Abstract
There is variability in guideline recommendations for assessment of the right ventricle (RV) with imaging as prognostic information after acute pulmonary embolism (PE). The objective of this study is to identify a clinical scenario for which normal CT-derived right-to-left ventricular (RV/LV) ratio is sufficient to exclude RV strain or PE-related short-term death. This retrospective cohort study included 579 consecutive subjects (08/2003-03/2010) diagnosed with acute PE with normal CT-RV/LV ratio (<0.9), 236 of whom received subsequent echocardiography. To identify a clinical scenario for which CT-RV/LV ratio was considered sufficient to exclude RV strain or PE-related short-term death, a multivariable logistic model was created to detect factors related to subjects for whom subsequent echocardiography detected RV strain or those who did not receive echocardiography and died of PE within 14 days (n = 55). The final model included five variables (c-statistic = 0.758, over-fitting bias = 2.52 %): congestive heart failure (adjusted odds ratio, OR 4.32, 95 % confidence interval, CI 1.88–9.92), RV diameter on CT >45 mm (OR 3.07, 95 % CI 1.56–6.03), age >60 years (OR 2.59, 95 % CI 1.41–4.77), central embolus (OR 1.96, 95 % CI 1.01–3.79), and stage-IV cancer (OR 1.94, 95 % CI 0.99–3.78). If these five factors were all absent (37.1 % of the population), the probability that “CT-RV/LV ratio is sufficient to exclude RV strain/PE-related short-term death” was 0.97 (95 % CI = 0.95–0.99). Normal CT-RV/LV ratio plus readily obtained five clinical predictors were adequate to exclude RV strain or PE-related short-term mortality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Right ventricular (RV) dysfunction is the main pathophysiology [1, 2] for the severe morbidity and mortality after acute pulmonary embolism (PE) [3, 4]. Transthoracic echocardiography (TTE) is accepted as a reference standard to assess RV status after acute PE [1, 2], based on its ability to evaluate RV size, systolic function, pressure, volume and RV wall motion [5]. TTE provides risk stratification; from a treatment perspective this translates into deciding the subset of patients for whom aggressive therapies such as thrombolysis or thrombectomy/embolectomy are warranted.
The cost and complication profile of the more aggressive treatment pathways underscores the need to accurately assess RV status. However, when the decision to initiate or to withhold aggressive therapies is clear from a clinical perspective or other ancillary data including the CT derived RV/left ventricular (LV) diameter ratio, TTE is unlikely to impact clinical management and may not be an appropriate use of resources. This study develops and examines a clinical scenario for which the CT derived RV/LV diameter ratio reported by the radiologist, combined with clinical information, adequately predicts the absence of short term mortality from acute PE.
Current guidelines vary regarding CT RV data as well as TTE utilization after acute PE, likely from the large void of data regarding how each is used, and their impact on clinical outcomes. This project represents a first step to fill that large void. The most recent American College of Cardiology Foundation/American Heart Association guideline states “Echocardiography identifies patients at increased risk of adverse outcomes from acute PE in many studies” [6]. In this document, the fibrinolysis recommendations include “Fibrinolysis may be considered (Class IIb) for patients with submassive acute PE judged to have clinical evidence of adverse prognosis (new hemodynamic instability, worsening respiratory insufficiency, severe RV dysfunction, or major myocardial necrosis) and low risk of bleeding complications”. The level of evidence was considered “less well established” (Level C).
American College of Chest Physicians (ACCP) guidelines have evolved over the past 25 years. The 8th edition (published in 2008) [7] notes, “Among patients without hemodynamic compromise, poor prognostic indicators include the following: (1) patients who appear ill, with marked dyspnea, anxiety, and low oxygen saturation; (2) elevated troponin, indicating right ventricular microinfarction; (3) right ventricular dysfunction on echocardiography; and (4) right ventricular enlargement on chest CT. These sick patients are at high risk for an adverse outcome and may derive benefit from thrombolytic therapy, even if they initially maintain systemic arterial pressure.” The 2012 ACCP guideline has the following more conservative role towards testing, perhaps in part from the circa 2012 initiative to deliver high value, cost-conscious care [8], “laboratory, ECG, echocardiography, and CT evidence of right ventricular dysfunction or enlargement, can supplement the clinical evaluation of instability; however, they are not sufficiently predictive to serve as indications for thrombolytic therapy on their own and we do not recommend that they are routinely measured”. While neither TTE nor CT are recommended for routine use [9, 10], this guideline also notes that “in selected patients whose initial clinical presentation or clinical course after starting anticoagulant therapy suggests a high risk of developing hypotension, we suggest administration of thrombolytic therapy”. Despite the impact of this decision and the relative frequency of acute PE, there is no explicit clinical prediction rule to identify the high risk subpopulation.
The 2014 European Society of Cardiology Guidelines state, “In patients at intermediate risk using a prediction score such as PE Severity Index [11], assessment of the right ventricle with echocardiography or CT, and of myocardial injury using a laboratory biomarker, should be considered (Class IIa)” [12]. The American College of Radiology Appropriateness Criteria includes the RV/LV diameter ratio as rationale for CT pulmonary angiography (CTPA) but does not address risk stratification by either TTE or CT for prognosis among patients diagnosed with acute PE. While, to our knowledge, there is no published comprehensive utilization data for TTE to assess the RV (e.g., how often TTE is used after acute PE diagnosis), its presence cardiology guidelines and general knowledge argues that it is commonly performed after acute PE. We hypothesize that TTE can be averted in some patients from whom CT-based data can be integrated into a risk stratification profile with sufficient prognostic information. For example, it may be the case that an obviously normal CT-RV/LV diameter ratio will be sufficient to exclude RV strain, at least in a subpopulation of patients. The purpose of this study is to develop a clinical prediction rule to identify the subpopulation for which CT-derived data can be used to predict normal RV function and survival from 14-day PE-related mortality among those patients who were diagnosed with acute PE and normal RV/LV diameter ratio by CTPA.
Methods
Study population
The Brigham and Women’s Hospital (BWH) Institutional Review Board (IRB) approved this HIPAA-compliant retrospective cohort study; written informed consent was waived since patient data was analyzed anonymously. Among all CTPA examinations performed at this large, urban teaching hospital between August 2003 and March 2010, all consecutive studies with a diagnosis of acute PE and a normal RV/LV diameter ratio [13, 14] (measurements described below) were included in this study.
CTPA and echocardiography
All CTPA examinations were performed in the craniocaudal direction with 16-, 64-, or 128-MDCT scanners without ECG-gating. Images were acquired with the section thickness of 1.0–1.25 mm at 80–130 kVp with effective milliampere of approximately 140–200 mAs, after intravenous administration of 75–100 mL iodinated (370 mg iodine per mL) contrast media at 3–4 mL/s, timed by bolus tracking at the main pulmonary artery.
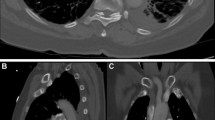
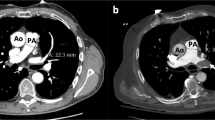
The CT-RV/LV diameter ratios were calculated from a reformatted 4-chamber image using a standardized double-oblique method [15, 16] on a dedicated 3D workstation (VitreafX; Vital Images, Minnetonka, MN). On the 4-chamber reformatted image, the RV and LV diameters were manually measured as the maximum distance from the interventricular septum to the endocardial border, perpendicular to the long axis of each ventricle. Official CT reports were reviewed to separate patients with respect to the most proximal extension of the embolus: central, lobar, segmental, or subsegmental pulmonary artery.
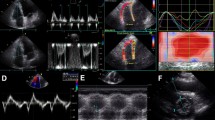
Clinical TTE examinations performed within 14 days of the positive CTPA were included in the study. All TTE were performed in a laboratory accredited by the Intersocietal Accreditation Commission and in accordance with the recommendations of the American Society for Echocardiography [5]. Standard two-dimensional 2–5 MHz phased array transducers were used to perform transthoracic studies. Each patient was examined in supine position, and the patient’s position was adjusted to the acoustic window being utilized.
All clinical TTE reports were reviewed for information on the RV by a physician who did not measure and who was unaware of the results from the RV/LV diameter ratios on the CTPA studies. RV strain was considered present when any of the following five findings were present: (1) reduced RV systolic function assessed qualitatively based on the RV wall motion; (2) RV hypokinesia/dyskinesia/akinesia; (3) pulmonary artery systolic pressure >36 mmHg [5, 17]; (4) moderate or severe dilatation of RV (description of RV size was based on 5 categories, determined by quantitatively or qualitatively assessed RV/LV end-diastolic diameter ratio: normal, borderline enlargement (corresponding to approximately RV/LV = 1), mild enlargement, moderate enlargement (corresponding to approximately RV/LV = 1.3), and severe enlargement [18, 19]); and (5) abnormal interventricular septal movement. When RV hypertrophy was observed, that case was not counted as “RV strain due to acute PE”. When the patient received multiple TTE studies during the period, the most abnormal TTE was included in the analysis.
Covariate collection
The following data were obtained from the electronic medical records: age, gender, race, hemodynamic instability at initial presentation (instable defined as cardiac arrest, shock, or systolic blood pressure <90 mmHg or a decrease >40 mmHg from the baseline), co-morbidities (cancer, congestive heart failure (CHF), coronary artery disease, atrial fibrillation, chronic lung disease, hypertension, coagulopathies, chronic renal failure, and stroke or transient ischemic attack), and recent surgery (≤30 days). For patients with cancer, further information regarding those patients who were stage-IV and those who underwent recent (≤2 weeks) chemotherapy treatment was also collected.
Study outcome
Death was confirmed by the Social Security Death Index, and the cause of death was determined by reviewing autopsy data, death certifications, electronic medical records, and the notes from the referring physicians, without knowledge of the imaging interpretations. Death was considered as PE-related by consensus of three physicians, when (1) either the autopsy data, death certification, or death report on the electronic medical record stated that the death was caused by PE; or (2) acute respiratory failure, cardiopulmonary arrest or shock was the cause of death, in the absence of other cardiopulmonary disease.
Patient classification
All patients were placed into one of four categories (Fig. 1) that formed two groups, “A” or “B”. For patients in Group A, the normal CT-RV/LV diameter ratio was considered sufficient to exclude critical RV strain. The two patient categories for Group A were (i) those patients who did not undergo TTE and did not die from PE within 14 days of diagnosis, and (ii) those patients who underwent TTE and the TTE findings showed no evidence of RV strain. For Group A patients, we used the premise that “no TTE performed with no short-term death indicates no critical RV dysfunction, and thus the normal CT-RV/LV diameter ratio is sufficient”. The remaining patients were placed in Group B: (i) patients for whom TTE identified RV strain despite a normal CT-RV/LV diameter ratio, and (ii) patients who did not have TTE but died from PE within 14 days of diagnosis. For Group B patients, the CT-RV/LV diameter ratio was considered insufficient because it was either discordant with reference standard TTE or it failed to predict PE related mortality.
Analytic methods
Univariate comparisons of clinical characteristics between groups were performed using the chi-squared statistic, unpaired Student’s t-test, or the Mann–Whitney U test, as appropriate.
To identify the significant variables that classified patients into the Group B, we ran a multivariate logistic regression including potential predictors from clinical factors and CTPA findings based on a forward stepwise method (p < 0.15) and clinical significance. We then calculated the probability of being in the Group A, using the various combinations of identified predictors. Model fit and discriminatory power were assessed by Hosmer–Lemeshow test and c-statistics.
The internal validation of the model was performed with a bootstrap method (1000 resamples). The percent difference between the apparent c-statistic (i.e., computed from the main model) and the bootstrapped c-statistic was calculated as an indicator of over-fitting bias.
The following sensitivity analyses were performed. First, we used 30-day PE-related mortality instead of 14-day PE-related mortality. Second, we defined RV strain based on TTE studies performed within 48 h of CTPA, instead of TTE performed within 14 days. Third, for those patients who received multiple TTE studies during the period, the one that was closest to the CTPA study was included in the analysis. All statistical analyses were performed using STATA version 10.1 (Stata Corp., College Station, TX).
Results
Among a total of 1887 consecutive CTPA studies with acute PE in the study period, 579 patients met the study criteria and were included in the study; 236 (40.8 %) patients received TTE (Fig. 1); 17 patients received two or more TTE examinations during the 14-day period. More than two-thirds of TTE examinations were performed within 48 h of the CTPA acquisition (Fig. 2). Central embolus, atrial fibrillation, CHF, and RV diameter on CT >45 mm were significantly more prevalent in patients receiving TTE (Table 1). Anticoagulation or IVC filter placement was performed more frequently in the population undergoing TTE.
Patient classification
Four patients who did not undergo TTE died from PE. Among 236 patients who underwent TTE, 21.6 % (51/236) showed RV strain (Table 2). Therefore, a total of 524 patients were classified into the Group A and 55 patients were classified into the Group B (Fig. 1). Among the patients who underwent TTE, there were five PE-related deaths; three patients had reported RV strain on TTE, and the remaining two patients had a normal RV on TTE.
Five predictors derived from multivariate logistic regression model
The final multivariate logistic regression model included five variables (Table 3); RV diameter on CT > 45 mm, CHF, age >60 years, and central embolus were the independent predictors for being in the Group B. Stage-IV cancer was near significant (Table 3). The model had a good discrimination (c-statistic = 0.758) with the Hosmer–Lemeshow statistic of 3.15 (lack of fit p value = 0.37). The bootstrapped c-statistic was 0.739, and thus the model was internally validated with the calculated over-fitting bias of 2.52 %.
Probability of Group A
The probability of being in Group A for the five predictors is summarized in Table 4; this includes all 13 combinations of those 5 predictors with a population frequency of 1.0 % or higher. For 215 patients (37.1 % of the entire population), all five predictors were negative, i.e. 215 patients with a normal CT-RV/LV diameter ratio had none of the following: age >60 years, congestive heart failure, stage-IV cancer, RV diameter >45 mm, and central embolus. Considering these 215 patients, the probability of being in Group A was 0.97, and the 95 % confidence interval was 0.95–0.99. None of these 215 patients died from PE, and RV strain was detected only in 1.9 % of these patients. RV strain was observed in 12.9 % of those patients for whom one or more of the predictors was positive. In other words, the positive predictive value of the model for CTPA to sufficiently excluding critical RV strain was 98.1 %, with sensitivity and specificity of 40.3 and 92.7 %, respectively.
Sensitivity analyses
When PE-related 30-day mortality was used instead of 14-day mortality, one additional death (at day 21) was identified. However this patient had normal RV on TTE, and thus was classified into Group A in the main analysis as well as in the sensitivity analysis. Therefore, this analysis was identical to the main analysis.
When defining RV strain based on TTE studies performed within 48 h of CTPA, the total number of the Group B became 47. The logistic model including five predictors identified by the main analysis still had a good discriminatory power in this population (c-statistic = 0.763). If all five predictors were negative, the probability of being in the Group A was unchanged at 0.97 (95 % CI 0.96–0.99), and RV strain was detected only in 0.9 % (n = 2) of these patients.
For all 17 patients who received multiple TTE studies during the period, the study closest in time to the CTPA was the most abnormal. Therefore, this sensitivity analysis had results identical to the main analyses.
Discussion
This study focuses on defining a clinical scenario for acute PE patients for which CT data alone can be used with clinical predictors to sufficiently assess for RV strain. While many studies focus on echocardiography, there remains uncertainty regarding the prognostic value of imaging the RV in acute PE, particularly in patients that are hemodynamically stable [20]. To our knowledge, there is no prior published data that uses a relatively large cohort of patients to derive a set of conditions that form a CT based clinical scenario. We propose that for acute PE patients with a RV/LV diameter ratio <0.9, age ≤60 years, no CHF, no stage-IV cancer, RV diameter ≤45 mm, and no central embolus, RV strain is very unlikely.
Extant guidelines endorsed by multiple societies have varied recommendations, but in general support a broad use of TTE to determine RV strain among patients with acute PE. Since a very large majority of patients suspected for acute PE undergo CTPA [21], there is a significant unmet need to investigate if the extra cost and human resources to perform TTE can, in theory, be saved if a normal CT-RV/LV diameter is used as part of a simple prediction rule to identify patients with favorable outcomes. There is also a great need for additional analyses to validate this new clinical scenario, dissect other clinical scenarios so that such recommendations can be incorporated into guidelines, and ultimately into clinical decision support tools [22].
Perhaps because a the paucity of literature and the fact that many clinical prediction rules may not have the high sensitivity needed to reassure clinicians [23], TTE is often performed to evaluate the RV status after acute PE [18, 19]. Utilization patterns are in general unknown, and without clear guidelines, it is likely that the decision to perform TTE follows local practice patterns. Since acute PE patients have a high mortality, a roughly 15 % mortality rate that is in fact higher than acute myocardial infarction [24, 25], it would not be surprising that many patients undergo TTE. In our cohort, TTE was performed in 41 % of the patients, in spite of a normal CT-RV/LV diameter ratio. This high utilization rate may also be based on the previous literature reporting that RV dysfunction detected by TTE is associated with poor prognosis, even if patient hemodynamics are stable [2, 24, 26] or the blood pressure is normal [27]. There is a large potential savings in healthcare resources if TTE can be averted in patients who fit in clinical scenario defined by the current study. This is particularly true for those patients who develop acute PE as hospital inpatients in countries where reimbursement is a fixed for specific diagnoses.
The comprehensive data collection also enables additional insights regarding TTE and its performance in predicting PE-related mortality. Among the 579 consecutive patients with normal CT derived RV/LV diameter ratio, 236 underwent TTE. Analyzing the presence versus absence of TTE derived RV strain using 30-day PE related mortality as reference standard, there were 3 true positives, 48 false positives, 182 true negatives, and 3 false negatives. The negative predictive value (NPV) of TTE was thus greater than 0.98 (182/185) and the specificity was 0.79 (182/230) with a 0.95 CI of (0.73–0.84). Using the same 30-day PE related mortality data, the normal CT derived RV/LV diameter ratio had 569 true negatives and 10 false negatives with the same NPV (0.98) as TTE.
Ideally, the current study would also include specific comments regarding TTE costs. While we unfortunately do not have these data, there is a cost-savings potential if TTE can be averted in a subpopulation of patients. There is a paucity of peer-review literature on this subject [28]. The current data supports that for patients with a normal RV/LV ventricular diameter ratio on CTPA who do not meet any of the following five criteria: age >60 years (demographic), RV diameter >45 mm and central embolus (CTPA), and congestive heart failure and stage-IV cancer (comorbidities), CTPA gives sufficient prognostic information and thus TTE may not be necessary. These data strongly support that radiologists must routinely reporting of CT-based RV data for all acute PE patients. In particular, the RV size (greater than 45 mm) is clinically important because the RV/LV diameter ratio alone can be limited in patients for whom both ventricles are dilated. While this occurs in patients with CHF, the RV size can increase dramatically and rapidly after acute PE. The presence of central embolus was used as a severity measure of PE because it is routinely reported and simple for the radiologist to identify. We acknowledge other metrics of clot burden such as obstruction index [29, 30] and clot volume [31, 32]. However, computation of these metrics is cumbersome, not part of routine clinical practice, and many studies do not show a significant correlation between the obstruction index [33–37] or clot volume [32] and outcomes. Stage-IV cancer is known to be strongly related to PE-related mortality [38]. Patients in group B were generally sicker and for in this group, the RV size can increase dramatically and rapidly. Thus, it would be reasonable to consider TTE in this subpopulation, even if the initial CTPA showed normal RV/LV.
Radiologists can positively impact clinical management decisions by providing substantial input to the five criteria model for patients with a normal RV/LV diameter ratio. This rule identified a clinical scenario (that included over one-third of our total patient cohort) with an only 1.9 % likelihood of RV strain. We recognize that a 1.9 % rate of RV strain could be considered as “high”. However, definitions of “RV strain” in the current study were chosen to be conservative (i.e., we included “mild” in the TTE definition for RV dysfunction). Therefore our prediction model was based on the minimized definition of patients for whom CT-RV/LV diameter ratio gives sufficient prognostic information. It is possible that the actual rate of patients whose CT-RV/LV diameter ratio is sufficient to exclude RV strain was higher than our reported rate.
The definition of RV dilatation by TTE varies slightly in the existing literature, but “the RV appearing larger than the LV” is the most common and recommended interpretation of RV dilatation [2, 5, 39], with an upper reference limit for the RV basal dimension of 42 mm (95 % CI 39–45 mm) [5]. As there is no widely recognized standard for a normal CT-RV/LV diameter ratio [40], we used 0.9 to identify the 579 patients from the 1887 in the study period. Using a ratio of 0.9 was the most strict (i.e. the lowest) cutoff used to define the normal CT-RV/LV diameter ratio [13, 14, 41–43]. This cutoff minimized the possibility that TTE showed RV dilatation among normal CT-RV/LV because of a difference in the definition between CT and TTE. Although we used a reformatted 4-chamber image to calculate ventricular ratio, measurements on axial images are known to give comparable prognostic value [40, 43].
We acknowledge several study limitations. First, the variables considered for the prediction model were simplified. Instead of including detailed vital signs, we applied a single indicator, hemodynamic instability. Also, we did not include laboratory tests such as white blood count, brain natriuretic peptide, or troponin, and/or ECG parameters. Future work could include additional variables, although the current model with only clinical and imaging information demonstrated good discriminatory power without laboratory data that itself has intrinsic costs (even if these are relatively inexpensive). Because of limited access to vital signs, we did not compare the created model with PE severity index [11], which will be considered in future work. Although the prediction criteria was simplified, there may be additional work to determine if a patient has stage-4 cancer; the other four variables are readily available after the CTPA scan. Second, all patients were obtained from a single large teaching hospital, and as noted above, TTE utilization is likely to vary widely among institutions. Third, study patients who underwent TTE could have been a selected population for whom clinicians feared an unfavorable course, despite an apparently normal CT derived RV/LV diameter ratio. Therefore the detection rate of RV dysfunction could have been lower if all patients with CT RV/LV diameter ratio <0.9 underwent TTE. However, this biased situation does not affect the conclusion, as the goal was to predict a subpopulation for whom CT data alone can be sufficient to exclude RV strain and/or PE-death. The data argues that even in this high risk population, patients without the five predictors are unlikely to have RV dysfunction or to die from PE. Fourth, regarding the TTE interpretation, data was retrospectively obtained by reviewing the finalized reports; information on RV size, systolic function, and wall motion was provided from qualitative interpretation. Information regarding inter-reader variability was not available. However, the cardiologists performing TTE at the institution are expertly trained to use standardized language, and thus little variability is expected. Regarding CT RV assessment, we have reported good inter-reader agreement [16]. Fifth, later generation CT technologies may, in theory, be able to detect smaller filling defects; however, variables in our prediction rule (central embolus or not, RV enlargement or not) are unlikely to be significantly affected by hardware differences. Finally, prediction of earlier mortality (e.g., 5-day mortality) would have been suitable for outcome to facilitate the identification of patients who need thrombolytic therapy, but this analysis was not possible due to limited number of outcomes. Similarly, stratification of patients by the covariates/comorbidities was not possible due to a small number of outcomes.
Conclusions
A new clinical scenario identifies an acute PE subpopulation for which a normal CT-RV/LV diameter ratio plus readily obtained predictors is adequate to exclude RV/strain or PE related short term mortality. After the external validation, this clinical prediction model may be incorporated into current clinical guidelines for appropriate utilization of resources in patients with acute PE.
References
Goldhaber SZ (2004) Pulmonary embolism. Lancet 363(9417):1295–1305. doi:10.1016/S0140-6736(04)16004-2
Grifoni S, Olivotto I, Cecchini P, Pieralli F, Camaiti A, Santoro G, Conti A, Agnelli G, Berni G (2000) Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation 101(24):2817–2822
Carson JL, Kelley MA, Duff A, Weg JG, Fulkerson WJ, Palevsky HI, Schwartz JS, Thompson BT, Popovich J Jr, Hobbins TE et al (1992) The clinical course of pulmonary embolism. N Engl J Med 326(19):1240–1245. doi:10.1056/NEJM199205073261902
Tapson VF (2008) Acute pulmonary embolism. N Engl J Med 358(10):1037–1052. doi:10.1056/NEJMra072753
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23(7):685–713 (quiz 786–688). doi:10.1016/j.echo.2010.05.010
Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, Vedantham S, White RJ, Zierler BK (2011) Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 123(16):1788–1830. doi:10.1161/CIR.0b013e318214914f
Hirsh J, Guyatt G, Albers GW, Harrington R, Schunemann HJ, American College of Chest P (2008) Antithrombotic and thrombolytic therapy: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 133(6 suppl):110S–112S. doi:10.1378/chest.08-0652
Qaseem A, Alguire P, Dallas P, Feinberg LE, Fitzgerald FT, Horwitch C, Humphrey L, LeBlond R, Moyer D, Wiese JG, Weinberger S (2012) Appropriate use of screening and diagnostic tests to foster high-value, cost-conscious care. Ann Intern Med 156(2):147–149. doi:10.7326/0003-4819-156-2-201201170-00011
Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ (2012) Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest physicians evidence-based clinical practice guidelines. Chest 141(2 suppl):7S–47S. doi:10.1378/chest.1412S3
Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR (2012) Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 141(2 suppl):e419S–e494S. doi:10.1378/chest.11-2301
Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J, Roy PM, Fine MJ (2005) Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 172(8):1041–1046. doi:10.1164/rccm.200506-862OC
Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D, Management of Acute Pulmonary Embolism of the European Society of C (2014) 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 35(43):3033–3069, 3069a–3069k. doi:10.1093/eurheartj/ehu283
Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ (2004) Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation 110(20):3276–3280. doi:10.1161/01.CIR.0000147612.59751.4C
Quiroz R, Kucher N, Schoepf UJ, Kipfmueller F, Solomon SD, Costello P, Goldhaber SZ (2004) Right ventricular enlargement on chest computed tomography: prognostic role in acute pulmonary embolism. Circulation 109(20):2401–2404. doi:10.1161/01.CIR.0000129302.90476.BC
Lu MT, Cai T, Ersoy H, Whitmore AG, Quiroz R, Goldhaber SZ, Rybicki FJ (2008) Interval increase in right-left ventricular diameter ratios at CT as a predictor of 30-day mortality after acute pulmonary embolism: initial experience. Radiology 246(1):281–287. doi:10.1148/radiol.2461062004
Kumamaru KK, Hunsaker AR, Wake N, Lu MT, Signorelli J, Bedayat A, Rybicki FJ (2012) The variability in prognostic values of right ventricular-to-left ventricular diameter ratios derived from different measurement methods on computed tomography pulmonary angiography: a patient outcome study. J Thorac Imaging 27(5):331–336. doi:10.1097/RTI.0b013e3182350a35
Moreno FL, Hagan AD, Holmen JR, Pryor TA, Strickland RD, Castle CH (1984) Evaluation of size and dynamics of the inferior vena cava as an index of right-sided cardiac function. Am J Cardiol 53(4):579–585. pii:0002-9149(84)90034-1
George E, Kumamaru KK, Ghosh N, Gonzalez Quesada C, Wake N, Bedayat A, Dunne RM, Saboo SS, Khandelwal A, Hunsaker AR, Rybicki FJ, Gerhard-Herman M (2014) Computed tomography and echocardiography in patients with acute pulmonary embolism. Part 2: prognostic value. J Thorac Imaging 29(1):W7–W12. doi:10.1097/RTI.0000000000000048
Wake N, Kumamaru KK, George E, Bedayat A, Ghosh N, Gonzalez Quesada C, Rybicki FJ, Gerhard-Herman M (2014) Computed tomography and echocardiography in patients with acute pulmonary embolism. Part 1: correlation of findings of right ventricular enlargement. J Thorac Imaging 29(1):W1–W6. doi:10.1097/RTI.0000000000000047
ten Wolde M, Sohne M, Quak E, Mac Gillavry MR, Buller HR (2004) Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med 164(15):1685–1689. doi:10.1001/archinte.164.15.1685
Hunsaker AR, Lu MT, Goldhaber SZ, Rybicki FJ (2010) Imaging in acute pulmonary embolism with special clinical scenarios. Circ Cardiovasc Imaging 3(4):491–500. doi:10.1161/CIRCIMAGING.109.855981
H.R. 4302 (ENR) (2014) An act to amend the Social Security Act to Extend Medicare Payments to Physicians and Other Provisions of the Medicare and Medicaid Programs, and for other purposes. http://www.gpo.gov/fdsys/pkg/BILLS-113hr4302enr/pdf/BILLS-113hr4302enr.pdf. Accessed February 15 2015
Kohn CG, Mearns ES, Parker MW, Hernandez AV, Coleman CI (2015) Prognostic accuracy of clinical prediction rules for early post-pulmonary embolism all-cause mortality: a bivariate meta-analysis. Chest 147(4):1043–1062. doi:10.1378/chest.14-1888
Goldhaber SZ, Visani L, De Rosa M (1999) Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 353(9162):1386–1389. pii:S0140673698075345
United States. Public Health Service. Office of the Surgeon General (2008) The Surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. U.S. Public Health Service, Office of the Surgeon General, Rockville, MD
Kreit JW (2004) The impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolism. Chest 125(4):1539–1545
Kucher N, Rossi E, De Rosa M, Goldhaber SZ (2005) Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mmHg or higher. Arch Intern Med 165(15):1777–1781. doi:10.1001/archinte.165.15.1777
Cook CH, Praba AC, Beery PR, Martin LC (2002) Transthoracic echocardiography is not cost-effective in critically ill surgical patients. J Trauma 52(2):280–284
Qanadli SD, El Hajjam M, Vieillard-Baron A, Joseph T, Mesurolle B, Oliva VL, Barre O, Bruckert F, Dubourg O, Lacombe P (2001) New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol 176(6):1415–1420
Mastora I, Remy-Jardin M, Masson P, Galland E, Delannoy V, Bauchart JJ, Remy J (2003) Severity of acute pulmonary embolism: evaluation of a new spiral CT angiographic score in correlation with echocardiographic data. Eur Radiol 13(1):29–35. doi:10.1007/s00330-002-1515-y
Furlan A, Patil A, Park B, Chang CC, Roberts MS, Bae KT (2011) Accuracy and reproducibility of blood clot burden quantification with pulmonary CT angiography. AJR Am J Roentgenol 196(3):516–523. doi:10.2214/AJR.10.4603
Furlan A, Aghayev A, Chang CC, Patil A, Jeon KN, Park B, Fetzer DT, Saul M, Roberts MS, Bae KT (2012) Short-term mortality in acute pulmonary embolism: clot burden and signs of right heart dysfunction at CT pulmonary angiography. Radiology 265(1):283–293. doi:10.1148/radiol.12110802
Ghaye B, Ghuysen A, Willems V, Lambermont B, Gerard P, D’Orio V, Gevenois PA, Dondelinger RF (2006) Severe pulmonary embolism:pulmonary artery clot load scores and cardiovascular parameters as predictors of mortality. Radiology 239(3):884–891. doi:10.1148/radiol.2392050075
Ghuysen A, Ghaye B, Willems V, Lambermont B, Gerard P, Dondelinger RF, D’Orio V (2005) Computed tomographic pulmonary angiography and prognostic significance in patients with acute pulmonary embolism. Thorax 60(11):956–961. doi:10.1136/thx.2005.040873
Araoz PA, Gotway MB, Harrington JR, Harmsen WS, Mandrekar JN (2007) Pulmonary embolism: prognostic CT findings. Radiology 242(3):889–897. doi:10.1148/radiol.2423051441
Araoz PA, Gotway MB, Trowbridge RL, Bailey RA, Auerbach AD, Reddy GP, Dawn SK, Webb WR, Higgins CB (2003) Helical CT pulmonary angiography predictors of in-hospital morbidity and mortality in patients with acute pulmonary embolism. J Thorac Imaging 18(4):207–216
Pech M, Wieners G, Dul P, Fischbach F, Dudeck O, Lopez Hanninen E, Ricke J (2007) Computed tomography pulmonary embolism index for the assessment of survival in patients with pulmonary embolism. Eur Radiol 17(8):1954–1959. doi:10.1007/s00330-007-0577-2
Exter PL, Gomez V, Jimenez D, Trujillo-Santos J, Muriel A, Huisman MV, Monreal M (2012) A clinical prognostic model for the identification of low-risk patients with acute symptomatic pulmonary embolism and active cancer. Chest. doi:10.1378/chest.12-0964
Kasper W, Konstantinides S, Geibel A, Tiede N, Krause T, Just H (1997) Prognostic significance of right ventricular afterload stress detected by echocardiography in patients with clinically suspected pulmonary embolism. Heart 77(4):346–349
Kumamaru KK, Lu MT, Ghaderi Niri S, Hunsaker AR (2012) Right ventricular enlargement in acute pulmonary embolism derived from CT pulmonary angiography. Int J Cardiovasc Imaging. doi:10.1007/s10554-012-0126-1
Kamel EM, Schmidt S, Doenz F, Adler-Etechami G, Schnyder P, Qanadli SD (2008) Computed tomographic angiography in acute pulmonary embolism: do we need multiplanar reconstructions to evaluate the right ventricular dysfunction? J Comput Assist Tomogr 32(3):438–443. doi:10.1097/RCT.0b013e3180ca7818
Scheffel H, Stolzmann P, Leschka S, Desbiolles L, Seifert B, Marincek B, Alkadhi H (2012) Ventricular short-axis measurements in patients with pulmonary embolism: effect of ECG-gating on variability, accuracy, and risk prediction. Eur J Radiol 81(9):2195–2202. doi:10.1016/j.ejrad.2011.03.067
Lu MT, Demehri S, Cai T, Parast L, Hunsaker AR, Goldhaber SZ, Rybicki FJ (2012) Axial and reformatted four-chamber right ventricle-to-left ventricle diameter ratios on pulmonary CT angiography as predictors of death after acute pulmonary embolism. AJR Am J Roentgenol 198(6):1353–1360. doi:10.2214/AJR.11.7439
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors disclose no conflict of interest.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was waived by the Institutional Review Board since patient data was analyzed anonymously.
Rights and permissions
About this article
Cite this article
Kumamaru, K.K., George, E., Ghosh, N. et al. Normal ventricular diameter ratio on CT provides adequate assessment for critical right ventricular strain among patients with acute pulmonary embolism. Int J Cardiovasc Imaging 32, 1153–1161 (2016). https://doi.org/10.1007/s10554-016-0887-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-016-0887-z