Abstract
A prognostic value of right ventricular (RV) systolic function assessed by echocardiography in patients with acute non-massive pulmonary embolism (PE) remains controversial. The hypothesis was RV free wall strain measured using speckle-tracking echocardiography might be a powerful prognostic factor in those patients. We aimed to evaluate the prognostic value of echocardiographic measurements of RV systolic function for clinical outcomes and to assess the correlation between the echocardiographic RV function parameters in patients with acute non-massive PE. Between November 2013 and September 2016, 144 consecutive patients diagnosed as acute non-massive pulmonary embolism were prospectively enrolled and echocardiographic evaluations were performed within 1 week of diagnosis to measure various parameters of RV systolic function. The primary endpoint was in-hospital events, the composite of in-hospital PE-related death, need of additive treatments such as thrombolysis or pulmonary artery thromboembolectomy, and need of inotropics due to unstable vital sign. Among patients (mean age 60.3 ± 14.7 years, 50% female) with acute non-massive PE, the in-hospital event rate was 11.1% (16 of 144 patients). In multivariate logistic regression analysis, after adjustment of confounding factors such as age, gender, and diabetes mellitus, RV free wall strain [odd ratio (OR) 1.12, 95% confidence interval (CI) 1.04–1.21, p = 0.002] and RV global wall strain (OR 1.20, 95% CI 1.07–1.35, p = 0.002) were independent predictors for in-hospital events. The event rates were significantly different between groups classified based on RV free wall strain with cut-off value of − 15.85% (p < 0.001). RV strain assessed with speckle-tracking echocardiography is an independent prognostic marker for in-hospital events in patients with acute non-massive PE. Our results may help identify high–intermediate risk patients who need a closer monitoring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute pulmonary embolism (PE) is a potentially lethal disease, and right ventricular (RV) dysfunction has been known to be a poor prognostic factor in patients with PE [1,2,3], and is associated with the extent of PE [4, 5]. RV dysfunction has been reported in ≥ 20% of patients with PE [6, 7], and echocardiography is a useful tool to evaluate RV systolic dysfunction. However, PE risk stratification approaches employ several definitions and different ways to evaluate or measure RV systolic function with echocardiography or with other modalities such as computed tomography (CT) and cardiac biomarkers [3,4,5,6,7,8,9,10,11].
There are many methods for assessing RV function; however, no single measurement is accepted as a gold standard. The echocardiographic criteria for RV dysfunction include RV dilation and/or an increased end-diastolic RV to left ventricular diameter ratio, hypokinesia of the free RV wall, increased velocity of the tricuspid regurgitation (TR) jet, or combinations of these conditions [8]. However, hypokinesia of the RV free wall is assessed subjectively and qualitatively, and RV dilatation does not necessarily mean RV systolic dysfunction. Although the RV ejection fraction can be measured through three-dimensional echocardiography, it is not always possible in patients with RV enlargement. Meta-analyses have shown that RV dysfunction detected with echocardiography is associated with an elevated risk of short-term mortality in patients without hemodynamic instability; however, its overall predictive power is low [8,9,10]. This might be attributed, at least in part, to differences in the definition of RV dysfunction among studies and to the lack of use of new quantitative methods such as tissue Doppler image and speckle-tracking echocardiography.
The hypothesis of this study was that RV free wall strain measured using speckle-tracking echocardiography was a powerful prognostic factor in those patients. We aimed to evaluate the prognostic value of echocardiographic measurements of RV systolic function for clinical outcomes and to assess the correlation between the echocardiographic RV function parameters in patients with acute non-massive PE.
Methods
Patients
The study was conducted at Asan Medical Center, a high-volume tertiary centre in Korea. Between November 2013 and September 2016, consecutive 144 patients diagnosed as acute non-massive pulmonary embolism were prospectively enrolled and echocardiographic evaluations were performed to measure various parameters of RV systolic function. The inclusion criteria were as follows: (1) age ≥18 years; (2) CT diagnosis of acute PE; and (3) echocardiography evaluation was performed within 1 week, since PE was diagnosed. Patients were excluded if they (1) presented with shock or hypotension at the admission, (2) had suboptimal echocardiographic images for the assessment of RV function, and (3) had a final diagnosis other than acute PE after work-up. At screening, all patients underwent clinical evaluation, including medical history, physical examination, including PE severity index (PESI), which was calculated as previously reported [8], electrocardiography, chest radiography, echocardiography, computed tomography (CT), and laboratory tests including troponin-I and brain natriuretic peptide. This study was approved by the Institutional Review Board in Asan Medical Center (2017–0612).
Echocardiography
All echocardiographic evaluations were performed with a GE Vivid E9 machine and a 3.5-MHz probe (GE, Vingmed Ultrasound, Horten, Norway). All echo-derived parameter analyses were performed by two experienced sonographers following the American Society of Echocardiography guidelines [11], with blinding of clinical data. To quantify the RV function, we measured the RV index of myocardial performance (RIMP), two-dimensional RV fractional area change (FAC), tricuspid annular plane systolic excursion (TAPSE), longitudinal Sʹ velocity of the tricuspid lateral annulus (Sʹ velocity), pulmonary vascular resistance (PVR), and eccentricity index (Fig. 1). RIMP was calculated as the RV isovolumic time divided by the ejection time [12, 13]. PVR was noninvasively calculated using the TR velocity and RV outflow tract time–velocity integral, as previously reported [14]. The eccentricity index was calculated as the left ventricular dimension parallel to the septum divided by another dimension perpendicular to the septum measured at the mid-papillary level [11, 15,16,17].
Offline speckle-tracking analyses were performed in EchoPAC (PC version 201, Horten, Norway) by two experienced sonographers. RV free or global longitudinal wall strain was measured from the RV-focused apical 4-chamber view using speckle-tracking echocardiography, as previously described [11, 16, 18]. The endocardial border was traced at end systole and end diastole. The quality of the tracking was confirmed visually from two-dimensional images of the strain traces. RV free wall strain was measured from the lateral annulus of the tricuspid valve to the RV apex, and RV global wall strain was measured from the whole RV myocardium including the interventricular septum.
Clinical outcomes
The primary outcome was defined as the in-hospital events, the composite of in-hospital PE-related death, need of additive treatments such as thrombolysis or pulmonary artery embolectomy, and need of inotropics due to unstable vital sign after the diagnosis of acute PE. The causes of deaths were carefully reviewed by two investigators, and the deaths due to other causes, such as concurrent severe infection (suspected or confirmed sepsis), or terminal malignancy (with death expected within days) were excluded.
Statistical analysis
Continuous variables were presented as means ± standard deviation or median (range), whereas categorical variables were described as numbers and percentages. The independent t test and Pearson’s Chi-square test (or Fisher’s exact test) were used to compare two groups for continuous variables and categorical variables, respectively. The effects of a variable on clinical events were evaluated using univariate logistic regression analysis. For multivariate logistic regression analysis, confounding factors such as age, gender, and diabetes mellitus were adjusted with echocardiographic parameters. Receiver operating characteristics (ROC) curve analyses were performed to assess the optimal cut-off values of echocardiographic parameters for predicting events. Kaplan–Meier survival analysis was performed to compare the development of clinical events between groups. The correlations between parameters were analysed by Spearman’s correlation coefficients. Statistical analysis was performed with Statistical Package for Social Sciences, version 17 (SPSS Inc., Chicago, IL, USA). All tests were two-sided, and a p value of <0.05 was considered significant.
Results
Baseline characteristics and clinical outcomes
Among 200 patients, 30 were not finally diagnosed as having acute PE and 18 were excluded because of unstable vital signs at the admission. Eight patients had suboptimal echocardiographic images for offline analysis, and they were excluded from the study. Consequently, 144 patients with acute non-massive PE were prospectively enrolled.
The median age of the 144 patients was 60.3 ± 1.2 years and 50% (72 patients) of them were women. The in-hospital event rate was 11.1% (16 events; eight resulted in in-hospital PE-related death; five received thrombolysis therapy; two underwent pulmonary artery embolectomy; and one used inotropics) (Supplement Table 1). The median time to the outcomes was 10.0 (0.1–41) days. Comparisons of the baseline characteristics between patients with and without events are presented in Table 1. In-hospital event group showed trend of a higher prevalence of diabetes mellitus and lower SaO2 level. PESI score was not significantly different between the two groups. Laboratory data and discharge medication were not significantly different between the two groups. Duration of hospital stay was shorter in in-hospital event group than no event group.
Echocardiographic parameters
Comparisons of echocardiographic parameters between the two groups are presented in Table 2. RV global wall strain and RV free wall strain showed higher values and TAPSE, S′ velocity, and eccentricity index showed significantly lower values, which meant decreased RV systolic function, in the in-hospital event group compared with the ‘no event’ group, while PVR, RIMP, TR maximal velocity, and FAC did not. The magnitude of RV longitudinal strain decreased in concordant with expectations for the group with in-hospital events.
Predictors of clinical outcomes
Univariate analyses of clinical factors and echocardiographic parameters in predicting in-hospital events are shown in Table 3. On univariate logistic regression analysis, RV global wall strain, RV free wall strain, PVR, TAPSE, S' velocity, FAC, and eccentricity index were significantly associated with in-hospital events. On multivariate logistic regression analysis after adjustment with clinical confounding factors of age, gender, and diabetes mellitus in the several models, RV global wall strain, RV free wall strain, PVR, TAPSE, Sʹ velocity, FAC, and eccentricity index were independently associated with in-hospital events (Table 4).
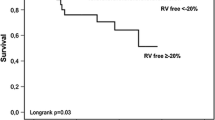
ROC curve analyses for predicting clinical events revealed that the areas under the curve (AUC) were 0.754 [95% confidence interval (CI) 0.621–0.887, p = 0.001] for RV free wall strain, 0.731 (95% CI 0.593–0.868, p = 0.003) for RV global wall strain, and 0.740 (95% CI 0.605–0.874, p = 0.002) for TAPSE (*[− 1]), while other parameters showed lower values (Fig. 2). The best cut-off values for predicting outcomes were − 15.85% for RV free wall strain (sensitivity 66.7%, specificity 79.8%, positive predictive value 27.8%, and negative predictive value 95.4%), − 18.95% RV global wall strain (sensitivity 80.0%, specificity 64.3%, positive predictive value 20.7%, and negative predictive value 96.5%) and 12.75 mm for TAPSE (sensitivity 40.0%, specificity 98.4%, positive predictive value 75.0%, and negative predictive value 93.4%). The event rates were significantly different between groups classified based on RV free wall strain with cut-off value of − 15.85% (30.5% vs. 5.7% of in-hospital events, p < 0.001, Fig. 3).
There were correlations among echocardiographic parameters of RV contractility. RV free wall strain showed significant but modest correlations with the PVR (R2 = 0.33, p < 0.0001), TAPSE (R2 = 0.29, p < 0.0001), S' velocity of the tricuspid annulus (R2 = 0.15, p < 0.0001), FAC (R2 = 0.41, p < 0.0001), and eccentricity index (R2 = 0.20, p < 0.0001), while the correlation with RIMP was not significant (Fig. 4).
Discussion
Prognostic indicators in acute PE
The rate of in-hospital events was 11.1% in our study, which is comparable to that in the previous reports in patients with non-massive PE [8]. The prognostic value of RV strain measured by speckle-tracking echocardiography in patients with acute PE is still controversial [15, 16, 19]. One study reported that four simple parameters that measure different aspects of the right ventricle (ratio of RV to left ventricular end-diastolic diameter, RV systolic pressure, TAPSE, and inferior vena cava collapsibility) were independently associated with mortality in patients presenting with acute PE; however, the RV strain values were not [19].
In contrast, other reports demonstrated the prognostic roles of RV strain [15, 16]. Vitarelli et al. demonstrated that mid-free wall RV longitudinal strain correlated with unfavourable outcomes in submassive PE [15]. Patients with submassive or intermediate-risk PE diagnosed as RV hypokinesia assessed using qualitative interpretation were included in the study. As the qualitative assessment of RV function may be subjective, the inclusion of patients may also be subjective. Therefore, we included patients with non-massive PE including patients with low risk in our study. Dahhan et al. demonstrated that the addition of echocardiographic parameters to clinical parameters may improve the risk prediction in acute PE [16]. In the current study involving a larger number of patients, we found that the RV free wall strain was the best prognostic imaging indicator in patients with acute non-massive PE.
In the current stage, the guidelines do not provide any cut-off values of quantitative parameters for RV function. The dichotomous decision about RV dysfunction would be subjective and consequently misleading for patient management, especially in patients with intermediate risk. Therefore, a more objective and quantitative value is required in this clinical situation, and the cut-off value of RV free wall strain of − 15.85%, which was the result of our study, can be useful for this purpose. As a decreased RV systolic function judged by RV free wall strain value of above − 15.85% may portend a poor short-term prognosis, we suggest that patients with non-massive PE at this high risk may warrant a more aggressive initial treatment strategy.
Quantitative assessment of RV function
Risk stratification in patients with PE is important in considering more aggressive initial treatments such as thrombolysis therapy or pulmonary artery embolectomy [20]. For risk stratification and further management, the assessment of RV function is one of the crucial factors [21]. The presence of RV systolic dysfunction likely represents a more advanced pathophysiologic stage of PE than PE without RV systolic dysfunction. Although there are many quantification methods for this purpose, RV systolic function is generally determined through a qualitative assessment in clinical practise. In this study, we found that there were significant but not strong correlations between RV free wall strain and other parameters, which might be caused by differences in methods measuring RV function. Indeed, all echocardiographic parameters have inherent limitations. The Sʹ velocity of the tricuspid annulus and TAPSE might be affected by angle dependence and tethering from apical contraction, and FAC might be influenced by the two-dimensional imaging plane. The eccentricity index is dependent not only on the RV contractility but also mainly on the difference between the RV and left ventricular pressures. Noninvasively calculated PVR is an important parameter in patients with pulmonary hypertension; however, it might not be directly linked to RV dysfunction. Finally, RIMP is not only a systolic parameter, but is also affected by diastolic dysfunction. Although the speckle-tracking image also depends on the imaging quality and temporal resolution, it measures RV myocardial systolic function without the confounding effect of angle dependency and tethering. In the current study, RV free wall strain showed significant but modest correlations with other parameters, and this result might be hypothesis generating that RV analysis of basal segments peak Sʹ velocity and TAPSE are more angle dependent and less comprehensive than RV free wall strain is. Our results on correlations make it plausible that the echocardiographic parameters of RV function are not interchangeable.
Study limitations
This current study has several limitations. First, our study investigated nonrandomized, observational data, and the overall findings should, therefore, be considered to be hypothesis-generating. The optimal patient number for this study was not calculated, because, before study, it was hard to determine groups of patients which would depend on the best predictive parameter. Consequently, this study might be underpowered. Second, the study does not provide information on which RV echocardiographic parameters obtained at the time of identifying PE that predict adverse outcomes within days after index PE, because echocardiography was performed within 1 week after the diagnosis. Third, the number of patients and events was limited. Although potential confounding factors were included in the multivariate model, additional confounding factors were not fully evaluated due to small number of clinical events. Therefore, further investigation in larger studies with a longer follow-up period is warranted. Further prospective study is also necessary to validate the clinical value of a stratified treatment strategy according to the RV systolic dysfunction judged using RV free wall strain values.
In conclusion, RV free wall strain assessed with speckle-tracking echocardiography is an independent prognostic marker for in-hospital events, such as in-hospital PE-related death, additive need of aggressive treatment, and need of inotropics due to unstable vital sign in patients with acute non-massive PE. Our results may help identify high-intermediate risk patients who need a closer monitoring.
References
Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, Vedantham S, White RJ, Zierler BK, American Heart Association Council on Cardiopulmonary CCP, Resuscitation, American Heart Association Council on Peripheral Vascular D, American Heart Association Council on Arteriosclerosis T, Vascular B (2011) Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 123:1788–1830
Goldhaber SZ, Visani L, De Rosa M (1999) Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 353:1386–1389
Kreit JW (2004) The impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolism. Chest 125:1539–1545
Chung T, Emmett L, Mansberg R, Peters M, Kritharides L (2007) Natural history of right ventricular dysfunction after acute pulmonary embolism. J Am Soc Echocardiogr 20:885–894
Hariharan P, Dudzinski DM, Rosovsky R, Haddad F, MacMahon P, Parry B, Chang Y, Kabrhel C (2016) Relation among clot burden, right-sided heart strain, and adverse events after acute pulmonary embolism. Am J Cardiol 118:1568–1573
Kucher N, Rossi E, De Rosa M, Goldhaber SZ (2005) Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm Hg or higher. Arch Intern Med 165:1777–1781
Grifoni S, Olivotto I, Cecchini P, Pieralli F, Camaiti A, Santoro G, Conti A, Agnelli G, Berni G (2000) Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation 101:2817–2822
Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M, Task Force for the D, Management of Acute Pulmonary Embolism of the European Society of C (2014) 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 35:3033-3069 (3069a–3069k)
Coutance G, Cauderlier E, Ehtisham J, Hamon M, Hamon M (2011) The prognostic value of markers of right ventricular dysfunction in pulmonary embolism: a meta-analysis. Crit Care 15:R103
Sanchez O, Trinquart L, Colombet I, Durieux P, Huisman MV, Chatellier G, Meyer G (2008) Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J 29:1569–1577
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23:685–713 (quiz 786–788)
Tei C, Dujardin KS, Hodge DO, Bailey KR, McGoon MD, Tajik AJ, Seward SB (1996) Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr 9:838–847
Topilsky Y, Khanna AD, Oh JK, Nishimura RA, Enriquez-Sarano M, Jeon YB, Sundt TM, Schaff HV, Park SJ (2011) Preoperative factors associated with adverse outcome after tricuspid valve replacement. Circulation 123:1929–1939
Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ (2003) A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol 41:1021–1027
Vitarelli A, Barilla F, Capotosto L, D'Angeli I, Truscelli G, De Maio M, Ashurov R (2014) Right ventricular function in acute pulmonary embolism: a combined assessment by three-dimensional and speckle-tracking echocardiography. J Am Soc Echocardiogr 27:329–338
Dahhan T, Siddiqui I, Tapson VF, Velazquez EJ, Sun S, Davenport CA, Samad Z, Rajagopal S (2016) Clinical and echocardiographic predictors of mortality in acute pulmonary embolism. Cardiovasc Ultrasound 14:44
Haddad F, Guihaire J, Skhiri M, Denault AY, Mercier O, Al-Halabi S, Vrtovec B, Fadel E, Zamanian RT, Schnittger I (2014) Septal curvature is marker of hemodynamic, anatomical, and electromechanical ventricular interdependence in patients with pulmonary arterial hypertension. Echocardiography 31:699–707
Risum N, Jons C, Olsen NT, Fritz-Hansen T, Bruun NE, Hojgaard MV, Valeur N, Kronborg MB, Kisslo J, Sogaard P (2012) Simple regional strain pattern analysis to predict response to cardiac resynchronization therapy: rationale, initial results, and advantages. Am Heart J 163:697–704
Khemasuwan D, Yingchoncharoen T, Tunsupon P, Kusunose K, Moghekar A, Klein A, Tonelli AR (2015) Right ventricular echocardiographic parameters are associated with mortality after acute pulmonary embolism. J Am Soc Echocardiogr 28:355–362
Wan S, Quinlan DJ, Agnelli G, Eikelboom JW (2004) Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation 110:744–749
Grifoni S, Vanni S, Magazzini S, Olivotto I, Conti A, Zanobetti M, Polidori G, Pieralli F, Peiman N, Becattini C, Agnelli G (2006) Association of persistent right ventricular dysfunction at hospital discharge after acute pulmonary embolism with recurrent thromboembolic events. Arch Intern Med 166:2151–2156
Funding
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF-2015R1A2A2A03003552) and a Grant (2015-7009) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest or financial disclosures to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, K., Kwon, O., Lee, EJ. et al. Prognostic value of echocardiographic parameters for right ventricular function in patients with acute non-massive pulmonary embolism. Heart Vessels 34, 1187–1195 (2019). https://doi.org/10.1007/s00380-019-01340-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-019-01340-1