Abstract
Impaired ventricular myocardial mechanics are observed in patients with repaired tetralogy of Fallot (rTOF). Effects of pulmonary valve replacement (PVR) on ventricular remodeling are controversial. The objective was to assess the impact of surgical PVR on ventricular mechanics in pediatric patients after rTOF. Speckle-tracking analysis was performed in 50 rTOF children, aged 12.6 ± 3.3 years, pre-operatively and 14.5 ± 2.2 months post-PVR. Early post-operative studies 2.2 ± 0.6 months post-PVR were performed in 28 patients. Cardiac magnetic resonance (CMR) pre- and post-PVR was collected. Mid-term post-PVR right ventricular (RV) longitudinal strain increased above pre-operative strain (−19.2 ± 2.7 to −22.0 ± 3.0%, p < 0.001) with increases observed in individual RV segments. Left ventricular (LV) strain did not differ at medium-term follow-up. LV and RV longitudinal strain was reduced early post-operatively, followed by recovery of biventricular systolic strain by mid-term follow-up. CMR RV end-diastolic indexed volumes correlated with RV strain pre-operatively (r = 0.432, p = 0.005) and at mid-term follow-up (r = 0.532, p = 0.001). Volume-loaded RVs had reduced early RV basal longitudinal strain compared to pressure-loading conditions. Reversed basal counterclockwise rotation was associated with lower mid-term global LV and basal RV strain compared to patients with normal rotation. An increase in mid-term global and regional RV strain beyond pre-operative values suggests positive RV remodeling and adaptation occurs in children post-PVR. Patients with larger pre-operative RV volumes had lower RV strain post-operatively. The impact of LV rotation on RV mechanics highlights the presence of ventriculo-ventricular interactions. These findings have important clinical implications in pediatric rTOF patients towards identifying pre-operative factors that predict RV post-operative remodeling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic pulmonary regurgitation after surgical repair of tetralogy of Fallot (rTOF) can result in progressive right ventricular (RV) dilatation and biventricular dysfunction leading to adverse clinical outcomes [1–4]. A reduction in both right and left ventricular (LV) strain parameters compared to controls have been described in young patients with rTOF [5, 6]. In current clinical practice surgical pulmonary valve replacement (PVR) is proposed to patients who become symptomatic or who develop progressive right ventricular (RV) dilation and/or RV dysfunction [7–9]. Determining the optimal timing of surgery, however, remains a challenge as it requires a balance between intervening too early, or too late to allow for RV recovery. Improvements in global LV strain and RV in subgroups are reported following percutaneous PVR [10] but overall the effects of surgical PVR on ventricular remodeling and myocardial deformation remain poorly defined. We hypothesized that PVR has a beneficial effect on ventricular remodeling characterized by improvements in myocardial deformation. The primary objective of our study was to assess the short and medium term effects of surgical PVR on ventricular function and myocardial strain in children after rTOF. Secondary objectives were to determine the effect of pre-operative RV volume loading as well as LV myocardial mechanics on post-operative strain.
Methods
This is a single-center retrospective cross-sectional study conducted with approval from the research ethics board. The institutional echocardiography database was screened for patients following rTOF and who had serial follow-up after surgical PVR between January 2008 and December 2014. Patients were included if they had a transthoracic echocardiogram (TTE) performed prior to and approximately 12 months following PVR without interval surgeries or interventions. If available, early post-operative studies performed within 1–3 months following PVR were collated. Patients were excluded if they had a primary diagnosis of an atrioventricular septal defect with TOF, absent pulmonary valve syndrome, if they required multiple staged operations prior to complete repair or were being paced during the TTEs. Studies with suboptimal quality TTE datasets unsuitable for strain analysis or had post-operative studies outside the desired time periods for analysis were excluded. Cardiac magnetic resonance (CMR) imaging information prior to and post-PVR were collected when available. Clinical and demographic details were obtained from the patients’ medical records.
Echocardiography
Clinical TTE studies were performed using Philips (IE33; Philips Ultrasound, Bothell, WA) or GE (Vivid 7 or 9; GE Medical Systems, Milwaukee, WI) machines. Images were stored on a Syngo Dynamics server (Siemens Medical Solutions, Malver, PA) in DICOM format at a default frame rate of 30 frames/sec for all studies. Two-dimensional (2D) measurements were analyzed off-line using Syngo Dynamics software and performed in accordance with previously published American Society of Echocardiography guidelines [11]. The 2D measurements included LV and RV end-diastolic dimensions (EDD), LV M-mode derived ejection fraction (EF), RV end-diastolic and end-systolic areas, RV fractional area of change (FAC) and tricuspid annular plane systolic excursion (TAPSE). The degree of pulmonary regurgitation (PR), tricuspid regurgitation, RV outflow tract (RVOT) obstruction and RV systolic pressures were also recorded.
Myocardial strain analysis was performed by speckle-tracking analysis using the TomTec Image-Arena platform (Version 4.6, TomTec Imaging systems, Unterschleissheim, Germany). Left ventricular longitudinal strain was calculated by tracing the endocardial border of the ventricles in the apical four-chamber, three-chamber and two-chamber views, and recorded if reliable segmental strain on visual assessment in at least ≥3 ventricular wall segments were present. The segments that did not have reliable tracking were excluded from analysis. Global longitudinal strain was calculated as an average of strain measurements in the three different longitudinal views. Circumferential LV strain was also calculated by contouring the endocardial border in the basal, mid and apical short-axis planes with global circumferential strain calculated as an average of strain from the three short-axis levels. Right ventricular longitudinal strain was obtained by averaging RV basal, mid and apical lateral wall measurements and recorded if segmental strain was present in at least two lateral RV segments. Regional strain parameters for the interventricular septum were incorporated into LV longitudinal strain calculations. Left ventricular rotation was recorded at basal and apical levels. A single observer performed all strain analyses and the results were re-evaluated by blinded re-analysis of 15 randomly selected studies 3 months after the initial analysis was performed.
CMR
Clinical CMR studies were performed with a 1.5T Avanto scanner (Siemens Medical Systems, Erlangen, Germany). Quantification of ventricular volumes and ejection fraction (EF) was performed using a cine short axis stack with manual contouring of the endocardial borders at end-diastole and end-systole. Pulmonary regurgitant fractions were derived from phase contrast velocity mapping of the main pulmonary artery. The presence of late end-diastolic forward flow as a marker of restrictive physiology was also noted. The CMR sequences were acquired and analyzed according to standardized CMR practice guidelines [12].
In order to evaluate the effect of volume and/or pressure loading effects on myocardial strain, a combination of echocardiographic and CMR data were used. The patients were subdivided into three groups—those with a predominantly volume-loaded or pressure-loaded RVs, or if they had a combination of both obstruction and PR. A volume-loaded ventricle was defined as an RVOT gradient <25 mmHg by TTE and a PR fraction of >20% by CMR, whereas a pressure-loaded ventricle was defined as RVOT gradient >40 mmHg and a PR fraction of <20%. The remainder of the cohort was considered to have a mixed volume and pressure-loaded ventricle. In the cases that CMR was not available pre-operatively, the degree of PR was judged by echocardiography and was graded from nil/trivial to severe.
Statistical methods
The statistical analysis was performed using IBM SPSS Statistics version 22 (IBM, Armonk, New York). Measurements were expressed as mean values with standard deviation (SD) for continuous variables that were normally distributed and absolute numbers with percentages for categorical variables. A two-tailed paired Student’s t-test was used to determine significant differences between pre-operative and post-operative measurements. The McNemar test was used to assess for significance between categorical variables for paired data and ANOVA was used to compare the differences between pressure, volume and mixed ventricular loading conditions. Pearson bivariate correlation was used to compare continuous pre- and post-operative variables. P values <0.05 were considered statistically significant. Intraobserver variability was assessed using Bland–Altman analysis, linear regression and calculation of the intraclass correlation coefficients.
Results
50 children, aged 12.6 ± 3.4 years at the time of pre-operative TTE were included. 33 (66%) of cases were male. Ten patients (20%) had associated syndromes, including 22q11.2 deletion in 5, VACTERL association in two, Trisomy 21 in one and Aarskog syndrome in one patient. Five (10%) patients required palliative Blalock-Taussig shunts prior to complete repair and 32 (64%) cases were repaired with a transannular patch. The mean age at primary TOF repair was 8.7 ± 6.2 months. A transannular patch was performed in 30 (60%) cases and 18 (36%) patients underwent a valve-sparing repair. The surgical details of the primary operation were not available in 2 cases performed overseas. The mean age at PVR was 12.6 ± 3.3 years and the types of prostheses included the Mosaic® bioprosthetic valve in 38 (76%) patients, Contegra® valved conduit in 6 (12%) patients and homograft bioprosthesis in 6 (12%) patients. The mean time frame between the pre-operative study (pre-PVR) and PVR was 2.1 ± 2.1 months and between PVR and the mid-term post-PVR echocardiogram (mid post-PVR) was 14.5 ± 4.2 months. Additional early strain data was collected in 28 patients who had post-operative echocardiograms 2.2 ± 0.6 months following PVR (early post-PVR). There were no significant differences in QRS duration or average heart rate between studies.
Echocardiographic measurements
Baseline demographics and two-dimensional (2D) echocardiographic measurements between pre-PVR and mid post-PVR echocardiograms are summarized in Table 1 and ventricular dimensions in Table 2. There was a reduction in RV dimensions and size, degree of PR, RVOT obstruction and estimated RV pressures between studies. Left ventricular dimensions and TAPSE increased post-operatively. There were no significant differences for LV EF or RV FAC between studies.
LV and RV myocardial strain
Pre-operative vs mid-term post-operative results
Left ventricular and RV myocardial deformation parameters as well as rotation were measured pre-operatively and mid post-PVR in all patients and highlighted in Table 3. There was an increase in RV regional and global longitudinal strain above pre-operative strain values mid post-PVR. The highest segmental strain values were observed in the basal RV segments and there was a progressive decrease in segmental strain from basal to apical segments. There were no significant changes in LV longitudinal strain or rotation parameters between pre-operative and mid-term post-operative studies.
Pre-operative vs early post-operative results
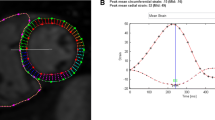
Further sub-analysis was performed in 28 (56%) patients who had additional early post-operative echocardiograms 2.1 ± 2.1 months after PVR. A reduction in RV basal longitudinal strain and global RV strain was initially observed in the early post-operative period. Longer-term follow-up subsequently showed an increase in both regional and global RV strain when compared to early post-PVR strain. Box plots illustrating interval changes in segmental and global RV strain results in this subgroup of patients are highlighted in Fig. 1.
Similarly to the RV, LV apical 4-chamber longitudinal strain fell in the early post-operative period following PVR (−21.5 ± 5.0 pre-operatively vs −18.9 ± 2.9% early post-operatively, p = 0.048). Between early and mid-term postoperative studies, an interval increase in LV apical 4-chamber longitudinal strain (−18.9 ± 2.9 vs −21.2 ± 3.2%, p = 0.01) and global longitudinal LV strain (−20.2 ± 2.7 vs −24.1 ± 2.6%, p = 0.001) were observed. However, overall LV strain parameters at mid-term follow-up did not increase significantly from pre-operative values.
Effect of volume vs pressure loading conditions
Using the cutoffs for pressure vs volume loading as stated in the “Methods”, the primary indications for PVR were predominantly due to volume loading in 28 (56%) patients, pressure loading in 4 (8%) patients and a combination of mixed pressure and volume loading in 18 (36%) patients. Pre-operative RV and LV strain parameters were not significantly different between groups, however, significantly lower early post-operative RV basal strain was observed in volume loading compared with pressure loading conditions (−15.8 ± 3.8 vs −25.9 ± 1.6% respectively, p = 0.01). There were no significant differences in longitudinal strain between loading conditions at mid-term follow-up.
Left ventricular rotation
Left ventricular rotation parameters were assessed and reversed counterclockwise basal rotation was observed in 12/50 (24%) patients prior to PVR. When patients with pre-operative counterclockwise basal rotation were compared to patients with usual clockwise basal rotation, the group with abnormal rotation had significantly lower basal, mid and global RV longitudinal strain early post-PVR. Basal RV longitudinal strain remained reduced at mid-term follow-up. Mid post-operative global LV longitudinal strain was also significantly decreased in patients who exhibited reversed basal rotation. Early post-operative LV strain parameters were not significant. Notably, patients with counterclockwise rotation had higher pre-operative apical rotation compared with patients with clockwise rotation. The significant parameters between normal and abnormal basal rotation groups are outlined in Table 4. Examples of normal and reversed basal rotation curves are illustrated in Fig. 2.
Five out of 12 (42%) patients with reversed counterclockwise rotation reverted to clockwise rotation at mid-term follow-up. There were no significant differences in echocardiographic 2D or strain parameters between patients who reverted to normal rotation compared to those who had persistently abnormal basal rotation at mid-term follow-up.
CMR parameters and RV size and function
Clinical CMRs were performed in 41 (82%) patients pre-operatively and 13 (26%) patients post-operatively. The average time frame from pre-operative CMR to PVR was 8.2 ± 6.7 months and between PVR to post-operative CMR was 2.0 ± 1.1 years. Patients who had an available post-operative CMR had significantly lower RVEDVi (218 ± 43 vs 143 ± 39 ml/m2, p < 0.001), RVESVi (131 ± 44 vs 85 ± 43 ml/m2, p < 0.001) and PR fraction (51 ± 10 vs 5 ± 12%, p < 0.001) post-operatively. The LVEDVi increased (81 ± 19 vs 98 ± 15 ml/m2, p = 0.02) post-PVR. There were no significant differences in LV or RV EF between pre- and post-operative CMR studies.
Pre-PVR RVEDVi volumes were further compared with RV longitudinal strain parameters. Pre-PVR RVEDVi was found to correlate with pre-operative RV global longitudinal strain (r = 0.432, p = 0.005). The correlation persisted between pre-operative RVEDVi and RV global longitudinal strain mid-term post-PVR (r = 0.532, p = 0.001), suggesting patients with larger pre-operative RV volumes tended to have lower RV deformation parameters post-operatively. Scatter plots illustrating the relationship between global RV pre- and post-operative strain and pre-operative RVEDVi are shown in Fig. 3.
Intra-observer variability
A blinded re-analysis of four randomly selected LV and RV strain parameters were performed in 15 randomly selected cases by a single observer approximately 3 months after the initial analysis. There was good overall agreement with acceptable reproducibility between both LV and RV measurements. The mean bias [95% confidence interval (CI)] for RV global pre-operative and post-operative repeated strain measurements were 0.23 (−0.42 to 0.88)%, p = 0.47 and 0.06 (−0.67 to 0.79)%, p = 0.87 respectively and corresponding intraclass correlation coefficients (95% CI) were 0.93 (0.81–0.98)%, p = <0.001 and 0.94 (0.82–0.98)%, p = <0.001 respectively.
Discussion
In our study we sought to determine the impact of PVR on ventricular myocardial deformation in pediatric patients with surgically corrected rTOF. Our results show that whilst an early reduction in regional and global RV strain is observed after PVR, myocardial function increases beyond pre-operative and early-post-operative values with longer term follow-up and suggests that positive RV remodeling occurs over time. An early reduction in LV longitudinal strain was followed by increases in both longitudinal and global LV strain at longer term follow-up after PVR, however, LV strain parameters at mid-term follow-up overall did not differ significantly from pre-operative results.
RV and LV myocardial mechanics following PVR
The impact of PVR on myocardial mechanics remains controversial. Harrild et al. assessed the effect of percutaneous PVR on strain mechanics using CMR and reported similar improvements in LV longitudinal strain to our study, but not RV strain parameters in their patients with predominantly pulmonary regurgitation [13]. The differences in RV strain may be attributed to their smaller patient cohort with a mix of cardiac lesions (including patients post Ross procedure and transposition of the great arteries), older age of assessment (median age 19.8 years) and shorter duration of follow-up (median 6 months). It is possible that pediatric patients may exhibit a higher capacity of RV remodeling than adults and the heterogeneous study population in Harrild’s study may account for differences in RV mechanics and myocardial strain. Knirsch et al. assessed 16 rTOF patients with surgical PVR and found similar reductions in early postoperative LV and RV longitudinal strain at 1 month with improvement in both parameters at 6 months follow-up [14]. In their study LV longitudinal strain improved to pre-operative levels and RV strain values did not reach pre-operative strain values. Our study provides insights that ongoing RV remodeling and adaptation to changes in loading conditions continue to occur in children with increasing time from surgery.
The recent effects of cardiopulmonary bypass and impact of surgery may explain the initial reduction in RV strain in the early post-operative period observed in our study. It may also, however, be in part related to the RV not yet re-adapting to the acute volume unloading following PVR. The reduction or removal of PR following PVR results in a significant reduction in RV stroke volume. In the early stages post-PVR the RV remains dilated and hence needs to deform less to generate RV output. As remodeling continues to occur and chamber sizes decrease over time, the RV subsequently increases its deformation in order to maintain the same RV cardiac output. This may explain the subsequent observed changes in RV myocardial deformation, above and beyond pre-operative strain data at medium term follow-up. Similar to the RV, peri-operative effects of surgery and bypass may directly explain the early reduction in LV longitudinal strain, although proposed mechanisms of LV dyssynchrony, impaired LV compliance, effect of RV dysfunction on LV function and RV-LV diastolic interactions may also lead to impaired LV deformation [5, 15–17].
In keeping with our previous studies, a progressive decrease in RV regional function from base to apex was observed pre-operatively [18]. This relationship of segmental RV function persisted after PVR. It has been reported that the apical trabecular segment of the RV has the highest volumes when compared to control subjects and pronounced apical broadening and eccentric bulging of the RV free wall is present in the setting of volume loaded RVs [19, 20]. It is postulated that these changes in RV geometry toward a rounded apical curvature may result in increased wall stress and reduced apical deformation,[18] hence leading to a reduction in apical strain.
We did not observe significant pre-operative to mid-term post-operative differences in myocardial strain between volume, pressure and mixed loading conditions. However, the early post-operative reduction of basal RV strain in volume-loaded ventricles again reflects a combination of transient injury from surgical insults, persistent RV dilation requiring less deformation to generate RV cardiac output and the volume unloading effects of acute preload and afterload reduction to the RV.
LV rotation parameters
Our study provides interesting observations regarding LV rotation, both prior to and at medium term follow-up after PVR. Firstly, we found that just under a quarter of our patients exhibited counterclockwise basal rotation at their pre-operative study and these patients had higher apical rotation compared to patients with clockwise rotation. This is consistent with previous findings from our group and by Takayasu et al. although the mechanisms involved may be multifactorial but are largely unclear [6, 21]. Reversed basal rotation persisted in 58% patients at mid-term follow-up and this subset of patients had significantly lower basal, mid and global RV longitudinal strain early post-PVR as well as lower basal RV strain and global LV longitudinal deformation at medium term follow-up. This suggests that counterclockwise basal rotation is associated with maladaptive ventricular remodeling post-operatively and further emphasizes the importance of ventriculo-ventricular interactions.
2D echocardiographic parameters following PVR
Reductions in baseline RV echocardiographic 2D measurements were observed, including a reduction in basal and mid RV diameter, RVEDD, end-diastolic and end-systolic areas as well as right area. Of note, the RVEDD z-score remained mildly increased, suggesting that the RV size does not completely normalize by the medium term following PVR. This is in keeping with a persistently elevated RVEDVi in the small number of patients who had a post-operative clinical CMR performed. A concurrent increase in LV size reflected by increased LVEDD and basal and mid diameters was also observed. These observed changes to LV dimensions coupled with improved longitudinal and global LV strain parameters at mid-term follow-up compared with early post-operative data, support the effects of enhanced LV filling and normalization of diastolic septal geometry [13, 22].
Similar to previous studies, we did not observe a change in systolic function with RV FAC, LV fractional shortening or EF on M-mode on medium-term follow-up [14, 23]. The differences observed with myocardial deformation supports that strain imaging is a more sensitive method for detecting subtle changes in ventricular systolic function that may otherwise be missed with standard clinical parameters [5, 6, 24–26].
Relationship of RV strain to pre-operative CMR RV volumes
The inverse correlation between pre-operative RV strain values and pre-operative RVEDVi indicate that patients with larger RVs trend toward lower RV strain. Although RV deformation improved post-operatively in the medium term, the patients who had larger RVs and lower strain parameters prior to PVR had persistently lower RV strain post-operatively, with a higher correlation observed between post-operative strain values and indexed volumes. We were not able to demonstrate a ceiling effect of RV volume in which RV remodeling does not occur. Our center has previously published similar correlations between RV volumes and function [18, 27]. The degree to which the observed increase in mid post-operative RV strain reflects a true improvement in myocardial function vs ongoing adaptation to reduced loading conditions with time remains to be elicited.
Limitations
This study was limited by its retrospective design and small sample size, and not all the study patients had early post-operative data at the eligible time frame for analysis. In addition, subdivision of patients into pressure, volume and mixed loading conditions lead to small patient subgroups for comparison and subsequently limits the strength of associations. Also, only a small number of our patients (26%) had clinical CMRs following PVR and time frames to the CMRs were different than to follow-up echocardiograms. Despite the small sample size, however, it was still possible to draw significant conclusions between pre-operative and post-operative PVR results for both CMR volumes and echocardiographic myocardial strain.
Conclusion
In summary, our study supports that RV deformation increases relative to pre-operative strain at medium term follow-up after PVR. These findings support the hypothesis that RV remodeling and adaptation occurs in pediatric patients with rTOF following PVR and that intervention may have a beneficial longer-term impact on RV function. Left ventricular strain and rotation abnormalities were observed and highlight the importance of ventriculo-ventricular interactions. Larger scale studies with long-term follow-up are warranted to identify pre-operative factors that may predict post-operative ventricular function and to differentiate patients with positive vs adverse RV remodeling. The effect of PVR on long-term RV adaptation may have important clinical implications towards determining the optimal timing of surgery in this subset of patients.
References
Geva T, Sandweiss BM, Gauvreau K, Lock JE, Powell AJ (2004) Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol 43:1068–1074
Murphy JG, Gersh BJ, Mair DD, Fuster V, McGoon MD, Ilstrup DM et al (1993) Long-term outcome in patients undergoing surgical repair of tetralogy of Fallot. N Engl J Med 329:593–599
Ferraz Cavalcanti PE, Sa MP, Santos CA, Esmeraldo IM, de Escobar RR, de Menezes AM et al (2013) Pulmonary valve replacement after operative repair of tetralogy of Fallot: meta-analysis and meta-regression of 3,118 patients from 48 studies. J Am Coll Cardiol 62:2227–2243
Ghai A, Silversides C, Harris L, Webb GD, Siu SC, Therrien J (2002) Left ventricular dysfunction is a risk factor for sudden cardiac death in adults late after repair of tetralogy of Fallot. J Am Coll Cardiol 40:1675–1680
Cheung EW, Liang XC, Lam WW, Cheung YF (2009) Impact of right ventricular dilation on left ventricular myocardial deformation in patients after surgical repair of tetralogy of fallot. Am J Cardiol 104:1264–1270
Dragulescu A, Friedberg MK, Grosse-Wortmann L, Redington A, Mertens L (2014) Effect of chronic right ventricular volume overload on ventricular interaction in patients after tetralogy of Fallot repair. J Am Soc Echocardiogr 27:896–902
Buechel ER, Dave HH, KellenBerger CJ, Dodge-Khatami A, Pretre R, Berger F et al (2005) Remodelling of the right ventricle after early pulmonary valve replacement in children with repaired tetralogy of Fallot: assessment by cardiovascular magnetic resonance. Eur Heart J 26:2721–2727
Geva T (2011) Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson 13:9
Oosterhof T, van Straten A, Vliegen HW, Meijboom FJ, van Dijk AP, Spijkerboer AM et al (2007) Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation 116:545–551
Harrild DM, Marcus E, Hasan B, Alexander ME, Powell AJ, Geva T et al. (2013) Impact of transcatheter pulmonary valve replacement on biventricular strain and synchrony assessed by cardiac magnetic resonance feature tracking. Circ Cardiovasc Interv 6:680–687
Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK et al (2010) Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the pediatric measurements writing group of the american society of echocardiography pediatric and congenital heart disease council. J Am Soc Echocardiogr 23:465–495 (quiz 576-7)
Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E (2008) Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson 10:35
Harrild DM, Berul CI, Cecchin F, Geva T, Gauvreau K, Pigula F et al (2009) Pulmonary valve replacement in tetralogy of Fallot: impact on survival and ventricular tachycardia. Circulation 119:445–451
Knirsch W, Dodge-Khatami A, Kadner A, Kretschmar O, Steiner J, Bottler P et al (2008) Assessment of myocardial function in pediatric patients with operated tetralogy of Fallot: preliminary results with 2D strain echocardiography. Pediatr Cardiol 29:718–725
Oosterhof T, Tulevski II, Vliegen HW, Spijkerboer AM, Mulder BJ (2006) Effects of volume and/or pressure overload secondary to congenital heart disease (tetralogy of fallot or pulmonary stenosis) on right ventricular function using cardiovascular magnetic resonance and B-type natriuretic peptide levels. Am J Cardiol 97:1051–1055
Liang XC, Cheung EW, Wong SJ, Cheung YF (2008) Impact of right ventricular volume overload on three-dimensional global left ventricular mechanical dyssynchrony after surgical repair of tetralogy of Fallot. Am J Cardiol 102:1731–1736
Fernandes FP, Manlhiot C, Roche SL, Grosse-Wortmann L, Slorach C, McCrindle BW et al (2012) Impaired left ventricular myocardial mechanics and their relation to pulmonary regurgitation, right ventricular enlargement and exercise capacity in asymptomatic children after repair of tetralogy of Fallot. J Am Soc Echocardiogr 25:494–503
Dragulescu A, Grosse-Wortmann L, Redington A, Friedberg MK, Mertens L (2013) Differential effect of right ventricular dilatation on myocardial deformation in patients with atrial septal defects and patients after tetralogy of Fallot repair. Int J Cardiol 168:803–810
van der Hulst AE, Roest AA, Holman ER, de Roos A, Blom NA, Bax JJ et al (2011) Real-time three-dimensional echocardiography: segmental analysis of the right ventricle in patients with repaired tetralogy of fallot. J Am Soc Echocardiogr 24:1183–1190
Sheehan FH, Ge S, Vick GW 3rd, Urnes K, Kerwin WS, Bolson EL et al (2008) Three-dimensional shape analysis of right ventricular remodeling in repaired tetralogy of Fallot. Am J Cardiol 101:107–113
Takayasu H, Takahashi K, Takigiku K, Yasukochi S, Furukawa T, Akimoto K et al (2011) Left ventricular torsion and strain in patients with repaired tetralogy of Fallot assessed by speckle tracking imaging. Echocardiography 28:720–729
Dragulescu A, Friedberg MK (2014) A tale of two ventricles: ventricular-ventricular interactions and LV dysfunction after surgical repair of Tetralogy of Fallot. Eur Heart J Cardiovasc Imaging 15:498–499
Oosterhof T, Vliegen HW, Meijboom FJ, Zwinderman AH, Bouma B, Mulder BJ (2007) Long-term effect of pulmonary valve replacement on QRS duration in patients with corrected tetralogy of Fallot. Heart 93:506–509
Weidemann F, Eyskens B, Mertens L, Dommke C, Kowalski M, Simmons L et al (2002) Quantification of regional right and left ventricular function by ultrasonic strain rate and strain indexes after surgical repair of tetralogy of Fallot. Am J Cardiol 90:133–138
Meris A, Faletra F, Conca C, Klersy C, Regoli F, Klimusina J et al (2010) Timing and magnitude of regional right ventricular function: a speckle tracking-derived strain study of normal subjects and patients with right ventricular dysfunction. J Am Soc Echocardiogr 23:823–831
Forsey J, Friedberg MK, Mertens L (2013) Speckle tracking echocardiography in pediatric and congenital heart disease. Echocardiography 30:447–459
Eyskens B, Brown SC, Claus P, Dymarkowski S, Gewillig M, Bogaert J et al (2010) The influence of pulmonary regurgitation on regional right ventricular function in children after surgical repair of tetralogy of Fallot. Eur J Echocardiogr 11:341–345
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors have no conflicts of interest to declare.
Ethical approval
The study was approved by our institutional ethics board and it was done in accordance with the institutional ethical standards and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was waved given the retrospective nature of the study.
Rights and permissions
About this article
Cite this article
Yim, D., Mertens, L., Morgan, C.T. et al. Impact of surgical pulmonary valve replacement on ventricular mechanics in children with repaired tetralogy of Fallot. Int J Cardiovasc Imaging 33, 711–720 (2017). https://doi.org/10.1007/s10554-016-1046-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-016-1046-2