict the ischemia in lesions with less than 50% stenosis [8]. An early study suggested that diffusely atherosclerotic coronary arteries without segmental stenosis caused continuous pressure fall along the arterial length [9]. The relationship between total plaque volume in vessel with a stenosis and ischemia is poorly understood in intermediate stenosis.
The purpose of this study was to compare vessel plaque volume to quantitative CTA for detecting flow-limiting coronary artery stenosis. We also compared to quantitative coronary angiography (QCA).
Methods
Patients
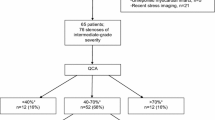
This study was a single-center retrospective study. There were 682 patients who underwent invasive coronary angiography (ICA) as a result of CTA from June 2012 to May 2014 in our hospital. Of them, 76 patients with intermediate lesions (30–69% diameter stenosis based on QCA) underwent FFR. We excluded patients with a target vessel that had an implanted stent, coronary artery bypass graft, or multi-lesions in the same vessel. We enrolled 49 patients with 55 intermediate lesions (Fig. 1). The study protocol was approved by the medical ethical committee and all patients were given written informed consent.
Multi detector computed tomography (MDCT) examination techniques
Patients with a heart rate more than 70 beats/min received 20 mg metoprolol; patients with a heart rate ≥65 beats/min received 0.125 mg/kg landiolol. Patients with systolic blood pressure ≥110 mmHg received nitroglycerine before CT examination. Imaging was performed with a whole-heart scan on a 320-row MDCT scanner (Toshiba AquilionOne Dynamic Volume CT, Otawara, Japan). The scanning coverage was from the middle ascending aorta to the upper abdomen. Imaging was achieved using an axial volumetric scanning technique without table movement because the scanner provides 16 cm of Z-axis volume using 0.5 mm detectors. The CT gantry rotation time was 350 ms. The tube voltage was 120 kV and the tube modulation was 300–400 mA for all patients. The 320-row CTA was performed by end-diastolic prospective scanning with an ECG-gated window of 65–85% phase of the R-R interval and single to three heartbeat wide-volume scanning. In patients with a heart rate ≤65 bpm, single heartbeat was used for image acquisition to allow an adaptive half scan; with heart rate >65 bpm, 2 or 3 heartbeats were used for image acquisition to allow adaptive multi-segment image reconstruction to be applied for improved temporal resolution. The patient received [0.07 × body weight (range 4.0–5.0) × 10] ml of Iopamidol 370 mg I/ml (Iopamiron-370; Bayer HealthCare, Osaka, Japan) followed by 30-ml normal saline injected into a superficial vein in the antecubital fossa with a 20 gauge needle via a dual-head power injector (Dual Shot GX, Nemoto Kyorindo, Tokyo, Japan) at a rate of (0.07 × body weight) ml/s (range, 4.0 to 5.0 ml/s). Bolus tracking in the ascending aorta was performed using a 200 Hounsfield unit (HU) threshold [10].
Quantitative analysis of CTA
All main epicardial coronary vessels with a lesion > 1.5 mm in diameter were analyzed (QAngioCT vs. 2.1 RC4, MEDIS™) according to an AHA 17 segment model. An automated luminal and vessel border detection were manually corrected when necessary. The proximal and distal edges of the lesion were selected to be the most adjacent points to the maximal stenosis in which there was minimal or no plaque, as shown in Fig. 2b. The target vessel means the vessel including the lesion. We defined quantitative plaque volume indices as follows: %plaque volume (%PV) = (total plaque volume/total vessel volume) × 100 and %lumen volume (%LV) = (total lumen volume/total vessel volume) × 100 [11]. CTA measures included lesion length, %diameter stenosis (%DS) and minimal lumen diameter (MLD). We also measured plaque volume, lumen volume, %PV, and %LV for the target vessel and lesion, respectively. The 49 patients with 55 intermediate lesions were analyzed for coronary artery plaque volume by two observers who did not know the results of the FFR.
a QCA analysis. b CTA analysis. a MLD and %DS were measured based on CAG. b The proximal and distal edges of the lesion were selected to be the most adjacent points to the maximal stenosis in which there was minimal or no plaque. The vessel means the target vessel with a lesion >1.5 mm in diameter. QCA quantitative coronary angiography, MLD minimal lumen diameter, %DS percent diameter stenosis, CTA CT angiography, PV plaque volume and LV lumen volume
We assessed the relation between APCs (coronary atherosclerotic plaque characteristics) and the ischemia. APCs included LAP (low attenuation plaque), PR (positive remodeling), and SC (spotty calcification). The definition of these APCs was shown in the previous paper [8]. LAP was defined as any voxel <30 Hounsfield units within a coronary plaque. A positive remodeling index was defined as a maximal lesion vessel diameter divided by proximal reference vessel diameter, with PR defined as a remodeling index ≥1.1. SC was defined by intra lesional calcific plaque <3 mm in the lesion that comprised <90°of the lesion circumference.
Invasive coronary angiography and fractional flow reserve
ICA was performed using standard techniques. Coronary segments were defined using a 19-coronary segment model. Significant coronary artery stenosis (obstructive CAD) was defined as ≥50% diameter stenosis by QCA as follows. FFR was measured using a 0.014-inch pressure sensor-tipped guidewire (PrimeWire PRESTIGE, Volcano Corporation, San Diego, California; PressureWireTM AerisTM, St. Jude Medical Systems, St. Paul, Minnesota) in intermediate diameter stenosis (30–69%), as previously described [12, 13]. The pressure tips were put at the distal of the lesion, not the distal of the target vessel. Hyperemia was induced with intracoronary bolus administration of papaverine hydrochloride 8 or 12 mg into the right or left coronary artery, respectively. FFR ≤0.80 was considered diagnostic of a flow-limiting lesion.
Quantitative analysis of invasive coronary angiography
All main epicardial coronary vessels with a lesion >1.5 mm in diameter were analyzed with dedicated software (QCA, vs. 7.1, MEDIS™). QCA measures included lesion length, %DS, and MLD as shown Fig. 2a.
Statistical analysis
Descriptive statistics were displayed using means and standard deviations for continuous variables and group proportions for categorical variables. Receiver operating characteristic (ROC) curves were used to determine the accuracy of CTA and ICA measures to predict flow-limiting coronary stenosis. The area under the receiver operating characteristic curve (AUC) was used to determine the accuracy of detecting a flow-limiting lesion. AUC values were compared using standard methods. All data were reported with 95% confidence intervals (CI). Additionally, a previous report showed that a high calcium score (>400) reduced the specificity of coronary stenosis [14]. Calcification overestimates coronary plaque volume. The CTA and ICA measures were assessed according to the presence of a high coronary artery calcium (CAC) score (>400).
The interobserver agreement for total coronary atheroma volume was determined for 20 randomly selected cases that were read by both observers. Bland–Altman plots were conducted to assess bias and interobserver variability.
Statistical significance was determined at p values <0.05. All analyses were conducted using the statistical software packages JMP (version 11 for Windows, SAS Institute Inc., Cary, NC, USA) and Stata (version 13.0, Stata Corp., College Station, TX, USA).
Results
Patient characteristics
Patient characteristics are shown in Table 1. Of all, 16 patients (33%) were FFR positive. There were no differences in patient characteristics between FFR positive and negative except smoking status.
QCA and CTA measures characteristics
We identified 18 ischemic lesions among 55 intermediate lesions. The correlations between FFR positive and negative are shown in Table 2. The lesion length measured by QCA and CTA had no difference between FFR positive and negative (12.2 vs. 11.3 mm, p = 0.57 for QCA; 22.5 vs. 17.4 mm, p = 0.19 for CTA). The calcium scores had no difference between FFR positive and negative (400 vs. 359, p = 0.66), but the medians of calcium score tended to be high. Vessel %PV in FFR positive was significantly higher than in FFR negative (67.6 vs. 62.7%, p = 0.018). Vessel %LV in FFR positive also was significantly lower than in FFR negative (31.9 vs. 37.8%, p = 0.013). There were no differences in lesion %PV and lesion %LV between FFR positive and negative (80.8 vs. 77.0%, p = 0.08 for lesion %PV; 19.2 vs. 23.0%, p = 0.08 for lesion %LV). MLD measured by QCA and CCTA had significant differences between FFR positive and negative (1.30 vs. 1.53 mm, p = 0.03 for QCA; 1.22 vs. 1.40 mm, p = 0.03 for CTA). There were no differences in %DS, positive remodeling, spotty calcification, or low attenuation plaque between FFR positive and negative.
Discrimination of flow-limiting lesion of intermediate stenosis by coronary CTA and QCA
The AUC and odds ratio (OR) of discrimination of flow-limiting lesion is shown in Fig. 3. The AUC for vessel %PV (0.76; 95% CI 0.61–0.87) was more accurate than for lesion %PV (0.65; 95% CI 0.48–0.79), for MLD measured by QCA (0.67; 95% CI 0.49–0.81), or MLD measured by CTA (0.69; 95% CI 0.53–0.82). OR of vessel %PV was 1.12 (95% CI 1.02–1.25). OR of MLD measured by QCA and CTA were also statistically significant (0.14; 95% CI 0.019–0.77 for QCA; and 0.15; 95% CI 0.020–0.77 for CTA).
AUC for discrimination of the flow-limiting lesions when FFR < 0.80 was diagnostic for flow-limiting lesion. AUC was highest for vessel %PV at 0.76. OR was highest for vessel %PV at 1.12. AUC area under the receiver operating characteristic, FFR fractional flow reserve, MLD minimal lumen diameter, OR odds ratio, %DS percent diameter stenosis, %PV percent plaque volume
The AUC for discrimination of the flow-limiting lesions according to low calcium score (≤400) is shown in Fig. 4. The AUC of vessel %PV improved from 0.76 to 0.82 (95% CI 0.57–0.94) compared with the AUC for all vessels with an intermediate lesion. OR of vessel %PV also improved from 1.12 to 1.55 (95% CI 1.13–2.47). On the other hand, the AUC of MLD measured by QCA and CTA decreased (0.59; 95% CI 0.34–0.80 for QCA, and 0.64; 95% CI 0.41–0.83 for CTA). The AUC for discrimination of the flow-limiting lesions according to calcium score (>400) is shown in Fig. 5. The AUC of vessels and lesion %PV—with vessels calcified severely—decreased compared with AUC for all vessels with intermediate lesion (0.71; 95% CI 0.47–0.86, 0.60; 95% CI 0.36–0.80). On the other hand, %DS and MLD measured by QCA improved (0.75; 95% CI 0.52–0.90, 0.75; 95% CI 0.49–0.90).
AUC for discrimination of the flow-limiting lesions According to calcium score ≤ 400. AUC of vessel %PV improved from 0.76 to 0.82 with calcium score less than 400. Abbreviations are same as in Fig. 3
AUC for discrimination of the flow-limiting lesions according to calcium score >400. AUC of vessel %PV with vessels calcified severely decreased from 0.76 to 0.71. Abbreviations are same as in Fig. 3
The AUC for discrimination of the flow-limiting lesion compared between %LV and %PV is shown in Fig. 6. The AUC of vessel %LV (0.76, 95% CI 0.62–0.87) was as accurate as vessel %PV. OR of the vessel %LV (0.89, 95% CI 0.79–0.97) was statistically significant. The AUC of lesion %LV was 0.65 (95% CI 0.48–0.79). The AUC of vessel and lesion %LV for discrimination of flow-limiting lesions according to calcium score (≤400) is shown in Fig. 7. The AUC improved from 0.76 to 0.84 (95% CI 0.61–0.94).
AUC for discrimination of the flow-limiting lesions compared with lumen volume. AUC of vessel %LV was as high as vessel %PV at 0.76. %LV percent lumen volume. Other abbreviations are same as in Fig. 3
Agreement
Bland–Altman analysis of 20 cases revealed excellent correlation between two observers for total vessel atheroma volume (r = 0.90; 95% CI 0.53–0.86; p < 0.001), as shown in Fig. 8a. The Bland–Altman plot implied a good interobserver concordance between reader 1 and 2. The mean difference was −162.2 mm3 (limits of agreement: −564.6 to 240.1), as shown in Fig. 8b.
The linear regression and Bland–Altman analysis in inter-observer agreement. a The liner linear regression graph in 20 cases revealed an excellent correlation between two observers for total vessel atheroma volume. b The Bland–Altman plot implied a good inter-observer concordance between reader 1 and 2. The mean difference was −162.2 mm3 (limits of agreement: −564.6 to 240.1)
Discussion
In this study, we indicated that vessel %PV was more accurate than lesion %PV and any other parameters measured by traditional CTA in intermediate stenosis, as seen in Fig. 2. Nakazato et al. reported that %APV, or lesion %PV in our study, improved the diagnostic performance of the ischemia in the intermediate lesions [7]. Our study suggests a new concept: that vessel %PV diagnoses the ischemia for intermediate stenosis. Vessel %PV could be considered as a predictor of stenosis in upcoming prospective studies. We discussed two reasons leading to our results. First, these results may depend on the narrowing of the lumen. In the process of atherosclerosis progression, the atheroma volume increased for preserving the lumen volume as positive remodeling at first. After that, the atheroma volume continued to increase, lumen volume decreased, and vessel diameter became small. At last, atherosclerotic vessels became negative remodeling [15]. The increase of vessel %PV might reflect the narrowing of the lumen under advanced atherosclerosis. This was also confirmed by the result that AUC of vessel %LV had the same result as that of vessel %PV. Second, our results may conform to mild diffuse atherosclerosis. Previous reports have shown that diffusely atherosclerotic coronary arteries cause continuous pressure fall along arterial length [9, 16]. In mild diffuse diseases, vessels with focal intermediate lesions had significantly lower FFR value than vessels without focal intermediate lesions [17]. Atherosclerotic arteries have been reported to have higher resistance than normal arteries and to associate the deficient endothelium-mediated vasodilation [9, 18]. There may have been a tandem lesion that was less than 30% diameter stenosis in the same vessel in our study. Increased vessel plaque volume besides the intermediate lesion in the target vessel might affect the flow-limiting lesions. The relationship between plaque volume in mild stenosis and FFR is poorly understood.
Our study also showed that vessel %PV, lesion %PV, vessel %LV, and lesion %LV were more accurate for the diagnosis of flow-limiting lesions with low calcium scores. Calcified plaque overestimates coronary artery stenosis according to the beam-hardening and blooming artifact [19]. On the other hand, MLD and %DS measured by QCA did not improve the AUC according to low calcium scores. This is appropriate because QCA estimates the lumen without the influence of calcified plaque. Based on these results, we would like to emphasize that vessel %PV—not MLD or %DS measured by QCA—has the closest relationship with the flow-limiting lesions in intermediate lesions.
We investigated the relation between ischemia and atherosclerotic plaque characteristics (APCs) including positive remodeling, low attenuation plaque, and spotty calcification. Our study showed that APCs had no significant differences between FFR positive and negative. On the other hand, previous studies have indicated that APCs improved the identification of ischemia [8]. This difference might be because the coronary arteries in our study were under advanced atherosclerosis and there was much calcified plaque. This was also shown by one report which found that plaque composition in acute coronary syndrome includes APCs and by another report which found that stable angina includes calcified plaque [20, 21]. The clinical implications were that the reduction of plaque volume may lead to prevention of myocardial ischemia. It is widely acknowledged that statins reduce the percent of atheroma volume [22, 23]. We should use statins to decrease vessel %PV.
Limitations
This study had some limitations. First, this study was performed in a single institution and the sample size was relatively small. In our study, many vessels had much calcified plaque. Non-calcified plaque might lead to other results. To further verify the results, future studies should be conducted in a larger sample size. Second, this study was a retrospective study. Although we selected the intermediate lesion on CAG, the relation between the intermediate stenosis on CTA and the vessel plaque volume may prove the usefulness of CTA. Third, we did not measure plaque volume by IVUS. Nonetheless, it was previously reported that plaque volume measured by QCT correlated with plaque volume measured by IVUS [24].
Conclusions
In coronary lesions with intermediate stenosis, vessel %plaque volume is more accurate than conventional stenosis assessment for detecting flow-limiting lesions. Vessel %plaque volume is more useful for diagnosis of ischemic heart disease than conventional quantitative measures in low calcium score.
References
Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’t Veer M et al (2009) Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 360:213–224
De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z et al (2012) Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 367:991–1001
Ko BS, Cameron JD, Leung M, Meredith IT, Leong DP, Antonis PR et al (2012) Combined CT coronary angiography and stress myocardial perfusion imaging for hemodynamically significant stenoses in patients with suspected coronary artery disease: a comparison with fractional flow reserve. JACC Cardiovasc Imaging 5:1097–1111
Rochitte CE, George RT, Chen MY, Arbab-Zadeh A, Dewey M, Miller JM et al (2014) Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study. Eur Heart J 35:1120–1130
Choi JH, Min JK, Labounty TM, Lin FY, Mendoza DD, Shin DH et al (2011) Intracoronary transluminal attenuation gradient in coronary CT angiography for determining coronary artery stenosis. JACC Cardiovasc Imaging 4:1149–1157
Min JK, Leipsic J, Pencina MJ, Berman DS, Koo BK, van Mieghem C et al (2012) Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 308:1237–1245
Nakazato R, Shalev A, Doh JH, Koo BK, Gransar H, Gomez MJ et al (2013) Aggregate plaque volume by coronary computed tomography angiography is superior and incremental to luminal narrowing for diagnosis of ischemic lesions of intermediate stenosis severity. J Am Coll Cardiol 62:460–467
Park HB, Heo R, o Hartaigh B, Cho I, Gransar H, Nakazato R et al (2015) Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: a direct comparison to fractional flow reserve. JACC Cardiovasc Imaging 8:1–10
De Bruyne B, Hersbach F, Pijls NH, Bartunek J, Bech JW, Heyndrickx GR et al (2001) Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but “Normal” coronary angiography. Circulation 104:2401–2406
Valentin J (2007) Managing patient dose in multi-detector computed tomography(MDCT). ICRP Publication 102. Ann ICRP 37:1–79
Papadopoulou SL, Neefjes LA, Garcia-Garcia HM, Flu WJ, Rossi A, Dharampal AS et al (2012) Natural history of coronary atherosclerosis by multislice computed tomography. JACC Cardiovasc Imaging 5:S28–S37
MacCarthy P, Berger A, Manoharan G, Bartunek J, Barbato E, Wijns W et al (2005) Pressure-derived measurement of coronary flow reserve. J Am Coll Cardiol 45:216–220
Pijls NH, Van Gelder B, Van der Voort P, Peels K, Bracke FA, Bonnier HJ et al (1995) Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation 92:3183–3193
Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E et al (2008) Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 52:1724–1732
Voros S, Rinehart S, Qian Z, Joshi P, Vazquez G, Fischer C et al (2011) Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc Imaging 4:537–548
Rodes-Cabau J, Gutierrez M, Courtis J, Larose E, Dery JP, Cote M et al (2011) Importance of diffuse atherosclerosis in the functional evaluation of coronary stenosis in the proximal-mid segment of a coronary artery by myocardial fractional flow reserve measurements. Am J Cardiol 108:483–490
Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R et al (2013) Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol 62:1639–1653
Zeiher AM, Schachlinger V, Hohnloser SH, Saurbier B, Just H (1994) Coronary atherosclerotic wall thickening and vascular reactivity in humans. Elevated high-density lipoprotein levels ameliorate abnormal vasoconstriction in early atherosclerosis. Circulation 89:2525–2532
Park MJ, Jung JI, Choi YS, Ann SH, Youn HJ, Jeon GN et al (2011) Coronary CT angiography in patients with high calcium score: evaluation of plaque characteristics and diagnostic accuracy. Int J Cardiovasc Imaging 27(Suppl 1):43–51
Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T et al (2007) Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 50:319–326
Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T et al (2009) Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 54:49–57
Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H et al (2004) Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study. Circulation 110:1061–1068
Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P et al (2011) Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 365:2078–2087
de Graaf MA, Broersen A, Kitslaar PH, Roos CJ, Dijkstra J, Lelieveldt BP et al (2013) Automatic quantification and characterization of coronary atherosclerosis with computed tomography coronary angiography: cross-correlation with intravascular ultrasound virtual histology. Int J Cardiovasc Imaging 29:1177–1190
Acknowledgements
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that all authors have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kato, N., Kishi, S., Arbab-Zadeh, A. et al. Relative atherosclerotic plaque volume by CT coronary angiography trumps conventional stenosis assessment for identifying flow-limiting lesions. Int J Cardiovasc Imaging 33, 1847–1855 (2017). https://doi.org/10.1007/s10554-017-1186-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-017-1186-z