Abstract
The aim of this study was to investigate heart rate variability (HRV) and left ventricular (LV) remodeling in uncomplicated diabetic patients. Furthermore, we sought to investigate the association between HRV indices and LV structural, functional and mechanical parameters. This cross-sectional study included 50 uncomplicated patients with type 2 diabetes and 40 healthy controls without cardiovascular risk factors. All study subjects underwent 24-h Holter monitoring, laboratory analyses and complete two-dimensional echocardiography examination (2DE). LV structure and diastolic function were significantly deteriorated in the diabetic patients comparing with the controls. LV global longitudinal, circumferential and radial strains were significantly reduced in the diabetic group. LV endocardial, mid-miocardial and epicardial longitudinal and circumferential strains were significantly decreased, whereas LV twist was significantly increased, in the diabetic patients; 24-h, daytime and nighttime heart rates were higher in the diabetic patients. All parameters of time and frequency domain of HRV were reduced in the diabetic subjects. LV mass index, mitral E/e′ ratio and 2DE LV endocardial and mid-miocardial longitudinal and circumferential strains correlated with HRV parameters. A multivariate regression analysis showed that E/e′ ratio and 2DE LV layer-specific strains were associated with HRV parameters independently of age, BMI, systolic blood pressure and LV mass index. HRV and LV mechanics are significantly deteriorated in uncomplicated diabetic individuals. Parameters of LV remodeling are independently associated with HRV indices, which could indicate the importance of HRV determination in diabetics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Patients with type 2 diabetes mellitus can develop heart failure without evidence of cardiac ischemia or presence of systemic hypertension [1]. One of the most important mechanisms responsible for cardiac remodeling in these subjects is cardiac autonomic neuropathy that is present in more than one-third of diabetic patients [2]. However, it is difficult to determine straightforward association between cardiac autonomic dysfunction and diabetic cardiomyopathy due to similar mechanisms that cause both conditions. Furthermore, there are no specific criteria to diagnose these disorders. Heart rate variability (HRV) may be used for non-invasive measurement of cardiac autonomic function, while advanced echocardiographic tools, such as speckle tracking imaging, may provide more detailed insight into cardiac function and mechanics in diabetic patients, and better prediction of adverse events in the growing population of diabetic patients [3]. The introduction of the advanced layer-specific strain imaging provides a new insight into LV mechanics in diabetic patients [4].
A recent study that involved 16,415 non-diabetic Hispanic/Latino adults showed that impairment in glucose homeostasis and abdominal obesity were associated with significantly lower HRV [5]. Similar findings regarding association between reduced HRV and insulin resistance and lower insulin sensitivity were revealed also in Japanese adults who were not taking medication for diabetes [6]. Perciaccante et al. revealed that insulin resistance was associated with sympathetic overactivity, especially during night [7], and Chyun et al. demonstrated that cardiac autonomic neuropathy could predict adverse cardiac outcomes in asymptomatic diabetic patients without known cardiac disease [8].
Our study group previously researched LV remodeling in diabetes and prediabetes [9, 10]. However, investigations that involve evaluation of LV remodeling and cardiac autonomic neuropathy in diabetic patients are scarce [11, 12], and usage of 24-h Holter monitoring for HRV is even more infrequent.
The aim of the present investigation was to evaluate cardiac autonomic function, evaluated with HRV, and LV functional and mechanical changes in diabetic patients. Additionally, we sought to determine the possible relationship between HRV and LV remodeling parameters in the whole study population.
Methodology
This prospective observational cross-sectional study included 50 consecutive normotensive uncomplicated patients with type 2 diabetes and 40 normotensive subjects free of cardiovascular diseases. The diagnosis of diabetes was based on the current recommendations [13]. Diabetes was defined if the blood glucose level was ≥7 mmol/l and HbA1c ≥6.5% or usage of antidiabetic treatment [13].
Exclusion criteria were antihypertensive treatment, heart failure, coronary artery disease, previous cerebrovascular events, atrial fibrillation, congenital heart disease, more than mild valvular heart disease, obesity (BMI ≥35 kg/m2), neoplastic disease, cirrhosis of the liver or kidney failure. Patients with >10% premature supraventricular or ventricular contractions were also excluded from the study due to HRV analysis (two subjects).
Clinic BP values were obtained in two separate visits 3 weeks apart. BP was measured by conventional sphygmomanometer in the morning hours by taking the average value of two consecutive measurements in the sitting position 10 min apart. BP was calculated as average values between all the measurements.
Anthropometric measures (height, weight) and laboratory analyses (level of fasting glucose, blood creatinine and urea, total cholesterol and triglycerides) were obtained from all the subjects included in the study. Body mass index (BMI) and body surface area (BSA) were calculated for each patient. The study was approved by the local Ethics Committee, and informed consent was obtained from all the participants.
24-h Holter monitoring
24-h Holter monitoring was performed with a three-channel digital Schiller Microvit MT-101 system (Schiller AG, Baar, Switzerland) and analyzed by the Schiller software (Schiller AG, Baar, Switzerland). The minimum duration of the recording was 18 h (after exclusion of non-sinusal cardiac cycles). Time-domain HRV parameters were calculated on the 24-h, daytime and nighttime recordings after excluding non-sinusal cardiac cycles, according to the guidelines [14]. SDNN was defined as a standard deviation from all normal RR intervals. SDANN, which reflects long-term HRV and therefore mainly sympathetic activity or sympathovagal balance, was defined as a standard deviation of the averaged normal RR intervals for all 5-min segments. rMSSD was calculated as the root mean square of the difference between the coupling intervals of adjacent RR intervals. pNN50 which reflects short-term beat-to-beat HRV and consequently primarily vagal activity was calculated as the proportion of adjacent RR intervals that varied by >50 ms. After power spectral density estimation, standard frequency-domain HRV measures were calculated for 24-h, daytime and nighttime [14]. Low frequency domain (LF) was defined between 0.04 and 0.15 Hz; high frequency domain (HF) was defined between 0.15 and 0.4 Hz; total spectral power (TP) for all intervals up to 0.4 Hz; and ratio of low to high frequency power (LF/HF).
Echocardiography
Echocardiographic examinations were performed by using a commercially available Vivid 7 (GE Vingmed, Horten, Norway) ultrasound machine equipped with a 2.5 MHz transducer. All echocardiographic data were analyzed off-line.
Aortic root diameter was assessed in all the patients according to the current guidelines. Reported values of all 2DE parameters were obtained as the average value of three consecutive cardiac cycles. LV diameters, posterior wall and septum thickness, were measured according to the current recommendations [15]. Relative wall thickness was calculated according to the formula. LV ejection fraction (EF) was calculated by using the biplane method. LV mass was calculated by using the Devereux formula [16], and indexed for the height powered to 2.7.
Pulsed-wave Doppler assessment of transmitral LV was obtained in the apical four-chamber view according to the guidelines [17]. Tissue Doppler imaging was used to obtain LV myocardial velocities in the apical four-chamber view, with a sample volume placed at the septal and lateral segments of the mitral annulus during early and late diastole (e′ and a′), and systole (s). The average of the peak early diastolic relaxation velocity (e′) of the septal and lateral mitral annulus was calculated, and the E/e′ ratio was computed.
2DE LV strain analysis
2DE strain imaging was performed by using three consecutive cardiac cycles [18], and a commercially available software Q-analysis (EchoPAC 201, GE-Healthcare, Horten, Norway) was used for 2DE strain analysis. The frame rate ranged between 50 and 70 Hz. 2DE speckle tracking analysis was done in three apical (four- and two-chamber, long-axis) views and parasternal short-axis view at the papillary muscle level.
Using the same software we traced short-axis views at basal and apical LV levels at the end-diastolic frame and obtained LV basal and apical rotation values (calculated by software). LV twist represents the summation of absolute values of basal and apical rotation and it was also provided by the software.
Multilayer longitudinal and circumferential strains were determined by modified 2DE strain software (Q-analysis). Multilayer longitudinal was assessed in apical four-chamber, two-chamber and apical long axis view, whereas multilayer circumferential strain was evaluated in short axis at the papillary muscles level. The automatic tracking of the endocardial contour was performed in end-systole. Automatically provided LV myocardium tracking by software was carefully verified and the region of interest was manually corrected to ensure optimal tracking of endocardium and epicardium and inclusion of the entire LV thickness including endocardial, mid-myocardial and epicardial layers in all the observed echocardiographic views. After delineating the region of interest, the software was used to divide the LV into six segments in four-chamber, two-chamber, apical long axis view and allow the investigation of three myocardial layers: endocardial, mid-myocardial and epicardial [18]. Then the global longitudinal and circumferential strain of the endocardial, mid-myocardial and epicardial myocardial layers, and the global radial strain of the LV were calculated [19]. LV global longitudinal and circumferential strains were calculated as the average of all three layer-specific strains (endocardial, mid-myocardial and epicardial), while global radial strain was assessed by the software. Global radial strain was assessed by Q-analysis at the level of papillary muscles.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD), and the Student t test was used to detect differences between the two groups for the variables that showed normal distribution. Differences in proportions were compared by using the χ2. Pearson’s correlation coefficients were used for determining the correlation between different demographic and echocardiographic parameters and HRV parameters. Almost all HRV parameters, except SDNN and SDANN, were transformed by natural logarithm, before using the t test or linear regressions because of their high positive skewed distribution. The variables which showed p value ≤0.10 were included into the stepwise multiple regression analyses. The p value <0.05 was considered statistically significant.
Results
There was no significant difference in gender distribution and blood pressure values between the two observed groups (Table 1). BMI and BSA were significantly higher in the diabetic patients. Blood levels of glucose and HbA1c were higher in the diabetic patients, which was not surprising considering the inclusion criteria. Creatinine and lipids levels were similar between the diabetic patients and the controls. Only the triglycerides level was higher in the diabetic patients. The creatinine level was similar between the groups, while the urea level was higher in the diabetic patients (Table 1). CRP and fibrinogen levels were significantly higher in the diabetic subjects.
Echocardiographic parameters
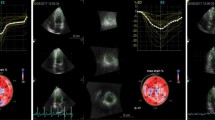
There is no difference in aortic root diameter between the observed groups (Table 2). LV diameters and ejection fraction are similar between the groups (Table 2). Interventricular septum thickness and LV mass index were higher in the diabetic patients, whereas relative wall thickness was similar between the groups (Table 2). All the parameters of LV diastolic function (transmitral E/A and DT) and LV diastolic filling were deteriorated in the diabetic patients (Fig. 1).
Tissue Doppler parameter of LV systolic function (s) was significantly lower in the diabetic individuals (Table 2).
2DE strain analysis
All differences in mechanical parameters between the observed groups were adjusted for age, BMI and the triglycerides level. Multidirectional LV mechanics (global longitudinal, circumferential and radial strain) is significantly deteriorated in the diabetic patients comparing with the controls (Table 3).
LV basal rotation did not differ between the controls and the diabetics, but apical rotation was significantly higher in the diabetic patients (Table 3). Consequently LV twist was also significantly higher in the diabetic patients (Table 3).
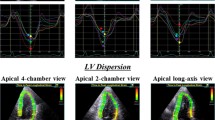
Endocardial, mid-myocardial and epicardial longitudinal and circumferential strains were significantly lower in the diabetic patients than in the controls (Table 3; Fig. 2).
24-h Holter monitoring
Heart rates during 24 h, day and night were higher in the diabetic patients than in the controls (Table 4). SDNN, SDANN, rMSSD and p50NN were significantly lower in the diabetic individuals. Similar results were obtained for 24-h, daytime and nighttime LF, HF and TP, which were reduced in the diabetic group (Table 4). There was no difference in 24-h, daytime and nighttime ratio between LF and HF (LF/HF).
Correlation and multivariate regression analysis
SDNN correlated with HbA1c, E/e′average ratio and 2DE LV endocardial longitudinal and circumferential strain. However, only HbA1c and and 2DE LV endocardial longitudinal strain remained independently associated with SDNN (Table 5).
rMSSD correlated with HbA1c, LV mass index, E/e′average ratio and 2DE LV endocardial longitudinal strain, but only HbA1c (β = −0.262, p < 0.001) and 2DE LV endocardial longitudinal strain (β = 0.173, p = 0.041) were independently associated with rMSSD (Table 5).
24-h LF correlated with age, HbA1c, LV mass index, E/e′average ratio and 2DE LV endocardial longitudinal and circumferential strain (Fig. 3). However, only HbA1c (β = –0.263, p < 0.001), E/e′average ratio (β = –0.184, p = 0.033) and 2DE LV endocardial longitudinal strain (β = 0.179, p = 0.030) were independent predictors of 24-h LF (Table 5).
24-h HF correlated with HbA1c, LV mass index and 2DE LV endocardial longitudinal and circumferential strain (Fig. 3). However, only HbA1c and endocardial longitudinal and circumferential strains were independently associated with 24-h HF (Table 5).
Discussion
The current investigation has provided several important findings which should be discussed: (a) HRV is impaired in the diabetic subjects; (b) LV longitudinal, circumferential and radial deformation are significantly impaired in the diabetic individuals; (c) LV apical rotation was significantly higher in the diabetic patients, which is why LV twist was also significantly increased in this group of patients; (d) all myocardial layers (endocardium, mid-myocardium and epicardium) are impacted by diabetes; (e) parameter of long-term glycemic control (HbA1c) correlated with HRV parameters; (f) LV layer-specific mechanics is associated independently with HRV.
Our study showed that HRV parameters were deteriorated in the diabetic patients, which confirms the previous studies regarding the autonomic imbalance in type 2 diabetes [5–8]. Namely, SDNN, SDANN, HF, LF and TP components were significantly lower in the diabetic subjects, which altogether indicate a significant impairment in autonomic nervous system activity. Earlier investigations revealed that HF component was mainly associated with vagal activity, whereas the significance of LF component was more controversial [14, 20]. However, most authors considered LF as a marker of both sympathetic and vagal influences [14, 21]. Interestingly, VLF could also be influenced by other neurohormonal systems such as the renin-angiotensin-aldosterone system [22]. These sympathovagal disturbances could potentially explain the increased risk of cardiovascular events, even in asymptomatic diabetic patients with normal blood pressure control [8]. Interestingly, due to the reduced powers of both LF and HF components, the LF/HF ratio was similar among the observed groups. Nevertheless, this does not eliminate the presence of impaired innervation in sympathetic and parasympathetic nerves in the diabetic patients. These results are concurrent with previous studies with diabetic patients [5–8, 23].
There are several mechanisms that could explain the relationship between autonomic disbalance and diabetes. Most of them refer to biohumoral systems such as renin-angiotensin-aldosterone and sympathetic nervous system or inflammatory processes. Lampert et al. showed that inflammatory parameters CRP and IL-6 were inversely associated with HRV variables [24]. These findings suggest that inflammatory and autonomic processes may be connected, and that many factors for coronary artery disease development may be the consequence of autonomic dysregulation. Endothelial dysfunction could also be one of the important steps that relates HRV and glucose metabolism in the diabetic patients [25].
Previous studies showed that LV structure, diastolic and systolic function, as well as mechanical function, were impaired in diabetic patients [25, 26]. Habek et al. previously indicated the relationship between LV diastolic function and HRV in diabetics [11]. This is entirely concurrent with our present results. Furthermore, our results for the first time showed significant decrease in layer-specific longitudinal and circumferential strain in diabetic patients. Additionally, our results for the first time demonstrated the association between HRV indices and layer-specific longitudinal and circumferential strain. Recently Enomoto et al. revealed decrease in endocardial radial strain, but not endocardial circumferential strain in diabetic patients [4]. Latest studies have shown that E/e′ ratio and LV longitudinal strain are the best negative predictive value for cardiovascular events in diabetic population free of cardiovascular disease [3], which increases the potential value of our present results that connect autonomic disbalance and LV remodeling in diabetic patients. To our knowledge, this is the first study that brings into relationship HRV and LV longitudinal strain in diabetic population. The association between endocardial longitudinal strain with HRV indices is particularly important because it confirms that endocardium is the most susceptible and vulnerable myocardial layer which is the first to show abnormalities, even in asymptomatic patients. Additionally, this is also significant from the technical point of view because some vendors and software packages that we use in echocardiography determine only endocardial strain. Therefore, our findings revealed that this kind of software could promptly determine subtle changes in LV myocardium.
Our findings showed that that LV apical rotation and LV twist were significantly increased in diabetic patients. The LV twist reflects impaired LV relaxation and diastolic function [27], as well as declined systolic function [28] as it was previously reported [29, 30]. In diabetic patients increased twist could serve as a compensatory mechanism that should maintain adequate diastolic filling in the condition of reduced longitudinal strain. This is supported by the fact that diabetic patients in our study in general did not show typical echocardiographic signs of increased LV filling pressure. Parameters of LV diastolic function were definitely worse in diabetic patients in our study, but still not in the range of abnormal LV diastolic function.
There are several important clinical implications of the current study. Assessment of LV global and multilayer longitudinal and circumferential strain, as well as estimation of HRV, may represent effective and sensitive methods in evaluation of preclinical cardiac damage in diabetic patients. LV strain and HRV can be used in the follow-up of diabetic patients, which represents an important clinical implication. Additionally, the tight glycemic control over a longer time period, evaluated by HbA1c level, is related with better cardiac autonomic function, which suggests that HbA1c can be a surrogate marker of autonomic nervous system dysfunction in clinical routine. The inverse relationship between LV mechanics and HRV indices shows that the assessment performed by one of these techniques can provide valuable information about the remaining one. This is very important for everyday clinical practice that frequently allows using only one of these methods.
The autonomic imbalance is associated with increased cardiovascular morbidity and mortality [31], as well as LV structural and functional changes [32] in global population, not only in diabetics [3, 33]. The most important clinical application of our study is the fact that our findings indicate the relationship between glucose metabolism, autonomic nervous system and LV structural, functional and mechanical remodeling that can potentially serve as an explanation for higher cardiovascular and overall morbidity and mortality in diabetic patients. It is important to emphasize that autonomic dysregulation can be efficiently and accurately assessed by HRV obtained from 24-h Holter monitoring or even in a shorter period of time, which represent additional clinical implications of the present investigation.
Considering the fact that diabetes represents one of the most important factors in the development of heart failure with preserved ejection fraction and ischemic heart disease and the fact that its negative influence cannot be detected by simple estimation of LV ejection fraction, we strongly recommend determination of LV longitudinal strain in all diabetic patients.
Limitations
The present investigation has several limitations. First, all the patients with comorbidities have been excluded, which decreases potential generalization of our results. Second, echocardiographic assessment of LV mechanics could be significantly influenced by the quality of ultrasound images. Third, HRV components are indirect measurements of autonomic nervous system and might be weaker predictors of the sympathovagal imbalance than direct measurements such as muscle sympathetic nerve activity. Fourth, this is the cross-sectional study, which does not allow determination of causal relationship between diabetes, HRV and LV remodeling.
Conclusion
The present research reveals that autonomic nervous function and LV structure, function and mechanics are significantly deteriorated in uncomplicated diabetic patients. The results demonstrate a significant relationship between HRV indices, parameter of long-term glucose control (HbA1c) and LV layer-specific deformation. Further follow-up studies with a larger population of diabetic patients are necessary to evaluate a long-term prognostic importance of the interaction between HRV and LV remodeling on cardiovascular morbidity and mortality in diabetic population.
References
Fang ZY, Prins JB, Marwick TH (2004) Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev 25:543–567
Balcıoğlu AS, Müderrisoğlu H (2015) Diabetes and cardiac autonomic neuropathy: clinical manifestations, cardiovascular consequences, diagnosis and treatment. World J Diabetes 6(1):80–91
Liu JH, Chen Y, Yuen M, Zhen Z, Chan CW, Lam KS, Tse HF, Yiu KH (2016) Incremental prognostic value of global longitudinal strain in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 15:22
Enomoto M, Ishizu T, Seo Y, Yamamoto M, Suzuki H, Shimano H, Kawakami Y, Aonuma K (2015) Subendocardial systolic dysfunction in asymptomatic normotensive diabetic patients. Circ J 79(8):1749–1755
Meyer ML, Gotman NM, Soliman EZ, Whitsel EA, Arens R, Cai J, Daviglus ML, Denes P, González HM, Moreiras J, Talavera GA, Heiss G (2016) Association of glucose homeostasis measures with heart rate variability among Hispanic/Latino adults without diabetes: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Cardiovasc Diabetol 15:45
Saito I, Hitsumoto S, Maruyama K, Nishida W, Eguchi E, Kato T, Kawamura R, Takata Y, Onuma H, Osawa H, Tanigawa T (2015) Heart rate variability, insulin resistance, and insulin sensitivity in Japanese adults: the Toon Health Study. J Epidemiol 25(9):583–591
Perciaccante A, Fiorentini A, Paris A, Serra P, Tubani L (2006) Circadian rhythm of the autonomic nervous system in insulin resistant subjects with normoglycemia, impaired fasting glycemia, impaired glucose tolerance, type 2 diabetes mellitus. BMC Cardiovasc Disord 6:19
Chyun DA, Wackers FJ, Inzucchi SE, Jose P, Weiss C, Davey JA, Heller GV, Iskandrian AE, Young LH, DIAD Investigators (2015) Autonomic dysfunction independently predicts poor cardiovascular outcomes in asymptomatic individuals with type 2 diabetes in the DIAD study. SAGE Open Med 3:2050312114568476
Tadic M, Ilic S, Cuspidi C, Stojcevski B, Ivanovic B, Bukarica L, Jozika L, Celic V (2015) Left ventricular mechanics in untreated normotensive patients with type 2 diabetes mellitus: a two- and three-dimensional speckle tracking study. Echocardiography 32(6):947–955
Tadic M, Ilic S, Cuspidi C, Kocijancic V, Celic V (2014) Prediabetes, diabetes and left heart deformation. Rev Esp Cardiol (Engl Ed) 67(12):1062–1064
Habek JC, Lakusic N, Kruzliak P, Sikic J, Mahovic D, Vrbanic L (2014) Left ventricular diastolic function in diabetes mellitus type 2 patients: correlation with heart rate and its variability. Acta Diabetol 51(6):999–1005
Sacre JW, Franjic B, Jellis CL, Jenkins C, Coombes JS, Marwick TH (2010) Association of cardiac autonomic neuropathy with subclinical myocardial dysfunction in type 2 diabetes. JACC Cardiovasc Imaging 3(12):1207–1215
American Diabetes Association (2013) Diagnosis and classification of diabetes mellitus. Diabetes Care 36:S67–S74
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J 17:354–381
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 28:1–39
de Simone G, Daniels SR, Devereux RB et al (1992) Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol 20:1251–1260
Quinones MA, Otto CM, Stoddard M et al (2002) Recommendations for quantification of Doppler echocardiography: A report from the Doppler quantification task force of the nomenclature and standards committee of the American Society of Echocardiography. J Am Soc Echocardiogr 15:167–184
Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G (2011) Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr 12:167–205
Leitman M, Lysiansky M, Lysyansky P, Friedman Z, Tyomkin V, Fuchs T, Adam D, Krakover R, Vered Z (2010) Circumferential and longitudinal strain in 3 myocardial layers in normal subjects and in patients with regional left ventricular dysfunction. J Am Soc Echocardiogr 23(1):64–70
Bernardi L, Spallone V, Stevens M, Hilsted J, Frontoni S, Pop-Busui R, Ziegler D, Kempler P, Freeman R, Low P, Tesfaye S, Valensi P, Toronto Consensus Panel on Diabetic Neuropathy (2011) Methods of investigation for cardiac autonomic dysfunction in human research studies. Diabetes Metab Res Rev 27:654–664
Huikuri HV, Stein PK (2013) Heart rate variability in risk stratification of cardiac patients. Prog Cardiovasc Dis 56(2):153–159
Bigger JT Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN (1992) Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 85(1):164–171
Tiftikcioglu BI, Bilgin S, Duksal T, Kose S, Zorlu Y (2016) Autonomic neuropathy and endothelial dysfunction in patients with impaired glucose tolerance or type 2 diabetes mellitus. Medicine (Baltimore) 95(14):e3340
Lampert R, Bremner JD, Su S, Miller A, Lee F, Cheema F, Goldberg J, Vaccarino V (2008) Decreased heart rate variability is associated with higher levels of inflammation in middle-aged men. Am Heart J 156(4):759.e1–e7
Ernande L, Bergerot C, Rietzschel ER, De Buyzere ML, Thibault H, Pignonblanc PG, Croisille P, Ovize M, Groisne L, Moulin P, Gillebert TC, Derumeaux G (2011) Diastolic dysfunction in patients with type 2 diabetes mellitus: is it really the first marker of diabetic cardiomyopathy? J Am Soc Echocardiogr 24(11):1268–1275
Roos CJ, Scholte AJ, Kharagjitsingh AV, Bax JJ, Delgado V (2014) Changes in multidirectional LV strain in asymptomatic patients with type 2 diabetes mellitus: a 2-year follow-up study. Eur Heart J Cardiovasc Imaging 15(1):41–47
Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK (2008) Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc Imaging 1(3):366–376
Maharaj N, Khandheria BK, Peters F, Libhaber E, Essop MR (2013) Time to twist: marker of systolic dysfunction in Africans with hypertension. Eur Heart J Cardiovasc Imaging 14(4):358–365
Ma H, Xie M, Wang J, Lü Q, Wang X, Lu X, Yang Y, Hu L (2008) Ultrasound speckle tracking imaging contributes to early diagnosis of impaired left ventricular systolic function in patients with type 2 diabetes mellitus. J Huazhong Univ Sci Technolog Med Sci 28(6):719–723
Karagöz A, Bezgin T, Kutlutürk I, Külahçıoğlu S, Tanboğa IH, Güler A, Karabay CY, Oduncu V, Aksoy H, Kırma C (2015) Subclinical left ventricular systolic dysfunction in diabetic patients and its association with retinopathy: a 2D speckle tracking echocardiography study. Herz 40(Suppl 3):240–246
Thayer JF, Yamamoto SS, Brosschot JF (2010) The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol 141(2):122–131
Kalam K, Otahal P, Marwick TH (2014) Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 100(21):1673–1680
Felício JS, Koury CC, Carvalho CT, Neto JF, Miléo KB, Arbage TP, Silva DD, de Oliveira AF, Peixoto AS, Junior AB, Dos Santos ÂK, Yamada ES, Zanella MT (2015) Present insights on cardiomyopathy in diabetic patients. Curr Diabetes Rev 12(4):384–395
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Vukomanovic, V., Tadic, M., Suzic-Lazic, J. et al. The relationship between heart rate variability and left ventricular layer-specific deformation in uncomplicated diabetic patients. Int J Cardiovasc Imaging 33, 481–490 (2017). https://doi.org/10.1007/s10554-016-1023-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-016-1023-9