Abstract
To investigate the impact of microvascular dysfunction assessed by angiography on myocardial deformation assessed by two-dimensional speckle-tracking echocardiography in ST-segment elevation myocardial infarction (STEMI). A total of 121 STEMI patients who received primary percutaneous coronary intervention were included. Thrombolysis in myocardial infarction, Myocardial Perfusion Frame Count (TMPFC), a novel angiographic method to assess myocardial perfusion, was used to evaluate microvascular dysfunction. Two-dimensional speckle-tracking echocardiography was performed at 3–7 days after reperfusion. The infarction related regional longitudinal (RLS) strains as well as circumferential (RCS) and radial (RRS) ones, along with global longitudinal, circumferential (GCS), and radial (GRS) strains were measured. Patients with microvascular dysfunction had decreased peak amplitude of RLS (p = 0.012), RCS (p < 0.001), RRS (p = 0.012) at the regional level and decreased peak amplitude of GCS (p = 0.005), GRS (p = 0.012) at the global level. The RCS to RLS and RCS to RRS ratios were significantly different between patients without than with microvascular dysfunction (1.28 ± 0.31 vs. 1.07 ± 0.47, p = 0.027 and 0.69 ± 0.33 vs. 0.56 ± 0.28, p = 0.047). Receiver operator characteristics curves identified a cutoff value of 94 frames for TMPFC to differentiate between normal and abnormal wall motion score index in the sub-acute phase of STEMI (AUC = 0.72; p < 0.001). In the sub-acute phase of STEMI, the presence of microvascular dysfunction in infarcted tissue relates to reduced global and regional myocardial deformation. RCS alterations were more significant than RLS and RRS between patients with than without microvascular dysfunction. TMPFC was useful to predict left ventricular systolic dysfunction in the sub-acute phase of STEMI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microvascular dysfunction (MVD) is a common complication after reperfusion therapy in patients with ST-segment elevation myocardial infarction (STEMI), which has been confirmed to be associated with increased post-procedural myocardial infarction occurrence, in-hospital mortality, adverse left ventricular (LV) remodeling and diminished recovery of LV function [1–5]. Traditional angiographic methods for assessment of myocardial tissue-level perfusion were thrombolysis in myocardial infarction (TIMI) myocardial perfusion grading (TMPG) and myocardial blush grading (MBG), which were limited by their subjective and categorical nature [6, 7]. In a previous study, we developed a novel and objective method named TIMI Myocardial Perfusion Frame Count (TMPFC), which measured the filling and clearance of contrast in the myocardium using cine-angiographic frame-counting to quantify myocardial tissue-level perfusion [5]. As a quantitative index for the assessment of myocardial perfusion, TMPFC proved to be a useful predictor of clinical prognosis [5, 8, 9]. Recent studies have revealed that myocardial deformation assessed using speckle-tracking echocardiography (STE) predicts LV remodeling and has additional prognostic value in acute myocardial infarction relative to traditional two dimensional (2D) echocardiography [10, 11]. However, the relationship between angiographically defined MVD and STE assessed by LV regional and global myocardial deformation remains unclear in the sub-acute phase of STEMI.
The objective of the present study was to investigate the relationship between angiographically defined MVD using TMPFC and myocardial deformation assessed by STE in the sub-acute phase of STEMI after reperfusion therapy.
Methods
Study population
Between October 2012 and January 2014, a total of 323 STEMI patients receiving reperfusion therapy were admitted to our center. STEMI was defined as chest pain of >30 min duration, and electrocardiographic (ECG) changes with ST-segment elevation of >2 mm in at least 2 precordial and >1 mm in the limb leads, and abnormal troponin levels or CK-MB at least twice the upper limit of normal [12]. After excluding 76 patients with onset-to-PCI time >12 h, and 77 patients treated with pharmacoinvasive strategy, the remaining 170 patients received primary percutaneous coronary intervention (pPCI) strategy. We further excluded 37 patients aged ≥75 years, and 22 patients with insufficient echocardiographic or angiography image quality. Finally, 121 STEMI patients aged <75 years treated with pPCI were included in the present analysis (Fig. 1). All patients were treated with standard therapeutic regimes according to guidelines. Informed consent was provided by each study subject and the study protocol was approved by the Institutional Review Board on Human Research.
Coronary angiography
Coronary angiography was performed using Seldinger technique via radial or femoral artery. To allow coronary angiographic assessment, a standard angiography was acquired immediately after the procedure (either coronary intervention or solo angiography) for each patient. The final angiographic imaging after PCI was used to assess infarction related regional myocardial perfusion.
Angiogram was obtained through a 6F guiding-catheter after intracoronary injection of 200 µg nitroglycerin to avoid coronary spasm and to produce maximal or near maximal coronary dilation without detriment to systemic hemodynamics [13].Contrast agent was manually injected (Iopamidol, 5–6 mL), and the sign of backflow into the aorta was mandatory as a proof of adequate contrast filling of the epicardial coronary artery. Projections were carefully chosen to minimize superimposing of non-infarcted myocardium; therefore, right anterior oblique view with essential caudal angulations was routinely used for left culprit artery (left anterior descending artery, LAD or left circumflex artery, LCX) and posterior–anterior oblique view with steep cranial angulations was selected for right coronary artery (RCA). Angiographic run was made long enough, i.e., until complete washout of myocardial blush in infarction zone or reaching a maximum of 10 s. Angiographic data were analyzed offline with a computer-based cardiovascular angiographic analysis system by two independent, experienced angiographers. Epicardial coronary flow was assessed by TIMI flow grade: grade 0, absent antegrade flow; grade 1, partial contrast penetration, incomplete distal filling; grade 2, patent with opacification of the entire distal artery, delayed contrast filling or washout; and grade 3, patent with normal flow [14]. Myocardial microvascular perfusion was assessed by TMPFC [5]. TMPFC is a simple quantitative index for assessing myocardial microvascular perfusion which was derived by modifying TMPG by the method of frame counting. The first frame of TMPFC was defined as that which clearly demonstrated the first appearance of myocardial blush beyond the infarction related artery (F1). The last frame of TMPFC was defined as that when contrast or myocardial blush disappeared (F2). TMPFC is the difference between F2 and F1 at filming rate of 30 frames/s (Fig. 2). In the present study, six patients showed persistence of myocardial blush in the distribution of the culprit artery, suggesting leakage of contrast medium into the extravascular space secondary to destruction of the microcirculation network structure—the most severe form of microvascular dysfunction [6, 7]. As described previously and following a similar principle to that used in the definition of corrected TIMI frame count (CTFC) [15], a frame count of 306, corresponding to the 99th percentile of TMPFC encountered in the present study, was assigned to these patients [5].
Example of TMPFC measurement. a Occlusion of right coronary artery (arrow). b First TMPFC frame (frame 24/134, F1): the frame that clearly demonstrated the first appearance of myocardial blush. c Myocardial blush peaked in frame 39/134. d Last TMPFC frame (frame 91/134, F2): the frame in which myocardial blush disappeared. TMPFC was therefore F2–F1 = 91–24 = 67 frame counts at a filming rate of 15 frames/s, and 134 (67 × 2) frames at the corrected filming rate of 30 frames/s
In our cardiac magnetic resonance (CMR) study, TMPFC is a more powerful tool for MVD prediction than TMPG and MBG, the value of 95 frames was the optimal cutoff point to predict MVD by receiver operator characteristics curve analysis [16]. Therefore, in the present study TMPFC > 95 frames was defined as angiographic MVD.
Echocardiography
Echocardiography was performed 3–7 days after reperfusion, with a commercially available system (Vivid E9, GE Vingmed Ultrasound, Horten, Norway). Conventional parasternal short axis views at basal, middle and apical levels as well as apical 4-chamber, 2-chamber and 3-chamber views were obtained. All the images were obtained at a frame rate of 60–90 frames/s. Three consecutive cardiac cycles in sinus rhythm were digitally stored for subsequent analysis during breath-hold. Traditional 2D-echocardiography parameters were measured by standard methods [17].
By qualitative visual assessment, we divided LV into 18 segments, each attributed a score reflecting: normal (1), hypokinesia (2), akinesia (3), dyskinesia (4), or aneurismal (5). Wall-motion score index (WMSI), an index of global wall motion abnormality, was calculated as a sum of all scores divided by 18 [18].
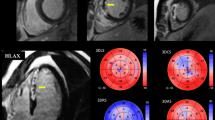
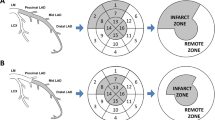
For 2D-strain analysis, an 18-segment LV model was obtained from apical four-, three-, and two-chamber views, and parasternal short axis views at basal, middle and apical levels by dedicated automated software (EchoPAC, Version 112; GE Healthcare). Peak systolic strain was defined as the peak positive or peak negative strain value during systole (Fig. 3). Global longitudinal strain (GLS), global circumferential strain (GCS), and global radial strain (GRS) values were calculated by averaging all segmental peak systolic strain values in an 18-segment model. Regional longitudinal strain (RLS), regional circumferential strain (RCS), and regional radial strain (RRS) were respectively calculated based on the perfusion territories of the 3 major coronary arteries in a 16-segment LV model [18] by averaging all segmental peak systolic strain values within each territory. Because the Echo PAC software produced strain values for 18 segments, we used 18 segments for RLS, RCS and RRS, by dividing the apical septal and inferior segments into two segments.
Statistical analysis
Summary statistics of continuous data with symmetric distribution are expressed as mean ± standard deviation (SD), while categorical data are expressed as counts and/or percentages. Comparisons between the groups were made using the independent samples t test. Categorical variables were compared using χ2 or Fisher’s exact tests. The correlation between each LV strain parameter and TMPFC was analyzed by Pearson’s correlation test. Receiver operator characteristics (ROC) curve analysis was performed on TMPFC to test whether it could differentiate between normal and abnormal WMSI. To obtain inter- and intra-observer reproducibility for TMPFC and strain parameters, two independent observers experienced in interpreting TMPFC evaluated the raw data from 20 randomly selected cases in a blinded fashion, and another two independent observers analyzed 2D strain, respectively. The reliability of the measurements (for both inter-observer and intra-observer variability) was evaluated by their reproducibility (intra-class coefficient of correlation, ICC), with values >0.8 considered excellent.
Statistical tests were considered significant at p < 0.05. Statistical analyses were performed using SPSS version 17.0 (SPSS, Inc., Chicago, IL) [19]. ROC curve analysis was done using MedCalc version 9.6.4.0 (MedCalc Software, Mariakerke, Belgium).
Results
Baseline characteristics
According to myocardial perfusion assessed by TMPFC, patients were divided into non-MVD group (TMPFC ≤ 95frames) and MVD group (TMPFC > 95 frames). In our CMR study, TMPFC = 95 frames was the optimal cutoff point to predict MVD by ROC analysis [16]. The baseline characteristics of patients between two groups are shown in Table 1. There was no difference between non-MVD and MVD groups in age, gender, heart rate, systolic and diastolic blood pressure, history of hypertension, hypercholesterolemia, infarction related artery (IRA), IRA lesion location and multi-vessel disease. MVD group had a higher proportion of diabetes patients, significantly longer pain-to-balloon time, higher peak CK-MB level and a lower proportion of post-PCI TIMI3 grade patients.
Echocardiographic findings and two-dimensional strains
Patients in MVD group had higher WMSI (1.5 ± 0.3 vs. 1.3 ± 0.3, p < 0.001) and lower left ventricular ejection fraction (LVEF; 50.9 ± 8.9 vs. 56.4 ± 8.9 %, p = 0.002) compared to patients in non-MVD group (Table 2). In 2D-strain analysis, patients in MVD group had decreased peak amplitude of RLS (−11.0 ± 5.6 vs. −13.7 ± 5.0 %, p = 0.012), RCS (−11.8 ± 5.7 vs. −16.2 ± 5.6 %, p < 0.001), RRS (23.5 ± 11.3 vs. 30.3 ± 16.7 %, p = 0.012) at the regional level and decreased peak amplitude of GCS (−14.2 ± 4.5 vs. −16.8 ± 4.2 %, p = 0.005), GRS (27.5 ± 9.9 vs. 33.7 ± 15.7 %, p = 0.012) at the global level, compared to patients in non-MVD group. However, GLS was not significantly different between two groups (−13.4 ± 4.9 vs. −14.9 ± 4.2 %, p = 0.116; Table 2).
Relationship between MVD and infarction related regional strain and global strain
A ratio of circumferential to longitudinal strain and circumferential to radial strain was calculated and related to myocardial reperfusion levels by TMPFC (Table 3). The RCS to RLS ratio was significantly different between patients without MVD and patients with MVD (1.28 ± 0.31 vs. 1.07 ± 0.47, p = 0.027). Because the peak amplitudes of RCS and RLS were both decreased in patients with MVD, the different RCS to RLS ratios can be explained by a more significant alteration of RCS than RLS between patients without than with MVD. The different RCS to RRS ratios also demonstrated that RCS alterations were more significant than RRS between patients without and with MVD (0.69 ± 0.33 vs. 0.56 ± 0.28, p = 0.047).
Correlation of TMPFC with two-dimensional strains, WMSI and LVEF
As shown in Fig. 4, the RLS (r = −0.24, p = 0.011), RCS (r = −0.40, p < 0.001), RRS (r = −0.35, p < 0.001), GCS (r = −0.38, p < 0.001) and GRS (r = −0.34, p < 0.001) were significantly correlated with the TMPFC. TMPFC was positively correlated with WMSI (r = 0.36, p < 0.001, Fig. 5a), and negatively correlated with LVEF(r = −0.31, p = 0.001, Fig. 5b).
Prognostic value of TMPFC on abnormal LV wall motion
To evaluate the ability of TMPFC to differentiate between normal and abnormal WMSI,receiver operator characteristics(ROC) curve analysis was performed. Normal WMSI (WMSI = 1) was obtained in 13 patients in sub-acute phase of STEMI in the present study. ROC curves identified a cutoff value of 94 frames for TMPFC to differentiate between normal and abnormal WMSI in sub-acute phase of STEMI, with sensitivity of 69 % and specificity of 69 % (area under the curve, AUC = 0.72; p < 0.001; Fig. 6).
Inter- and intra-observer variability
Excellent inter-observer and intra-observer agreements for TMPFC were achieved with the corresponding intraclass coefficients (ICC) as follows: Inter-observer ICC = 0.97 (95 % CI 0.93–0.99) and intra-observer ICC = 0.98 (95 % CI 0.96–0.99).
RLS, RCS, RRS were sufficiently reproducible to be integrated in the reliability analysis. The inter-observer and intra-observer ICC for RLS were 0.92 (95 % CI 0.82–0.97) and 0.95 (95 % CI 0.88–0.98), respectively, while those for RCS were 0.93 (95 % CI 0.84–0.97) and 0.98 (95 % CI 0.96–0.99), and those for RRS were 0.97 (95 % CI 0.92–0.99) and 0.99 (95 % CI 0.97–0.99), respectively.
Discussion
The key conclusions of this study can be formulated as follows: (1) the presence of MVD in infarcted tissue relates to reduced global and regional myocardial deformation; (2) RCS alterations were more significant than RLS and RRS between patients with than without MVD; and (3) TMPFC was a useful method to predict LV systolic dysfunction in sub-acute phase of STEMI after PCI.
Despite improvement in reperfusion strategies, patients who experience MVD following STEMI are known to exhibit a higher prevalence of early post-infarction complications, adverse left ventricular remodeling, recurrent hospitalizations, heart failure and mortality [3–5, 20]. In the present study, restoration of myocardial perfusion was assessed angiographically by TMPFC, which measures the filling and clearance of contrast in the myocardium using cine-angiographic frame-counting to quantify myocardial tissue-level perfusion. TMPFC allows quantification of TMPG, and can accurately predict the outcomes in STEMI patients undergoing reperfusion therapy [5, 8, 9]. In our CMR study, TMPFC is a more powerful tool for MVD prediction than TMPG and MBG; the value of 95.5 frames was the optimal cutoff point to predict MVD [13] and we therefore selected TMPFC > 95 frames to define angiographic MVD. In the present study, MVD group had a higher proportion of diabetes patients, significantly longer pain to balloon time, higher peak CK-MB level and fewer post-PCI TIMI3 grade patients, which was consistent with previous studies [5–8].
2D strain imaging is a new technique that uses standard B-mode images for speckle tracking. Strain analysis has also been shown to be a useful tool in evaluating regional ventricular function, infarct size, myocardial viability, and recovery after medical or mechanical reperfusion, as well as the subtle changes related to myocardial ischemia in patients with acute myocardial infarction (AMI) [21–24]. However, most of these studies focused on longitudinal strain (LS) [10, 11, 21, 23, 24]. In this study, we analyzed both three infarction related regional strains and three global strains, and compared WMSI, LVEF and each strain, which were LV systolic function parameters, with early myocardial perfusion. The results of the present study showed that patients in MVD group had higher WMSI, lower LVEF, lower peak amplitude of infarction related regional strains and global strains in sub- acute phase of STEMI. These six strain parameters correlated with TMPFC. The latter finding confirmed the concept that myocardial perfusion level was an important factor that influences post-infarction LV function. However, there was not an established cutoff value of TMPFC that could differentiate between normal and abnormal myocardial contraction. In the present study, there were 13 patients with normal visual WMSI (WMSI = 1). We proposed an optimal threshold value of 94 frames for TMPFC to predict LV systolic dysfunction (WMSI > 1) in sub-acute phase of STEMI, which is close to the value of 95.5 frames, the optimal cutoff point to predict MVD in our CMR study [16]. The threshold might help cardiologists to evaluate early on which patients will suffer LV systolic dysfunction and choose optimal therapy strategy.
We identified that RCS alterations were more significant than RLS in patients with MVD in the present study. Myocardial fibers are mostly oriented longitudinally in subendocardial layers, circumferentially in mid-layers, and radially in subepicardial layers [25]. The myocardial necrosis in AMI progresses from subendocardial layers to subepicardial layers. Subendocardial layers are more susceptible to ischemia [26]. In the early phase, LS was mostly responsible for the segmental dysfunction, but after its partial recovery, in the sub-acute phase, the function of stunned myocardium in mid and subepicardial layers recovered earlier than subendocardial layers which have more unviable myocardium [27]. Segments with functional recovery demonstrated similar LS but higher circumferential strain (CS) compared with non-improving segments [25], which might explain by the fact that patients in non-MVD group had more functional recovery segments than those in MVD group, resulting in more significant CS than LS alterations. A recent study by Chan et al. [28] confirmed the utility of assessing myocardial deformation heterogeneity in the detection of ischemia. RS was reduced in transmural infarct segments and relatively preserved in subendocardial infarct segments, CS was decreased in transmural as compared with subendocardial infarcts and normal myocardium, whereas LS was reduced in all infarcts irrespective of their extent. Our results suggest that function of stunned myocardium in mid-layers recovered earlier than that in endocardial layers in the early healing phase of acute myocardial infarction.
Study limitations
Several limitations of the current analysis deserve to be mentioned. First, because of the relatively small patient sample, only surrogate but not clinical endpoints were analyzed. Second, as a novel echocardiographic modality for quantitative evaluation of myocardial motion and contractility, the normal values for STE-derived strain have not been defined yet, and there are no established cut-off values that could differentiate between normal and abnormal myocardial contraction. Thus, we could not work out the cut-off for TMPFC based on a ROC analysis with strain parameters. Third, cardiac output and systemic vascular resistance which might influence microvascular perfusion were not available from our study database, and we will further investigate these potential influencing factors in a larger population in the future.
Conclusions
In the sub-acute phase of STEMI, the presence of MVD in infarcted tissue relates to reduced global and regional myocardial deformation. TMPFC may be useful to predict LV systolic dysfunction in the sub-acute phase of STEMI. RCS alterations were more significant than RLS and RRS between patients with or without MVD, which might indicate that the function of stunned myocardium in mid-layers recovered earlier than that in endocardial layers in the early healing phase of acute myocardial infarction.
References
Yang Y, Sun Y, Yi W et al (2014) A review of melatonin as a suitable antioxidant against myocardial ischemia-reperfusion injury and clinical heart diseases. J Pineal Res 57:357–366
Huang B, Wang X, Yang Y et al (2015) Association of admission glycaemia with high grade atrioventricular block in ST-segment elevation myocardial infarction undergoing reperfusion therapy: an observational study. Medicine (Baltimore) 94:e1167
Pu J, Shan P, Ding S et al (2011) Gender differences in epicardial and tissue-level reperfusion in patients undergoing primary angioplasty for acute myocardial infarction. Atherosclerosis 215:203–208
Ding S, Li Z, Ge H et al (2015) Impact of early ST-segment changes on cardiac magnetic resonance-verified intramyocardial haemorrhage and microvascular obstruction in ST-elevation myocardial infarction patients. Medicine (Baltimore) 94:e1438
Ding S, Pu J, Qiao ZQ et al (2010) TIMI myocardial perfusion frame count: a new method to assess myocardial perfusion and its predictive value for short-term prognosis. Catheter Cardiovasc Interv 75:722–732
Gibson CM, Cannon CP, Murphy SA et al (2000) Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation 101:125–130
Van’t Hof AW, Liem A, Suryapranata H et al (1998) Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade: Zwolle Myocardial Infarction Study Group. Circulation 97:2302–2306
Pu J, Shan PR, Ding S et al (2011) Factors affecting thrombolysis in myocardial infarction myocardial perfusion frame count: insights of myocardial tissue-level reperfusion from a novel index for assessing myocardial perfusion. Chin Med J (Engl) 124:873–878
Pu J, Ding S, Shan P et al (2010) Comparison of epicardial and myocardial perfusions after primary coronary angioplasty for ST-elevation myocardial infarction in patients under and over 75 years of age. Aging Clin Exp Res 22:295–302
Vitarelli A, Martino F, Capotosto L et al (2014) Early myocardial deformation changes in hypercholesterolemic and obese children and adolescents: a 2D and 3D speckle tracking echocardiography study. Medicine (Baltimore) 93:e71
Antoni ML, Mollema SA, Delgado V et al (2010) Prognostic importance of strain and strain rate after acute myocardial infarction. Eur Heart J 31:1640–1647
Thygesen K, Alpert JS, White HD et al (2007) Universal definition of myocardial infarction. Circulation 116:2634–2653
Conti CR, Feldman RL, Pepine CJ et al (1983) Effect of glyceryl trinitrate on coronary and systemic hemodynamics in man. Am J Med 74:28–32
Simes RJ, Topol EJ, Holmes DR Jr et al (1995) Link between the angiographic substudy and mortality outcomes in a larger and randomized trial of myocardial reperfusion. Importance of early and complete infarct artery reperfusion. GUSTO-I investigators. Circulation 91:1923–1928
Gibson CM, Murphy SA, Rizzo MJ et al (1999) Relationship between TIMI frame count and clinical outcomes after thrombolytic administration. Thrombolysis in Myocardial Infarction (TIMI) Study Group. Circulation 99:1945–1950
Ge H, Ding S, An D et al (2015) Frame counting improves the assessment of post-reperfusion microvascular patency by TIMI myocardial perfusion grade: evidence from cardiac magnetic resonance imaging. Int J Cardiol 203:360–366
Cheitlin MD, Alpert JS, Armstrong WF et al (1997) ACC/AHA guidelines for the clinical application of echocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Clinical Application of Echocardiography). Developed in collaboration with the American Society of Echocardiography. Circulation 95:1686–1744
Lang RM, Bierig M, Devereux RB et al (2005) Chamber Quantification Writing Group, American Society of Echocardiography’s Guidelines and Standards Committee, European Association of Echocardiography: recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18:1440–1463
Jia EZ, Chen ZH, An FH et al (2014) Relationship of renin-angiotensin-aldosterone system polymorphisms and phenotypes to mortality in Chinese coronary atherosclerosis patients. Sci Rep 4:4600
Li J, Ma T, Mohar D et al (2015) Ultrafast optical-ultrasonic system and miniaturized catheter for imaging and characterizing atherosclerotic plaques in vivo. Sci Rep 5:18406
Xu TY, Sun JP, Lee AP et al (2015) Left atrial function as assessed by speckle-tracking echocardiography in hypertension. Medicine (Baltimore) 94:e526
Pirat B, Khoury DS, Hartley CJ et al (2008) A novel feature-tracking echocardiographic method for the quantitation of regional myocardial function: validation in an animal model of ischemia-reperfusion. J Am Coll Cardiol 51:651–659
Munk K, Andersen NH, Nielsen SS et al (2011) Global longitudinal strain by speckle tracking for infarct size estimation. Eur J Echocardiogr 12:156–165
Mistry N, Beitnes JO, Halvorsen S et al (2011) Assessment of left ventricular function in ST-elevation myocardial infarction by global longitudinal strain: a comparison with ejection fraction, infarct size, and wall motion score index measured by non-invasive imaging modalities. Eur J Echocardiogr 12:678–683
Jones CJ, Raposo L, Gibson DG (1990) Functional importance of the long axis dynamics of the human left ventricle. Br Heart J 63:215–220
Greenbaum RA, Ho SY, Gibson DG, Becker AE, Anderson RH (1981) Left ventricular fibre architecture in man. Br Heart J 45:248–263
Carasso S, Agmon Y, Roguin A et al (2013) Left ventricular function and functional recovery early and late after myocardial infarction: a prospective pilot study comparing two-dimensional strain, conventional echocardiography, and radionuclide myocardial perfusion imaging. J Am Soc Echocardiogr 26:1235–1244
Chan J, Hanekom L, Wong C et al (2006) Differentiation of subendocardial and transmural infarction using two-dimensional strain rate imaging to assess short-axis and long-axis myocardial function. J Am Coll Cardiol 48:2026–2033
Acknowledgments
This work was supported by National Natural Science Foundation of China (81270282, 81330006 and 81400261), Shanghai Leading Talents Program (LJ10007), Program for New Century Excellent Talents in University from Ministry of Education of China (NCET-12-0352), Shanghai Shuguang Program (12SG22), Program of Shanghai Committee of Science and Technology (15411963600 and 14JC1404500), Shanghai Emerging Technology Program (SHDC12013119), Shanghai Municipal Education Commission Gaofeng Clinical Medicine Grant Support (20152209), Shanghai Jiaotong University (YG2013MS420) and Shanghai Jiaotong University School of Medicine (15ZH1003 and 14XJ10019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no potential conflict of interest.
Rights and permissions
About this article
Cite this article
He, B., Ding, S., Qiao, Z. et al. Influence of microvascular dysfunction on regional myocardial deformation post-acute myocardial infarction: insights from a novel angiographic index for assessing myocardial tissue-level reperfusion. Int J Cardiovasc Imaging 32, 711–719 (2016). https://doi.org/10.1007/s10554-015-0834-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-015-0834-4