Abstract
This study was aimed at determining whether late gadolinium enhancement (LGE) in conjunction with Galectin-3 (Gal-3) level offered more precise prognosis of non-ischemic cardiomyopathy (NICM) in comparison to LGE alone. Results of LGE and Gal-3 expression in 192 patients with NICM, including 85 subjects with dilated cardiomyopathy (DCM) and 107 with hypertrophic cardiomyopathy (HCM), were examined. As suggested by the characteristics of LGE and Gal-3 levels, patients were divided into four groups: LGE positive + low Gal-3 (n = 10 for DCM, n = 15 for HCM), LGE positive + high Gal-3 (n = 25 for DCM, n = 51 for HCM), LGE negative + low Gal-3 (n = 32 for DCM, n = 29 for HCM), LGE negative + high Gal-3 (n = 18 for DCM, n = 12 for HCM). Primary endpoints over the follow-up period included major adverse cardiac events (MACEs). Kaplan–Meier survival analysis and univariate Cox proportional hazard models were used to analyze the survival status of patients with NICM. The optimal cut-off value of Gal-3 level for two types of NICM was determined by receiver operating characteristic analysis (13.38 U/L for DCM and 14.40 U/L for HCM). The combination of LGE and Gal-3 levels offered a more significant prognostic value than using LGE alone for both DCM and HCM (DCM P = 0.001 < 0.012; HCM P = 0.037 < 0.040). Moreover, the Cox proportional hazard model suggested that both LGE status [Hazard ratio (HR) = 2.62, P = 0.017] and Gal-3 level (HR = 1.16, P = 0.013) were significant predictors of MACEs in DCM, while they did not appear to have significant prognostic values for HCM (P = 0.06 and 0.64). Furthermore, the multivariate analysis only confirmed LGE as an independent element in predicting prognosis of DCM (HR = 12.19, P = 0.026). In conclusion, LGE status was an independent indicator of DCM prognosis, yet the insignificant role of LGE in HCM prognosis could be limited by sample size.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-ischemic cardiomyopathy (NICM) includes two subtypes of dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM), which affects approximately 0.05 and 0.2 % of the world population, respectively [1, 2]. DCM is a universal cardiovascular disease that results in almost 33 % of heart failure (HF) cases. Although various therapeutic strategies have been developed for DCM, conspicuous morbidity and mortality of DCM are still considered as major issues to be solved for the purpose of minimizing social and economic costs resulted from DCM [3]. On the other hand, HCM is a genetic cardiomyopathy which is characterized by substantial increase in both interstitial and replacement fibrosis which may become patchy or diffuse [4]. Cardiac fibrosis which is triggered by both neurohormonal activation and myocardial vulnerability is a key mechanism to the progression of myocardial remodeling which is responsible for arrhythmias, sudden cardiac death (SCD) and adverse remodeling of left ventricular (LV) [5–7]. Therefore, it is worthwhile to precisely identify myocardial fibrosis (MF) which is a key signal for both DCM and HCM.
To date, several clinicopathologic correlation studies have indicated that MF may proffer a substratum for adverse cardiovascular events including malignant ventricular arrhythmias and SCD [8, 9]. Endomyocardial biopsy has been considered as a conventional approach for clinical assessment of MF, but it has several inherent limitations including invasiveness, small myocardial sample size and possible complications [10]. More recently, cardiovascular magnetic resonance (CMR) imaging technique has been rapidly developed as a preferred imaging tool for characterization and diagnosis of DCM and HCM. CMR provides clinicians with superior clinical assessment of cardiac morphology and function [6, 11]. Apart from that, delayed enhancement of CMR (DE-CMR) is known to be compatible with myocardial scarring and the enhancement degree of CMR is associated with the severity of cardiac functional abnormalities particularly contributed by NICM [12].
CMR with late gadolinium enhancement (LGE) has emerged as a powerful and noninvasive tool that is able to accurately identify and quantify ventricular MF [13]. This imaging modality not only offers incremental characterization of tissues but also identifies the presence of NICM in about two-thirds of HCM patients and at least one-third of DCM patients [14]. As suggested by previous studies on CMR, ventricular LGE was able to forecast several adverse events of DCM patients including failure-related hospitalization and death [15–18]. Since LGE depends on different signal intensity between focal MF and normal myocardium, LGE exhibited limited ability to identify diffuse interstitial fibrosis which is very common in patients with DCM [19]. Thus, patients with high risk of NICM are likely to be misdiagnosed by LGE and the feasibility of LGE combined with other techniques should be clarified.
Galectin-3 (Gal-3) is a β-galactoside-binding lectin secreted by activated macrophages. In addition, Gal-3 has been suggested to be associated with several mechanism pathways of MF observed in patients with HF and it is correlated with extracellular matrix update [20, 21]. Gal-3 also has been hypothesized as a risk marker and mediator for both fibrosis and inflammation that are critical to LV remodeling process [22, 23]. As suggested by several studies, up-regulated Gal-3 was observed in cardiomyopathy, myocarditis and hypertensive heart disease models which were constructed on rats whereas suppressed LV systolic function and disclosed aortic stenosis were identified in hypertrophied human ventricular myocardium [20, 24, 25]. Other studies also provided evidence that Gal-3 exhibited a prognostic role in both predicting LV remodeling status and forecasting mortality of chronic HF patients [26–28].
Nevertheless, significant relationship between LGE and clinical assessment of DCM/HCM has not been clarified. Furthermore, researches have not been carried out for determining the feasibility of LGE-CMR combined with Gal-3 in evaluating patients with NICM. As a result, we conducted this study to assess the prognostic value of CMR combined with Gal-3 level for patients with NICM (HCM and DCM).
Materials and methods
Ethics statement
All study procedures were agreed by the Institutional Ethics Committee of the First Hospital of Chenzhou. Informed consent was obtained from patients prior to study commencement.
Patient samples
The prospective observational study recruited a total of 192 NICM patients with 85 DCM cases and 107 HCM cases who received both LGE-CMR and cardiac catheterization imaging at the First Hospital of Chenzhou.
As suggested by the criteria of the American Heart Association, DCM patients were diagnosed with the follow conditions: patients received optimal medical therapy with left ventricular ejection fraction (LVEF) <45 %, LV diastolic dimension >55-mm, traditional signs or symptoms of HF, absence of obvious coronary artery disease revealed by perfusion imaging or other approaches [3]. As suggested by HCM guidelines released by European Society of Cardiology in 2014, any radiographical results that suggested ventricular wall thickness of one or more LV myocardial segments to be ≥15 mm among adult patients could serve as the evidence of HCM development [29]. Subjects with ischemic cardiomyopathy, myocarditis, hypertensive heart disease, valvular heart disease and secondary cardiomyopathy were excluded. In addition, patients with chronic or acute inflammatory diseases, severe chronic kidney disease and hematological malignancies which may have potential influence on Gal-3 level were excluded.
CMR protocol
CMR examinations were carried out using a 1.5-T scanner (Symphony Maestro Upgrade, Germany) in conjunction with a steady-state acquisition imaging (TrueFisp) system including ECG-triggered breath-hold gradient-echo. Patients were placed with supine position and morphologic images were examined in cardiac short axis and two/three/four chamber long axis. LV outflow tract was viewed using fast-field echo cine images. Typical imaging parameters were set as: 6-mm slice thickness, 4-mm gap, 256 × 192 matrix, 1.5-ms TE, 40–45-ms TR and 50° flip angle. After gadolinium had been injected into peripheral bolus for 10 min (0.2 mmol/kg of body weight, Shering AG, Germany), MF and/or scar was evaluated on DE multislice long-axis, short-axis and four-chamber views. Typical imaging parameters were set as: 6-mm slice thickness, 4-mm gap, 256 × 192 matrix and the inversion time was optimized according to previously research methods [30].
LGE analysis
LGE analysis was performed by two blinded investigators and a third investigator was consulted in the case of disagreement. CAAS MRV 3.4 software (Pie Medical, Netherlands) was implemented for LGE analysis. Both epicardial and endocardial contours were created for assessing myocardial mass. MF was presented when myocardium signal in any region was increased due to other reasons rather than image artifact on two orthogonal or contiguous slices. MF patterns were categorized into the following conditions when they were identified: diffuse, sub-endocardial based, sub-epicardial based, RV insertion site, mid-wall striae and mid-wall patchy.
Quantification of the LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), LVEF and LV mass were conducted using semi-automated contour tracing of epicardial and endocardial borders on imaging datasets of sequential short axis. Mass and volume measurements were indexed to body surface area (BSA). Technique of Signal Threshold versus Reference Myocardium (STRM) was used to quantify the extent of MF [14, 31]. Scar signal was assessed by three separate thresholds above the mean signal in the normal region which was the largest contiguous region of homogenously nulled myocardium. Total area of MF was calculated as: the accumulated area with enhancement signals multiplied by the slice thickness and then the total area of MF was presented as a percentage with respect to the total LV mass.
Blood samples and Gal-3 evaluation
Gal-3 was measured using serum samples which were immediately centrifuged and stored at −80 °C prior to the analysis. Serum Gal-3 levels were measured using an enzyme-linked immunosorbent assay (ELISA) which was carried out by a specific kit (Waltham, USA) and the detection threshold was set as 1.13 ng/ml.
Follow-up study and endpoints
Follow-up study was conducted through telephone interviews or outpatient record reviews and relevant data were obtained during the follow-up period (i.e. 7 years). All patients were advised to continue their medications in order to prevent HF. Regular follow-up interviews with/without standard 12-lead ECG were carried out in the outpatient clinic every 2–4 months. Besides that, patients were recommended to undergo 24-h ambulatory Holter monitoring and arrhythmia management if they had palpitations or unusual symptoms. Ventricular tachycardia was diagnosed if Holter or ECG suggested more than 100 beats of ventricular depolarization per minute for more than three consecutive times. Endpoints of the follow-up study included major adverse cardiac events (MACEs) including cardiac death, arrhythmic event (ventricular fibrillation and ventricular tachycardia) and aggravated HF. The aggravated HF was defined as that the functional class of HF increased by ≥1 degree based on evaluations that extra drugs were prescribed for HF, or that symptomatology was indicated to be worsened.
Statistical analysis
All statistical analyses results were obtained from SPSS 18.0 software (Chicago, Illinois, USA). Data were presented in the form of mean ± standard deviation (SD). The two-tailed student’s t test, one-way analysis of variance (ANOVA) or non-parametric test was used to analyze between-group comparisons, whereas the Chi square test was used for assessing differences in categorical variables between two groups. Cut-off values of Gal-3 were determined based on results from Receiver Operating Characteristic (ROC) curve analysis. The Kaplan–Meier method was used to create survival curves and difference in survival times among groups was assessed by the log-rank test. Hazard ratio (HR) of adverse cardiac events and their 95 % CIs were calculated using the univariate Cox proportional hazards regression model. P < 0.05 provided sufficient evidence of statistical significance.
Results
Clinical characteristics
Baseline clinical characteristics of patients with DCM and those with HCM were shown in Table 1. There were no significant differences in age, BMI, NYHA class, indexed LVEDV, indexed LV mass and LVEF between the LGE positive and LGE negative group among DCM patients (all P > 0.05). Besides, patients with LGE positive had significantly higher indexed LVESV and higher Gal-3 level (all P < 0.05) compared to patients with LGE negative.
Similarly, no significant differences were found in age, BMI, NYHA class, indexed LVEDV, indexed LVESV, indexed LV mass, LVEF or Gal-3 level between the LGE positive and LGE negative group in HCM patients (all P > 0.05). In addition, patients with HCM exhibited remarkably higher levels of Gal-3, indexed LV mass and LVEF as well as lower levels of indexed LVEDV and indexed LVESV in comparison to DCM patients.
Cut-off value of Gal-3 for predicting cardiac events
As suggested by the results from follow-up study, 26 cardiac events were observed among 85 patients in the DCM group. ROC curve suggested that the optimal cut-off value of Gal-3 for predicting cardiac events was 13.28 U/L in the DCM group. This cut-off value was associated with a sensitivity of 0.769, specificity of 0.610 and area under the ROC curve (AUC) of 0.64 for predicting cardiac events (Fig. 1a). Then DCM patients were sub-grouped into four groups as follows: LGE positive + low Gal-3 (Gal-3 < 13.38 U/L, n = 10), LGE positive + high Gal-3 (Gal-3 > 13.38 U/L, n = 25), LGE negative + low Gal-3 (Gal-3 < 13.38 U/L, n = 32) and LGE negative + high Gal-3 (Gal-3 > 13.38 U/L, n = 18).
On the other hand, 22 cardiac events were observed among 107 HCM patients. As suggested by the ROC curve, the cut-off value of Gal-3 for predicting cardiac events was 14.40 U/L in the HCM group, with a sensitivity of 0.864, specificity of 0.482 and area under the ROC curve (AUC) of 0.63 (Fig. 1b). Then patients in the HCM group were also allocated into four groups: LGE positive + low Gal-3 (Gal-3 < 14.40 U/L, n = 15), LGE positive + high Gal-3 (Gal-3 > 14.40 U/L, n = 51), LGE negative + low Gal-3 (Gal-3 < 14.40 U/L, n = 29), LGE negative + high Gal-3 (Gal-3 > 14.40 U/L, n = 12).
There were 4 cardiac deaths, 16 arrhythmic events and 6 aggravated HFs in the DCM group whereas 3 cardiac deaths, 14 arrhythmic events and 5 aggravated HFs were observed in the HCM group (Tables 2, 3). For patients with DCM, the LGE positive + high Gal-3 group was more likely to have cardiac events compared to the other three groups (P = 0.004, Table 2). A similar trend with respect to the likelihood of cardiac events was observed in patients with HCM (P = 0.004, Table 3).
Prognostic value of CMR and Gal-3
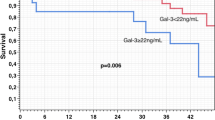
Kaplan–Meier survival analysis by CMR or Gal-3 status was displayed in Fig. 2. The LGE positive group had significantly lower cardiac event-free survival rate compared with the LGE negative group in DCM patients (P = 0.012, Fig. 2a), while the high Gal-3 group exhibited remarkably lower survival rate compared with the low Gal-3 group (P < 0.001, Fig. 2b). Meanwhile, similar trends were observed in HCM patients (all P < 0.05, Fig. 2c, d).
Kaplan–Meier analyses of major adverse cardiac event-free survival status. a MACE-free survival status of DCM patients with and without LGE. b MACE-free survival status of DCM patients with low and high levels of Gal-3. c MACE-free survival status of HCM patients with and without LGE. d MACE-free survival status of HCM patients with low and high levels of Gal-3. DCM dilated cardiomyopathy, MACE major adverse cardiac event, HCM hypertension cardiomyopathy, LGE late gadolinium enhancement, Gal-3 galectin-3
Kaplan–Meier survival analysis which combined CMR with Gal-3 was suggested in Fig. 3. The LGE negative + low Gal-3 group had significantly higher survival rate compared with other groups among DCM patients (all P < 0.05), the LGE positive + low Gal-3 group had insignificantly higher rate than the LGE positive + high Gal-3 group but lower rate than the LGE negative + higher Gal-3 group (all P > 0.05, Fig. 3a). For HCM patients, survival rate in the LGE positive + low Gal-3, LGE negative + low Gal-3 and LGE negative + high Gal-3 group were very similar (all P > 0.05), whereas the LGE positive + high Gal-3 group exhibited significantly lower survival rate than the LGE negative + low Gal-3 group(P = 0.04, Fig. 3b).
As suggested by univariate Cox regression analysis, LGE presence, age, Gal-3 and indexed LVESV were all significant predictors of all cardiac events in DCM patients, while only age and BMI were associated with cardiac events in HCM patients (all P < 0.05 Table 4). Patients with presence of LGE, older age and higher levels of indexed LVESV and BMI were associated with higher risk of cardiac events (OR > 1). Nonetheless, after execution of multivariate analysis, it appeared that merely LGE presence and age were notably correlated with prognosis of DCM, yet no significance emerged when HCM was considered.
Discussion
DCM and HCM were two types of myocardium dysfunctions that belonged to NICM [32], and CMR provided a noninvasive approach for detecting and characterizing both DCM and HCM [33]. Besides that, LGE was always used as an important indicator of CMR because it was able to precisely visualize infiltration areas and MF [34]. We analyzed different clinical characteristics of NICM patients based on LGE status (positive or negative) and concluded that no significant differences in age, BMI, NYHA class, indexed LVEDV, indexed LV mass and LVEF between the two groups were observed in DCM or HCM patients. As suggested by a single-center cohort study in China conducted by Li et al., no significant difference in mortality was observed among DCM patients with different genders or ages [35]. However, DCM patients with LGE positive exhibited significantly higher level of indexed LVESV compared to patients with LGE negative and this trend was not observed in HCM patients. Additionally, HCM patients exhibited significantly lower indexed LVEDV and LVESV levels along with much higher indexed LV mass and LVEF in comparison to DCM patients. Similarly, Cheng et al. reported that lower LVEDV and LVESV levels were identified in DCM patients compared with healthy individuals whereas no significant difference in age or gender distributions was observed between these two groups of individuals [36].
On the other hand, whether LGE was an independent risk factor for NICM or whether LGE is associated with other established prognostic factors [37] still remained elusive [38]. A cohort study conducted revealed that difference in LGE and clinical results among DCM patients were not significant [39]. Another study conducted by Vergaro et al. suggested that LGE which was able to cardiac fibrosis has emerged as a powerful predictor of LV remodeling and LGE was useful for evaluating risk of DCM [40]. Apart from that, LGE was particularly associated with hospitalization and mortality resulted from HF in NICM patients [41]. Our study demonstrated that LGE positive group in both DCM and HCM patients exhibited significantly lower cardiac event-free survival rate. As suggested by the univariate Cox regression model, we discovered that LGE presence was a significant predictor of cardiac events in DCM patients whereas its prediction was not significantly reflected in HCM patients.
Galectin-3 was involved in multiple immune reactions including activation and migration of different cells as well as cell apoptosis [42]. Selecn et al. reported that Gal-3 level was correlated with the degree of LV hypertrophy [43]. As suggested by our experiments, the LGE positive group in both HCM and DCM patients had remarkably higher level of Gal-3 and this trend was consistent with the study conducted by Selecn et al. who concluded that Gal-3 level was increased in both NICM and HF patients [40, 43, 44].
Results from Kaplan–Meier survival analysis indicated that higher levels of Gal-3 were associated with lower survival rate and higher MACE rate in both DCM and HCM patients. The prognostic value of Galectin-3 for predicting events such as HF in the long-term was confirmed by Benjimin [45]. Though the number of research conducted on this topic has increased, it is still challenging to obtain evidence on the intrinsic relationship between Gal-3 and MRI in patients with NICM [40]. We sub-divided DCM and HCM patients into four groups by LGE status and Gal-3 level in our study in order to examine how these two factors influenced the survival status of HCM and DCM patients. As suggested by the Kaplan–Meier survival analysis, the LGE positive + high Gal-3 group exhibited significantly lower survival rate in comparison to other groups and this provided evidence of their prognostic values for NICM patients. Other studies also suggested that the presence of cardiac fibrosis was associated with right ventricular dysfunction which may be triggered by higher prevalence of right ventricular dilation and systolic impairment in HF patients [46]. On top of that, Freed concluded that the presence of LGE was correlated with restricted right ventricular function assessed by MRI [47]. Our study supported the notion that LGE status together with Gal-3 level were capable of predicting clinical outcomes in NICM patients and this conclusion was consistent with the notion that Gal-3 was directly involved in cardiac remodeling and progression of heart failure syndromes.
Conclusions
This study enabled us to clarify the relationship between LGE and Gal-3 in predicting the survival status of NICM patients whereas a few limitations should be addressed with great caution. For instance, issues such as loss of follow-up may cause missing data and affect the completeness of data collection which may have significant impact on the statistical analysis. As a result of this, how Gal-3 and LGE are related to the pathophysiology and progression of NICM should be further studied in order to address these limitations.
References
Smith N, Steeds R, Masani N et al (2015) A systematic approach to echocardiography in hypertrophic cardiomyopathy: a guideline protocol from the british society of echocardiography. Echo Res Pract 2:G1–G7. doi:10.1530/ERP-14-0115
Khan R, Massel D, Stirrat J et al (2013) Myocardial fibrosis and quality of life in patients with non-ischemic cardiomyopathy: a cardiovascular magnetic resonance imaging study. Int J Cardiovasc Imaging 29:395–404. doi:10.1007/s10554-012-0107-4
Maron BJ, Towbin JA, Thiene G et al (2006) Contemporary definitions and classification of the cardiomyopathies: an american heart association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation 113:1807–1816. doi:10.1161/CIRCULATIONAHA.106.174287
Basso C, Thiene G, Corrado D et al (2000) Hypertrophic cardiomyopathy and sudden death in the young: pathologic evidence of myocardial ischemia. Hum Pathol 31:988–998. doi:10.1053/hupa.2000.16659
Mann DL, Bristow MR (2005) Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation 111:2837–2849. doi:10.1161/CIRCULATIONAHA.104.500546
Masci PG, Schuurman R, Andrea B et al (2013) Myocardial fibrosis as a key determinant of left ventricular remodeling in idiopathic dilated cardiomyopathy: a contrast-enhanced cardiovascular magnetic study. Circ Cardiovasc Imaging 6:790–799. doi:10.1161/CIRCIMAGING.113.000438
Olivotto I, Maron BJ, Appelbaum E et al (2010) Spectrum and clinical significance of systolic function and myocardial fibrosis assessed by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol 106:261–267. doi:10.1016/j.amjcard.2010.03.020
Wu TJ, Ong JJ, Hwang C et al (1998) Characteristics of wave fronts during ventricular fibrillation in human hearts with dilated cardiomyopathy: role of increased fibrosis in the generation of reentry. J Am Coll Cardiol 32:187–196
Pogwizd SM, McKenzie JP, Cain ME (1998) Mechanisms underlying spontaneous and induced ventricular arrhythmias in patients with idiopathic dilated cardiomyopathy. Circulation 98:2404–2414
Perazzolo Marra M, De Lazzari M, Zorzi A et al (2014) Impact of the presence and amount of myocardial fibrosis by cardiac magnetic resonance on arrhythmic outcome and sudden cardiac death in nonischemic dilated cardiomyopathy. Heart Rhythm 11:856–863. doi:10.1016/j.hrthm.2014.01.014
White JA, Patel MR (2007) The role of cardiovascular mri in heart failure and the cardiomyopathies. Cardiol Clin 25(71–95):vi. doi:10.1016/j.ccl.2007.02.003
Ahn MS, Kim JB, Joung B et al (2013) Prognostic implications of fragmented qrs and its relationship with delayed contrast-enhanced cardiovascular magnetic resonance imaging in patients with non-ischemic dilated cardiomyopathy. Int J Cardiol 167:1417–1422. doi:10.1016/j.ijcard.2012.04.064
Mewton N, Liu CY, Croisille P et al (2011) Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol 57:891–903. doi:10.1016/j.jacc.2010.11.013
Bruder O, Wagner A, Jensen CJ et al (2010) Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 56:875–887. doi:10.1016/j.jacc.2010.05.007
Masci PG, Barison A, Aquaro GD et al (2012) Myocardial delayed enhancement in paucisymptomatic nonischemic dilated cardiomyopathy. Int J Cardiol 157:43–47. doi:10.1016/j.ijcard.2010.11.005
Lehrke S, Lossnitzer D, Schob M et al (2011) Use of cardiovascular magnetic resonance for risk stratification in chronic heart failure: prognostic value of late gadolinium enhancement in patients with non-ischaemic dilated cardiomyopathy. Heart 97:727–732. doi:10.1136/hrt.2010.205542
Wu KC, Weiss RG, Thiemann DR et al (2008) Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol 51:2414–2421. doi:10.1016/j.jacc.2008.03.018
Assomull RG, Prasad SK, Lyne J et al (2006) Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol 48:1977–1985. doi:10.1016/j.jacc.2006.07.049
de Leeuw N, Ruiter DJ, Balk AH et al (2001) Histopathologic findings in explanted heart tissue from patients with end-stage idiopathic dilated cardiomyopathy. Transpl Int 14:299–306
Sharma UC, Pokharel S, van Brakel TJ et al (2004) Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 110:3121–3128. doi:10.1161/01.CIR.0000147181.65298.4D
Weir RA, Petrie CJ, Murphy CA et al (2013) Galectin-3 and cardiac function in survivors of acute myocardial infarction. Circ Heart Fail 6:492–498. doi:10.1161/CIRCHEARTFAILURE.112.000146
Dumic J, Dabelic S, Flogel M (2006) Galectin-3: an open-ended story. Biochim Biophys Acta 1760:616–635. doi:10.1016/j.bbagen.2005.12.020
Yang RY, Rabinovich GA, Liu FT (2008) Galectins: structure, function and therapeutic potential. Expert Rev Mol Med 10:e17. doi:10.1017/S1462399408000719
Reifenberg K, Lehr HA, Torzewski M et al (2007) Interferon-gamma induces chronic active myocarditis and cardiomyopathy in transgenic mice. Am J Pathol 171:463–472. doi:10.2353/ajpath.2007.060906
Thandavarayan RA, Watanabe K, Ma M et al (2008) 14-3-3 protein regulates ask1 signaling and protects against diabetic cardiomyopathy. Biochem Pharmacol 75:1797–1806. doi:10.1016/j.bcp.2008.02.003
de Boer RA, Lok DJ, Jaarsma T et al (2011) Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med 43:60–68. doi:10.3109/07853890.2010.538080
Lok DJ, Van Der Meer P, de la Porte PW et al (2010) Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol 99:323–328. doi:10.1007/s00392-010-0125-y
Lok DJ, Lok SI, Bruggink-Andre de la Porte PW et al (2013) Galectin-3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clinical Res Cardiol 102:103–110. doi:10.1007/s00392-012-0500-y
Elliott PM, Anastasakis A, Borger MA et al (2014) [2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy]. Kardiol Pol 72:1054–1126. doi:10.5603/KP.2014.0212
Kim RJ, Shah DJ, Judd RM (2003) How we perform delayed enhancement imaging. J Cardiovasc Magn Reson 5:505–514
Gao P, Yee R, Gula L et al (2012) Prediction of arrhythmic events in ischemic and dilated cardiomyopathy patients referred for implantable cardiac defibrillator: evaluation of multiple scar quantification measures for late gadolinium enhancement magnetic resonance imaging. Circ Cardiovasc Imaging 5:448–456. doi:10.1161/CIRCIMAGING.111.971549
Morgan RB, Kwong R (2015) Role of cardiac MRI in the assessment of cardiomyopathy. Curr Treat Options Cardiovasc Med 17:53. doi:10.1007/s11936-015-0410-1
Johnston DL, Rokey R, Okada RD (1987) Nuclear magnetic resonance imaging of the cardiovascular system. Herz 12:51–67
Masci PG, Schuurman R, Barison A et al (2013) Response to letters regarding article, “myocardial fibrosis as a key determinant of left ventricular remodeling in idiopathic dilated cardiomyopathy: a contrast-enhanced cardiovascular magnetic study”. Circ Cardiovasc Imaging 6:e79. doi:10.1161/CIRCIMAGING.113.001217
Li X, Cai C, Luo R et al (2015) The usefulness of age and sex to predict all-cause mortality in patients with dilated cardiomyopathy: a single-center cohort study. Clin Interv Aging 10:1479–1486. doi:10.2147/CIA.S88565
Cheng L, Zhao R, Jin Z et al (2015) Association of genetic polymorphisms on btnl2 with susceptibility to and prognosis of dilated cardiomyopathy in a chinese population. Int J Clin Exp Pathol 8:10488–10499
Ismail TF, Prasad SK, Pennell DJ (2012) Prognostic importance of late gadolinium enhancement cardiovascular magnetic resonance in cardiomyopathy. Heart 98:438–442. doi:10.1136/heartjnl-2011-300814
Rodriguez-Capitan J, Garcia-Pinilla JM, Ruiz-Zamora I et al (2015) Reply to the letter “prognostic value of late gadolinium enhancement in cardiomyopathy: causative risk factor or surrogate marker?” Int J Cardiol 181:102–103. doi:10.1016/j.ijcard.2014.12.007
Rodriguez-Capitan J, Garcia-Pinilla JM, Ruiz-Zamora I et al (2014) Long-term prognostic value of late gadolinium enhancement in a cohort of patients with nonischemic dilated cardiomyopathy. Int J Cardiol 177:17–19. doi:10.1016/j.ijcard.2014.09.110
Vergaro G, Del Franco A, Giannoni A et al (2015) Galectin-3 and myocardial fibrosis in nonischemic dilated cardiomyopathy. Int J Cardiol 184:96–100. doi:10.1016/j.ijcard.2015.02.008
Ise T, Hasegawa T, Morita Y et al (2014) Extensive late gadolinium enhancement on cardiovascular magnetic resonance predicts adverse outcomes and lack of improvement in LV function after steroid therapy in cardiac sarcoidosis. Heart 100:1165–1172. doi:10.1136/heartjnl-2013-305187
Gucuk Ipek E, Akin Suljevic S, Kafes H et al (2015) Evaluation of galectin-3 levels in acute coronary syndrome. Ann Cardiol Angeiol doi:10.1016/j.ancard.2015.09.046
Yakar Tuluce S, Tuluce K, Cil Z et al (2015) Galectin-3 levels in patients with hypertrophic cardiomyopathy and its relationship with left ventricular mass index and function. Anatol J Cardiol. doi:10.5152/AnatolJCardiol.2015.6191
Cuspidi C, Tadic M, Sala C (2015) Galectin-3 and hypertensive heart disease. J Clin Hypertens (Greenwich). doi:10.1111/jch.12756
French B, Wang L, Ky B et al (2015) Prognostic value of galectin-3 for adverse outcomes in chronic heart failure. J Card Fail. doi:10.1016/j.cardfail.2015.10.022
Guglin M, Verma S (2012) Right side of heart failure. Heart Fail Rev 17:511–527. doi:10.1007/s10741-011-9272-0
Freed BH, Gomberg-Maitland M, Chandra S et al (2012) Late gadolinium enhancement cardiovascular magnetic resonance predicts clinical worsening in patients with pulmonary hypertension. J Cardiovasc Magn Reson 14:11. doi:10.1186/1532-429X-14-11
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All Authors have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Hu, DJ., Xu, J., Du, W. et al. Cardiac magnetic resonance and galectin-3 level as predictors of prognostic outcomes for non-ischemic cardiomyopathy patients. Int J Cardiovasc Imaging 32, 1725–1733 (2016). https://doi.org/10.1007/s10554-016-0958-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-016-0958-1