Abstract
As the number of implanted biventricular pacemakers increases, the coronary sinus (CS) has evoked much interest amongst cardiologists. A dilated CS could prompt the existence of many diseases. The normal CS diameter is uncertain, especially in children. A total of 446 Chinese healthy children were prospectively enrolled in this study. The superior and inferior diameter of the CS was measured from the CS ostium 1 cm from the end of ventricular systole in the modified apical 4-chamber view. Seven models were tested to determine the relationships between parameters of body size and CS diameter. Heteroscedasticity was tested by the White and Breusch–Pagan tests. A multiple linear regression model should be gender as a covariate along with BSAStevenson, in order to evaluate the influence of gender on the measurements. The formula of Stevenson was best-fit. The predicted values and Z-score boundaries for measurement of the CS diameter were calculated. Bland–Altman plot regression showed that the 95 % limits of agreement for inter- and intra-observer measurements were not significantly different. We report new, reliable echocardiographic Z scores for the CS diameter derived from a large population of healthy Chinese children. The Z scores can be used in echocardiographic examinations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As the number of implanted biventricular pacemakers and defibrillators have increased, the coronary sinus (CS) has evoked much interest amongst cardiologists. Transthoracic echocardiography (TTE) is the simplest and quickest technology by which to evaluate the CS. A dilated CS leads to many diseases, such as a persistent left superior vena cava, an anomalous pulmonary venous connection, and an unroofed CS [1–3]. For example, varying degrees of CS dilatation serve as important clues in the diagnosis of different types of unroofed CS [3]. Previous studies have shown that measurement of the CS diameter using M-mode echocardiography in 133 healthy adults is actually an oblique diameter [4]. No studies have shown the normal range of CS diameters in children. Indeed, the normal CS diameter is unknown, especially in children.
Methods
Study subjects
All subject children were referred to the Pediatric Cardiology Outpatient Clinics of Shengjing Hospital and First Affiliated Hospital of China Medical University between May 2014 and January 2016. Most of the children were evaluated by physicians in our hospital for systolic murmurs, chest pain, or shortness of breath, and those who underwent complete TTE were considered for inclusion. Some of the children were considered healthy at the time of birth in the Department of Obstetrics. Subjects were included if the physical examination, electrocardiogram, and echocardiographic results were normal. Subjects were excluded if they had a fever, cough, other respiratory disease, and/or evidence of any structural anomaly, ventricular hypertrophy [5], body mass index ≥95th percentile for children ≥2 years of age, or weight-for-length Z-score ≥2 on the basis of the World Health Organization’s Child Growth Standards for children <2 years of age [6, 7]. Heights and weights of all subjects were measured. This study was approved by the Ethics Committee of China Medical University. Written informed consent was obtained from legal guardians of all subjects for the publication of this study and any accompanying images.
Echocardiographic measurements
Echocardiography was performed using a Philips iE33 system (Philips Medical Systems, Bothell, WA, USA) and a 1.5/5 or 4/12 MHz phased array probe. All images were obtained from standard views according to the recommendations for quantification methods during the performance of a pediatric echocardiogram [8]. All images were digitally stored and analyzed offline using customized software (Qlab; Philips Medical Systems).
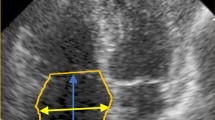
CS measurements are routinely obtained for all new pediatric subjects in our department. In the standard apical 4-chamber view, we tilt the probe down into the short view and acquire the modified apical 4-chamber view. The modified apical 4-chamber view shows the long axis of the CS. The superior and inferior diameter of the CS was measured from the CS ostium 1 cm from the end of ventricular systole [9] (Fig. 1).
Statistical analysis
To test the normality of age, height, weight, and CS diameter, the Kolmogorov–Smirnov and Shapiro–Wilk tests were used. If the distribution of these parameters was normal, independent t tests were used. If the distribution was non-normal, the rank-sum test with the Mann–Whitney U test were used.
Due to the lack of recognition with the best-fit BSA formula for Chinese children, seven formulae were used in this study, as follows:
BSADuBois [10]: height 0.725 × weight 0.425 × 0.007184
BSADreyer [11]: weight 0.6666 × 0.1
BSABoyd [12]: 0.0003207× (1000 × weight)[0.7285−0.0188 × log(1000 × weight)] × height 0.3
BSAHaycock [13]: weight 0.5378 × height 0.3964 × 0.024265
BSAMosteller [14]:√[(height × weight)/3,600]
BSAGehan [15]:0.0235 × height0.42246 × weight0.51456
BSAStevenson [16]: 0.0061 × height + 0.0128 × weight-0.1529
Seven models were tested to determine the relationships between parameters of body size and CS diameter, including linear (y = a + bx), logarithmic (y = a + b × ln[x], ln(y) = a + bx), exponential (ln(y) = a + b × ln[x]), and square root equations (y = a + b×√x, √y = a + bx, √y = a + b×√x). To determine whether or not there was heteroscedasticity, the White and the Breusch-Pagan tests were used to describe the behavior of variance of the residuals. Among the models, the best-fit formula was the model with the highest R 2 and homoscedasticity [17]. Scatter plot graphs of the BSA were drawn to compare the best-fit formula with others.
To test the normality of the BSAbest−fit, the Kolmogorov–Smirnov and Shapiro–Wilk tests were used. If the distribution of the parameters was normal, independent t tests were used between the male and female children groups. If the distribution of the parameters was non-normal, the rank-sum with Mann–Whitney U tests were used.
Regression was used to model the relationship of the CS diameter with three different independent variables (height, weight, and BSAbest−fit). The seven models were re-tested. Among the models, the best-fit formula was the model with the highest R 2, homoscedasticity, and a normal distribution of residuals. Then, the Z-score was calculated, as follows: Z = [observed CS-mean CS]/SE; Z = [observed CS-mean CS]/√MSE.
To assess inter- and intra-observer agreement with echocardiographic measurements, we used the method described by Bland and Altman. Forty subjects were randomly selected for the analyses. For the inter-observer agreement assessment, the first observer performed the analyses. The second observer repeated the analyses within 24 h. For assessment of the intra-observer agreement, the analyses were repeated twice by the first observer within 1 week.
We used Eview 7.0 and SPSS 17.0 to analyze the data. We used GraphPad Prism 5 and Matlab 2014 to depict the results in the figures. A P value <0.05 was considered statistically significant.
Results
Population
Four hundred forty-six consecutive children, including neonates and infants (233 males and 213 females), met the inclusion and exclusion criteria. Age, height, and weight had a non-normal distribution in the three groups (Table 1). The differences between the male and female groups with respect to age, height, and weight were not statistically significant (P = 0.503, 0.218, and 0.572 > 0.05, respectively).
CS diameter distribution
The CS diameter was 5.49 ± 0.90 mm and normally distributed in the combined male and female groups (Kolmogorov–Smirnov, P = 0.061; Shapiro–Wilk, P = 0.151). The CS diameter was 5.50 ± 0.91 mm and normally distributed in the male group (Kolmogorov–Smirnov, P = 0.055; Shapiro–Wilk, P = 0.167). The CS diameter was 5.46 ± 0.90 mm and normally distributed in the female group (Kolmogorov–Smirnov, P = 0.097; Shapiro–Wilk, P = 0.148). The difference in CS diameters between the male and female groups was not statistically significant (t = 0.639, P = 0.523 > 0.05).
Preliminary models
The results suggested that the highest R 2 was the ln(y) = a + b × ln[x] model [Stevenson formula (R 2 = 0.693, SE = 0.094); Table 2]. The residual variance had homoscedasticity (White test, P > 0.05). Thus, the Stevenson formula was selected as the best-fit formula.
BSADuBois, BSAHaycock, and BSAMosteller were also shown to be the most similar formulae to BSAStevenson (−5 to 5 %), followed by BSABoyd and BSAGehan (−5 to 10 %), then BSADreyer (−15 to 15 %) (Fig. 2).
Gender
BSA was normally distributed in the combined male and female group and the male group. BSA had a near-normal distribution in the female group (P = 0.017). The difference in BSA between the male and female groups were not statistically significant (P = 0.079 > 0.05) (Table 3).
To evaluate the influence of gender on the measurements, we used a multiple linear regression model with gender as a covariate along with BSA based on the Stevenson formula.
Based on the multiple linear regression equation, CS = 3.402 + 1.934 × BSA + 0.008 × Gender, while the P value of gender was 0.8668 > 0.05. Thus, there was no significant effect of gender on the model for measurement of the CS diameter.
Final models
Regression was used to model the relationship between the CS diameter with three different independent variables (height, weight, and BSAStevenson). The best-fit model for the CS diameter measurement was the exponential model (ln(y) = a + b × ln[x]) because the exponential model satisfied the assumption of homoscedasticity and normality of residuals and had the highest R 2 (Table 4).
The CS diameter had a heteroscedastic positive relationship with BSA (White test, P = 0.042 < 0.05).
The regression equation for the CS diameter and BSA was as follows:
The residual had a normal distribution, of which the mean value was 0.0003 and the standard deviation was 0.094.
The predicted values and Z-score boundaries for measurement of the CS diameter are presented in Fig. 3 and Table 5. The predicted values and Z-score boundaries (Z = 2 and Z = − 2) for the CS diameter with the best-fit model were based on the BSA (Fig. 4).
The Z-score is a normal distribution, of which the mean value was −0.003 and the standard deviation was 0.999.
Reproducibility
The Bland–Altman plot regression showed that the 95 % limits of agreement for inter- and intra-observer measurements were not significantly different (Fig. 5).
Discussion
CS dilatation is an imaging finding which may be caused by a persistent left superior vena cava, elevated right atrial pressure disease, anomalous pulmonary venous connection, and unroofed CS. The prevalence of CS dilatation was 0.294 % (295/100,180) in our 15-year study, exceeding the prevalence of Kawasaki disease (0.134 % [134.4/100,000]) in 2011 [18]. Indeed, some physicians may not pay sufficient attention to this finding.
Determining the normal range of the CS diameter is a prerequisite for diagnosing diseases which cause CS dilatation. The ranges of normal values of hearts and great vessels have been one of the hot spots in recent years, especially with respect to the coronary artery. The importance of accurate pediatric nomograms has recently been addressed by various investigators, with recommendations to use Z-scores for various reasons [19, 20]. To date, there are no published studies regarding the CS diameter in healthy children.
Although calculate the parameter of children is an arduous work due to growth and other factors, previous studies have summarized a relatively mature statistical method using multiple regression models of BSA to fit normal values. Then, residual associations, residual heteroscedasticity, and distribution of residual or normalized values were analyzed one-by-one [21]. We designed our research according to the above statistical method.
At the beginning of the current study, we demonstrated which formula was the best-fit formula. In contemporary pediatric echocardiographic research, normalization to BSA is the standard approach. Several BSA formulae have been reported [10–16]. At present, researchers have not reached a consensus regarding the best-fit formula in children. There are few studies involving a comparison of the differences between several formulae [17, 22], and it was concluded that BSAStevenson provides the best fit for most cardiac structures. No research, however, has demonstrated that this formula is suitable for Chinese children. The results of our study showed that BSAStevenson is the best-fit formula for Chinese children. At a BSA <0.5 m2 when compared with the BSAStevenson, all other formulae overestimated the BSA. At a BSA >0.5 m2 when compared with the BSAStevenson, the BSADuBois, BSABoyd, BSAMosteller, and BSAGehan overestimated the BSA, while the BSADreyer underestimated the BSA. BSAHaycock is similar to BSAStevenson. These results are similar to a previous study [23]. In Chinese children, the BSA is overestimated when traditional formulae (BSADuBois, BSABoyd, BSAGehan, and BSAHaycock) are used based on Yu et al. [24].
BSA is a function of both weight and height. We must ascertain whether or not a model including weight and height as independent predictors would be a better fit than BSA alone. We tried these models in preliminary analyses. Height and weight do not have a higher R 2 than the BSA. Computation of the Z-score using two independent variables is more complex because the Z-score cannot be displayed in a 2-dimentional figure or table for clinical use.
Although we showed that the exponential model (ln(y) = a + b × ln[x]) was best for the calculation, similar cardiac parameters were used in previous studies [17, 22, 25]. In fact, there was virtually no difference when Z-score equations were derived from BSA estimated with different equations, and misclassification was rare [5]. The exponential model was selected because it is homoscedasticity and convenient for fitting with the BSA [26].
The Z-score is a standardized score that indicates how many standard deviations a value is above or below the mean in a normally distributed population. By definition, Z scores must also be normally distributed with a mean value of zero and a standard deviation of one [5]. According to our study results, the Z-score is normally distributed with a mean value of −0.003 and the standard deviation was 0.999. This Z-score indicates that our calculation was relatively accurate.
Reproducibility tests showed that echocardiography is a reliable imaging tool for the measurement of CS diameter, and echocardiography has been shown not only to be efficacious for the diagnosis of persistent left superior vena cava or unroofed CS, but to evaluate the pulmonary artery pressure in pediatric patients [27].
Limitations
This research was designed to measure the CS diameter of Chinese children. Thus, the study lacked data from subjects of different races. The equation and Z-score boundaries may need to be slightly adjusted to apply to different races.
The total number of healthy children was small [28]. Due to the rapid change in infancy and small infant sample of our study (including 42 infants), it was difficult to obtain normal values in very young children. The equation and Z-score boundaries may need further investigation [29].
Conclusions
BSAStevenson may be more appropriate than other traditional formulae for Chinese children. New, reliable echocardiographic Z scores of the CS diameter derived from a large population of healthy Chinese children are reported. The Z scores can be used in echocardiographic examinations.
References
D’Cruz IA, Shala MB, Johns C (2000) Echocardiography of the coronary sinus in adults. Clin Cardiol 23:149–154
Katre R, Burns SK, Murillo H, Lane MJ, Restrepo CS (2012) Anomalous pulmonary venous connections. Semin Ultrasound CT MR 33:485–499
Xie MX, Yang YL, Cheng TO, Wang XF, Li K, Ren PP, Lü Q, Lin H, Li L (2013) Coronary sinus septal defect (unroofed coronary sinus): echocardiographic diagnosis and surgical treatment. Int J Cardiol 168:1258–1263
Kronzon I, Tunick PA, Jortner R, Drenger B, Katz ES, Bernstein N, Chintiz LA, Freedberg RS (1995) Echocardiographic evaluation of the coronary sinus. J Am Soc Echocardiogr 8:518–526
Dallaire F, Dahdah N (2011) New equations and a critical appraisal of coronary artery Z score in healthy children. J Am Soc Echocardiogr 24:60–74
World Health Organization. The WHO child growth standards. http://www.who.int/childgrowth/en. Accessed 26 Aug 2014
Centers for Disease Control and Prevention. Growth charts. http://www.cdc.gov/growthcharts. Accessed 26 Aug 2014
Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T (2010) Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the pediatric measurements writing group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 23:465–495
Ezhumalai B, Satheesh S, Anantha A, Pakkirisamy G, Balachander J, Selvaraj RJ (2014) Coronary sinus diameter by echocardiography to differentiate atrioventricular nodal reentrant tachycardia from atrioventricular reentrant tachycardia. Cardiol J 21:273–278
DuBois D, DuBois EF (1916) A formula to estimate the approximate suface area if height and weight be known. Arch Intern Med 17:863–871
Dreyer G, Ray W (1912) Further experiments upon the blood volume of mammals and its relation to the surface area of the body. Phil Trans R Soc Lond 202:191–212.
Boyd E (1935) The growth of the surface area of the human body. Greenwood, Westport
Haycock GB, Schwartz GJ, Wisotsky DH (1978) Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr 93:62–66
Mosteller RD (1987) Simplified calculation of body surface area. New Engl J Med 317:1098
Gehan EA, George SL (1970) Estimation of human body surface area from height and weight. Cancer Chemother Rep 54:225–235
Stevenson PH (1937) Height-weight-surface formula for the estimation of surface area in Chinese subjects. Chin J Physiol 3:327–330
Cantinotti M, Scalese M, Murzi B, Assanta N, Spadoni I, Festa P, De Lucia V, Crocetti M, Marotta M, Molinaro S, Lopez L, Iervasi G (2014) Echocardiographic nomograms for ventricular, valvular and arterial dimensions in caucasian children with a special focus on neonates, infants and toddlers. J Am Soc Echocardiogr 27(179–191):e2
Kim KY, Kim DS (2016) Recent advances in Kawasaki disease. Yonsei Med J 57:15–21
Cantinotti M, Lopez L (2013) Nomograms for blood flow and tissue Doppler velocities to evaluate diastolic function in children: a critical review. J Am Soc Echocardiogr 26:126–141
Cantinotti M, Scalese M, Molinaro S, Murzi B, Passino C (2012) Limitations of current echocardiographic nomograms for left ventricular, valvular, and arterial dimensions in children: a critical review. J Am Soc Echocardiogr 25:142–152
Mawad W, Drolet C, Dahdah N, Dallaire F (2013) A review and critique of the statistical methods used to generate reference values in pediatric echocardiography. J Am Soc Echocardiogr 26:29–37
Sluysmans T, Colan SD (2005) Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol 99:445–457
Verbraecken J, Van de Heyning P, De Backer W, Van Gaal L (2006) Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism 55:515–524
Yu C-Y, Yu-Hung L, Chiou W-K (2003) The 3D scanner for measuring body surface area: a simplified calculation in the Chinese adult. Appl Ergon 34:273–278
Gautier M, Detaint D, Fermanian C, Aegerter P, Delorme G, Arnoult F, Arnoult F, Milleron O, Raoux F, Stheneur C, Boileau C, Vahanian A, Jondeau G (2010) Nomograms for aortic root diameters in children using two-dimensional echocardiography. Am J Cardiol 105:888–894
Cantinotti M, Scalese M, Murzi B, Assanta N, Spadoni I, De Lucia V, Crocetti M, Cresti A, Gallotta M, Marotta M, Tyack K, Molinaro S, Iervasi G (2014) Echocardiographic nomograms for chamber diameters and areas in Caucasian children. J Am Soc Echocardiogr 27:1279–1292
Gunes Y, Guntekin U, Tuncer M, Kaya Y, Akyol A (2008) Association of coronary sinus diameter with pulmonary hypertension. Echocardiography 25:935–940
Cantinotti M, Kutty S, Giordano R, Assanta N, Murzi B, Crocetti M, Marotta M, Iervasi G (2015) Review and status report of pediatric left ventricular systolic strain and strain rate nomograms. Heart Fail Rev 20:601–612
Cantinotti M, Assanta N, Crocetti M, Marotta M, Murzi B, Iervasi G (2014) Limitations of current nomograms in pediatric echocardiography: just the tip of the iceberg—a call for standardization. J Am Soc Echocardiogr 27:339
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Song, G., Liu, J., Qiao, W. et al. Regression equations of Z score and echocardiographic nomograms for coronary sinus in healthy children. Int J Cardiovasc Imaging 32, 1687–1695 (2016). https://doi.org/10.1007/s10554-016-0960-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-016-0960-7