Abstract
Evaluating mechanical valves with computed tomography (CT) can be problematic because artifacts from the metallic components of valves can hamper image quality. The purpose of this study was to determine factors affecting the image quality of cardiac CT to improve assessment of mechanical aortic valves. A total of 144 patients who underwent aortic valve replacement with mechanical valves (ten different types) and who underwent cardiac CT were included. Using a four-point grading system, the image quality of the CT scans was assessed for visibility of the valve leaflets and the subvalvular regions. Data regarding the type of mechanical valve, tube voltage, average heart rate (HR), and HR variability during CT scanning were compared between the non-diagnostic (overall image quality score ≤2) and diagnostic (overall image quality score >2) image quality groups. Logistic regression analyses were performed to identify predictors of non-diagnostic image quality. The percentage of valve types that incorporated a cobalt-chrome component (two types in total) and HR variability were significantly higher in the non-diagnostic image group than in the diagnostic group (P < 0.001 and P = 0.013, respectively). The average HR and tube voltage were not significantly different between the two groups (P > 0.05). Valve type was the only independent predictor of non-diagnostic quality. The CT image quality for patients with mechanical aortic valves differed significantly depending on the type of mechanical valve used and on the degree of HR variability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Traditionally, transthoracic echocardiography (TTE) has been the standard method for assessing and establishing valve function after mechanical valve replacement. However, mechanical valve assessment by TTE has its limitations, such as suboptimal visualization of valve motion due to acoustic shadowing and wide variation in transprosthetic pressure gradients (PGs). Increased transprosthetic PG may be caused by left ventricular outflow tract (LVOT) obstruction, measurement error, a high flow state, or pressure recovery [1]. Even though cine fluoroscopy is used to evaluate valve leaflet motion, obtaining a perpendicular image of the valve leaflet with fluoroscopy and accurately measuring the opening angle can be difficult.
Recently, cardiac computed tomography (CT) has been shown to be valuable for evaluating mechanical valves, particularly in regard to evaluation of leaflet motion, measurement of opening and closing angles [2, 3], and identifying the causes of prosthetic valve obstruction (e.g., pannus, thrombus, or vegetation) [4, 5]. However, CT evaluation of the mechanical valve can be difficult because artifacts from the metallic components of valves can hamper image quality, especially in valves with cobalt-chrome components [2, 4–7]. However, there have been no assessments of the relevant factors affecting CT image quality of mechanical aortic valves in a large population.
The purpose of this study was to identify factors affecting the image quality of cardiac CT scans to determine their utility in mechanical aortic valve assessment.
Methods
Patients
The Institutional Review Board of Severance Hospital approved this retrospective study and informed consent was waived. We retrospectively searched our database for cardiac CT examinations performed between March 2010 and August 2014. Among 22,824 consecutive patients who underwent CT during this period, we included 181 patients who underwent aortic valve replacement (AVR) with mechanical valves before receiving their CT scans. Thirty-seven patients without multiphase CT data were excluded from this study. A total of 144 patients were included in the final analysis. Demographic data and information on mechanical valves were collected from electronic medical records. The clinical conditions for which patients underwent cardiac CT included suspected coronary artery disease (n = 77), suspected prosthetic aortic valve dysfunction on TTE (n = 40), suspected dysfunction of the mitral or tricuspid valve on TTE (n = 11), postoperative evaluation after AVR (n = 7), pulmonary vein evaluation before radiofrequency ablation (n = 6), and evaluation of coronary bypass graft (n = 3).
Image acquisition
All CT scans were performed with a dual-source CT scanner (SOMATOM Definition Flash; Siemens Healthcare, Erlangen, Germany). In the absence of contraindications, patients with a heart rate (HR) >65 beats/min (bpm) received a 50 mg dose of an oral beta-blocker (metoprolol tartrate) 1 h before examination and were administered a 0.3 mg sublingual dose of nitroglycerin just before the scan. Scans were performed using a retrospective electrocardiogram (ECG)-gated data acquisition mode. We did not use ECG-based tube current modulation to allow for images of the mechanical valve during the entire cardiac cycle; for each patient, the appropriate time interval between contrast agent injection and initiation of scanning was determined by the timed bolus technique. After a bolus injection of 10 mL of iopamidol (Pamiray: 370 mg of iodine/mL; DongKook Pharmaceutical, Seoul, Korea) followed by 20 mL of saline at 5 mL/s, optimal delay times were determined by automatic evaluation of the contrast enhancement in the ascending aorta. All CT scans were performed using the triple-phase injection method (70 mL of iopamidol followed by 30 mL of 30 % blended iopamidol with saline and 20 mL of saline at 5 mL/s). From March 2010 to July 2011, the tube potential and tube-current–time product were designated according to a BMI-based protocol described in previous studies [8, 9]. From August 2011, the automatic tube potential selection with tube current modulation was conducted using the CARE kV™ software (Siemens Healthcare) and simultaneous application of CARE Dose4D (Siemens Healthcare) [8, 9].
From the raw data sets, images were generated using filtered back projection. Image reconstruction was performed with a medium kernel (b36f), and a reconstruction slice thickness of 0.75 and 0.5 mm increments. For all patients, ten transverse datasets were reconstructed at every 10 % increase of the cardiac cycle (from 0 to 90 % of the RR interval at 10 % increments). Reconstructed images were transferred to an image server and analyzed using dedicated three-dimensional software (Aquarius iNtuition, version 4.4.11; TeraRecon, Foster City, CA, USA).
Image analyses
All CT analyses were performed by two observers who were blinded to all clinical information. To resolve disagreements over image analysis, a final assessment was reached through a consensus reading. Mechanical valves were evaluated using multiplanar reformatted images in cine mode. To visualize the mechanical valves, the default window for the vertebrae was selected with additional adjustment to the window level and width to minimize blooming, at the discretion of the reader. For CT analysis, three common views were created: (1) A short axis image of the mechanical aortic valve was created in a direction similar to that of the surgeon’s view. (2) A long-axis view of the LVOT was created parallel to the axis of the mechanical valve leaflets. (3) The coronal section view was created perpendicular to the left ventricular long-axis view.
The image quality of the scans for mechanical aortic valve evaluation was assessed for the subvalvular and valvular regions using a four-point scale: 1 = poor visualization, 2 = fair visualization, 3 = good visualization, and 4 = excellent visualization, as described in a previous study [7]. The criteria for different scores by region were also formulated. For the subvalvular region: 1 = no discernible subvalvular details widely beyond the prosthesis; 2 = no or limited details within 5 mm of the prosthesis; 3 = adequate details within 5 mm of the prosthesis; 4 = perfect details. For the valvular region: 1 = presence of severe metal artifacts and the leaflet is hardly visible; 2 = presence of moderate artifacts and the leaflet is identifiable in some of the reconstructed phases but opening and closing angle measurements were not obtained reliably; 3 = presence of mild artifacts and images allow for a view of opening and closing angle measurements but they are partially blurred; 4 = presence of minimal artifacts and visualization of valvular angles were excellent in both diastole and systole, allowing for reliable measurements. The overall image quality score was determined as the mean score of the valvular and subvalvular regions. We divided patients into two groups according to their overall image quality score: non-diagnostic overall image quality (score ≤2) or diagnostic overall image quality (score >2). We decided on >2 as the cutoff for diagnostic quality because either the measured value of opening and closing angles or the presence of pannus could not be accurately evaluated in cases with a mean score ≤2.
For those patients with images of diagnostic quality, the phase that showed the least number of artifacts among the ten cardiac cycles (0–90 %) was recorded for both the systole (opening phases of valve) and the diastole (closing phases of valve). The best phases for observing the coronary artery were separately recorded.
Statistical analyses
Statistical analyses were performed using MedCalc for Windows, version 12.7.0.0 (MedCalc Software, Ostend, Belgium). Normally distributed data were identified using the Shapiro–Wilk W-test. Continuous variables are presented as the mean ± standard deviation and were compared using the independent t test for normally distributed data or a Mann–Whitney U test for non-normally distributed data. For categorical variables, we used the Chi square test or Fisher’s exact test. Intra- and inter-observer variability for image-quality assessment was evaluated using kappa statistics. The type of mechanical valve, tube voltage for CT (80, 100, 120, and 140 kV), average HR, HR variability during CT scanning, and presence of other valve replacement or repair (e.g., mitral valve or tricuspid valve) were compared between the non-diagnostic and diagnostic image quality groups. Thus, HR variability refers to the maximal HR spread during CT scanning. Logistic regression analyses were performed to identify the factors that predict non-diagnostic image quality. Significant factors from univariate analyses were analyzed for independence in multivariate analyses. P values less than 0.05 were considered statistically significant.
Results
Our final study population included 144 patients whose clinical and CT data are summarized in Table 1. A total of ten different types of mechanical valves were included (CarboMedics = 28, St. Jude Medical Regent = 26, St. Jude Medical = 23, ATS open pivot = 15, Duromedics = 13, On-X = 12, Sorin = 11, MIRA = 10, Björk-Shiley = 4, Medtronic Hall = 2).
Image quality analyses
The median image quality scores were 4.0 for valvular regions and 3.0 for subvalvular regions (interquartile range for both: 3.0–4.0; Table 2). The overall median image quality score was 3.5 (interquartile range 3.0–4.0). The weighted kappa values for intra-observer variability were 0.919 for the subvalvular region (standard error = 0.023) and 0.933 for the valvular region (standard error = 0.028), indicating excellent agreement. The weighted kappa values for the image quality scores from both observers were 0.905 for the subvalvular region (standard error = 0.026) and 0.913 for the valvular region (standard error = 0.034). Twenty-one patients (14.6 %) with image-quality scores ≤2 were placed in the non-diagnostic group.
Patients who received Duromedics or Björk-Shiley valves represented a larger proportion of the non-diagnostic quality group (15/21, 71.4 %) than the diagnostic quality group (2/123, 1.6 %, P < 0.0001; Table 3; Fig. 1). The HR variability was significantly higher in the non-diagnostic quality group than in the diagnostic quality group (median 34 vs. 6 bpm, P = 0.0125; Fig. 2). Average HR, tube voltage, and the presence of other valve replacement or repair were not significantly different between the two groups (P > 0.05). Multivariate logistic regression analysis showed that valve type was the only independent predictor of non-diagnostic image quality (Table 4, P < 0.0001, odds ratio = 165, 95 % confidence interval 26.3–1037).
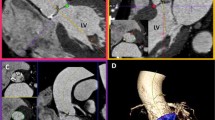
Examples of CT images showing the image quality of 10 different types of mechanical aortic valves. Long axis and short axis view CT images show valvular and subvalvular regions of ten different types of mechanical aortic valves. CT images in DM (subvalvular region) and BS (valvular and subvalvular regions) valves are of non-diagnostic quality. BS Björk-Shiley, CM CarboMedics, CT computed tomography, DM Duromedics, MH Medtronic-hall, SJR St. Jude Medical Regent, SJM St. Jude Medical
Examples of CT images showing different image quality according to HR variability in St. Jude Medical Regent valves. Long axis (top and middle) view of CT images shows valvular regions (for valve opening and closing), and short axis view of images (bottom) shows subvalvular regions of St. Jude Medical Regent aortic valves with different HR variabilities. bpm beats per minute, CT computed tomography, HR heart rate
Among the 123 diagnostic-quality images, the best diastolic phases were: 0 % of the cardiac cycle, n = 5 cases (4.1 %); 70 %, n = 15 cases (12.2 %); 80 %, n = 39 cases (31.7 %); and 90 %, n = 64 cases (52.0 %; Fig. 3). The best systolic phases were: 10 % of the cardiac cycle, n = 10 cases (8.1 %); 20 %, n = 51 cases (41.5 %); 30 %, n = 52 cases (42.3 %), and 40 %, n = 10 cases (8.1 %). The best phase for the coronary artery was the same as the best systolic phase in 13 cases (10.6 %) or the diastolic phase in 18 cases (14.6 %). In 92 cases (74.8 %), the best phase for the coronary artery was not the same as either the best systolic or diastolic phase.
Discussion
Our study shows that specific valve types—Duromedics and Björk-Shiley—and HR variability during CT scanning were observed significantly more frequently in the non-diagnostic image quality group than in the diagnostic image quality group. Tube voltage, average HR, and the presence of other valve replacement or repair were not significantly different between the two groups. Having had valve replacement with either a Duromedics or Björk-Shiley valve was the only independent predictor of non-diagnostic image quality.
Previous studies concluded that valve types with cobalt–chrome components were more likely to degrade CT image quality [2, 4–7]. In our study, all images of patients with Duromedics or Björk-Shiley valves, except for two, were of non-diagnostic quality, which is a finding that is consistent with those of previous studies. Although those studies used high tube voltage (mostly 120 kV, but 140 kV in a few studies) for their CT imaging to avoid metal-related artifacts, the image quality in our study was not hampered by our use of low-tube voltage. Rhythm irregularity (i.e., high HR variability) was significantly correlated with non-diagnostic image quality in our study, although it was not an independent predictor in multivariate logistic analyses.
The presence of artifacts on CT images depends on valve materials, motion, and image noise. Scatter, photon starvation, edge effects, beam hardening, and motion may be responsible for these artifacts [10, 11]. The degree of artifacts seems to be related to the radio-opaque parts, such as the metal alloy of the prosthetic ring and the tungsten impregnated carbon leaflets. Some valve types with cobalt-chrome components (e.g., Duromedics, Björk-Shiley, and Allocarbon) have been reported to cause severe metal-related artifacts [7, 12]. This is in line with our finding that the strongest determining factor of image quality in our study was the type of mechanical aortic valve implanted. Most of the Duromedics and Björk-Shiley valves included in our study received an image-quality score of 1 or 2, especially in the subvalvular region than in the valvular region. To evaluate the formation of subprosthetic pannus from CT, visibility of not only the valve leaflet, but also of the subvalvular region, is important. The current most commonly used mechanical valves consist of titanium and carbon rather than of a cobalt-chrome alloy. Therefore, the limited utility of CT scanning for cobalt-chrome valve types is not likely to be a serious limitation for future evaluations.
In addition to the metallic components of mechanical aortic valves, motion artifacts caused by a fast HR or arrhythmia can decrease image quality [7], because leaflet motion and annular motion along with heart movements may enhance artifacts [13, 14]. It may be important to clarify the effects of arrhythmia on image quality, because aggressively lowering HR with beta blockers during scanning is contraindicated in some patients with prosthetic valves, as they may have poor left ventricular function and/or conduction abnormalities. In our study, 47.9 % of patients (69 of 144 patients) had high HR variability greater than 19 bpm during CT scanning, and 23.2 % of them had non-diagnostic image quality scores. However, previous studies have not validated the effect of HR variability on image quality [7]. This might be because those studies had only small sample sizes.
Although a fast HR and arrhythmia can degrade image quality, the type of mechanical aortic valve was the only independent predictor of non-diagnostic image quality in our study. A possible reason is that, among some patients who have a fast HR, motion artifacts may have been reduced by CT data reconstruction during the end systolic phase [14]. Although image quality in patients with irregular HRs may not have always improved with CT data that were reconstructed for every 10 % increase of the cardiac cycle, image reconstruction or acquisition using the absolute delay strategy may be helpful in reducing artifacts related to cardiac motion [15].
Usually, cardiac CT is acquired and reconstructed for coronary artery evaluation, and prospective ECG gating during middiastole (60–70 % of the RR interval) or end-systole (30–50 %) is widely used [16, 17]. For the mechanical valve, retrospective ECG gating without ECG-based tube-current modulation is largely used to evaluate the mechanical valve during the entire cardiac cycle, despite the increased radiation dose. A previous study reported the usefulness of prospective gating over retrospective gating to reduce radiation dosage, while also noting the advantages this techniques had for improving image quality. However, in our study, the best phase was 20–30 % of the RR interval (83.7 %) for the prosthetic valve opening and 80–90 % of the RR interval (82.7 %) for the valve closing, and none of the two best phases were equal to the best phase for the coronary artery in 74.8 % of the included cases. This result was consistent with the result from a previous in vitro study, which reported that leaflet motion affects valve-related artifacts and that good image quality can be expected when the leaflet is closed (during 80–90 or 0 % of the RR interval) and opened (30–50 % of the RR interval) [13]. Therefore, retrospective ECG gating without ECG-based tube current modulation is regarded as most useful for CT evaluation of prosthetic valves by covering the best phases for both the prosthetic valve and the coronary artery, because the optimal phases for the coronary artery and the prosthetic valve are usually different. To overcome radiation-dose increases by retrospective gating, other dose-reduction techniques, such as iterative reconstruction or low-tube potential selection, are more beneficial than prospective gating or ECG-based tube current modulation.
Previous studies regarding prosthetic valve imaging mostly used high-tube voltage (120 or 140 kV) to reduce image noise and then to avoid metal-related artifacts [2–5, 18]. However, the tube voltage setting in our study did not affect image quality. This result suggests that, although low-tube voltage may increase image noise, the degree of increased noise was not critical enough to have an impact on overall image quality. Instead, valve type or an irregular HR seems to determine overall image quality. Besides tube voltage, image noise in prosthetic valve CT imaging may be caused by interpolation in the retrospective ECG-gated helical scan mode [19–21]. One study found that prospective-triggered axial scanning reduced artifacts in prosthetic valve CT imaging, compared to retrospective gating, because less image noise in the axial scan was achieved by avoiding multisegmental reconstructions and helical interpolation of helical scans [14]. Although the helical scan was used in our study, we used dual-source CT, which has better temporal resolution to reduce motion artifacts than does single-source CT, and the majority of our cases (123 of 144 cases, 85.4 %) had diagnostic quality images. Although the data from the cases in our study were reconstructed with filtered-back projection, the use of iterative reconstruction can be strategic for improving image quality in terms of reducing image noise [22, 23]. We suggest that the added value of iterative reconstruction to image quality could be investigated in a future clinical study.
Our study has several limitations. First, it is a nonrandomized retrospective study, and all data were obtained from a single institution. However, to avoid bias in patient selection, analyses of CT images were conducted blindly without any clinical information. Second, the potential hazards associated with radiation exposure from CT scanning can be a limitation. In particular, radiation dose can be a concern in terms of retrospective gating without tube-current modulation, regardless of the indications from the CT scan. The mean dose-length product from CT scans in our study was 701.5 ± 340.1 mGy cm, which was more than two or three times the dose of prospective gating in our institution [8, 9]. To minimize the increase in radiation dose by retrospective gating, we have used a dose reduction technique with an automatic tube potential selection with tube current modulation software for most of our CT examinations.
In conclusion, the CT image quality for patients with mechanical aortic valves was significantly different depending on the type of mechanical valve and HR variability during CT scanning. Valve type was the only independent predictor; thus, we conclude that evaluation of Duromedics and Björk-Shiley valves is not feasible with CT scanning due to severe metal-related artifacts, but cardiac CTs can be used to evaluate the majority of commercially available mechanical aortic valves.
References
Bach DS (2010) Echo/Doppler evaluation of hemodynamics after aortic valve replacement: principles of interrogation and evaluation of high gradients. JACC Cardiovasc Imaging 3(3):296–304
Konen E, Goitein O, Feinberg MS, Eshet Y, Raanani E, Rimon U, Di-Segni E (2008) The role of ECG-gated MDCT in the evaluation of aortic and mitral mechanical valves: initial experience. AJR Am J Roentgenol 191(1):26–31
LaBounty TM, Agarwal PP, Chughtai A, Bach DS, Wizauer E, Kazerooni EA (2009) Evaluation of mechanical heart valve size and function with ECG-gated 64-MDCT. AJR Am J Roentgenol 193(5):W389–W396
Tsai IC, Lin YK, Chang Y, Fu YC, Wang CC, Hsieh SR, Wei HJ, Tsai HW, Jan SL, Wang KY, Chen MC, Chen CC (2009) Correctness of multi-detector-row computed tomography for diagnosing mechanical prosthetic heart valve disorders using operative findings as a gold standard. Eur Radiol 19(4):857–867
Symersky P, Budde RP, de Mol BA, Prokop M (2009) Comparison of multidetector-row computed tomography to echocardiography and fluoroscopy for evaluation of patients with mechanical prosthetic valve obstruction. Am J Cardiol 104(8):1128–1134
Habets J, Mali WP, Budde RP (2012) Multidetector CT angiography in evaluation of prosthetic heart valve dysfunction. Radiographics 32(7):1893–1905
Habets J, Symersky P, van Herwerden LA, de Mol BA, Spijkerboer AM, Mali WP, Budde RP (2011) Prosthetic heart valve assessment with multidetector-row CT: imaging characteristics of 91 valves in 83 patients. Eur Radiol 21(7):1390–1396
Park YJ, Kim YJ, Lee JW, Kim HY, Hong YJ, Lee HJ, Hur J, Nam JE, Choi BW (2012) Automatic tube potential selection with tube current modulation (APSCM) in coronary CT angiography: comparison of image quality and radiation dose with conventional body mass index-based protocol. J Cardiovasc Comput Tomogr 6(3):184–190
Suh YJ, Kim YJ, Hong SR, Hong YJ, Lee HJ, Hur J, Choi BW (2013) Combined use of automatic tube potential selection with tube current modulation and iterative reconstruction technique in coronary CT angiography. Radiology 269(3):722–729
van der Schaaf I, van Leeuwen M, Vlassenbroek A, Velthuis B (2006) Minimizing clip artifacts in multi CT angiography of clipped patients. AJNR Am J Neuroradiol 27(1):60–66
Boas FE, Fleischmann D (2011) Evaluation of two iterative techniques for reducing metal artifacts in computed tomography. Radiology 259(3):894–902
Symersky P, Budde RP, Prokop M, de Mol BA (2011) Multidetector-row computed tomography imaging characteristics of mechanical prosthetic valves. J Heart Valve Dis 20(2):216–222
Symersky P, Budde RP, Westers P, de Mol BA, Prokop M (2011) Multidetector CT imaging of mechanical prosthetic heart valves: quantification of artifacts with a pulsatile in vitro model. Eur Radiol 21(10):2103–2110
Symersky P, Habets J, Westers P, de Mol BA, Prokop M, Budde RP (2012) Prospective ECG triggering reduces prosthetic heart valve-induced artefacts compared with retrospective ECG gating on 256-slice CT. Eur Radiol 22(6):1271–1277
Rist C, Johnson TR, Muller-Starck J, Arnoldi E, Saam T, Becker A, Leber AW, Wintersperger BJ, Becker CR, Reiser MF, Nikolaou K (2009) Noninvasive coronary angiography using dual-source computed tomography in patients with atrial fibrillation. Invest Radiol 44(3):159–167
Sun Z, Ng KH (2012) Prospective versus retrospective ECG-gated multislice CT coronary angiography: a systematic review of radiation dose and diagnostic accuracy. Eur J Radiol 81(2):e94–e100
Kim HY, Lee JW, Hong YJ, Lee HJ, Hur J, Nam JE, Choi BW, Kim YJ (2012) Dual-source coronary CT angiography in patients with high heart rates using a prospectively ECG-triggered axial mode at end-systole. Int J Cardiovasc Imaging 28(Suppl 2):101–107
Chenot F, Montant P, Goffinet C, Pasquet A, Vancraeynest D, Coche E, Vanoverschelde JL, Gerber BL (2010) Evaluation of anatomic valve opening and leaflet morphology in aortic valve bioprosthesis by using multidetector CT: comparison with transthoracic echocardiography. Radiology 255(2):377–385
Horiguchi J, Fujioka C, Kiguchi M, Yamamoto H, Kitagawa T, Kohno S, Ito K (2009) Prospective ECG-triggered axial CT at 140-kV tube voltage improves coronary in-stent restenosis visibility at a lower radiation dose compared with conventional retrospective ECG-gated helical CT. Eur Radiol 19(10):2363–2372
Wilting JE, Timmer J (1999) Artefacts in spiral-CT images and their relation to pitch and subject morphology. Eur Radiol 9(2):316–322
Yang WJ, Pan ZL, Zhang H, Pang LF, Guo Y, Chen KM (2011) Evaluation of coronary artery in-stent restenosis with prospectively ECG-triggered axial CT angiography versus retrospective technique: a phantom study. Radiol Med 116(2):189–196
Habets J, Symersky P, Leiner T, de Mol BA, Mali WP, Budde RP (2012) Artifact reduction strategies for prosthetic heart valve CT imaging. Int J Cardiovasc Imaging 28(8):2099–2108
Sucha D, Willemink MJ, de Jong PA, Schilham AM, Leiner T, Symersky P, Budde RP (2014) The impact of a new model-based iterative reconstruction algorithm on prosthetic heart valve related artifacts at reduced radiation dose MDCT. Int J Cardiovasc Imaging 30(4):785–793
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there are no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was waived by approval of the Institutional Review Board of Severance Hospital.
Rights and permissions
About this article
Cite this article
Suh, Y.J., Kim, Y.J., Hong, Y.J. et al. Factors affecting computed tomography image quality for assessment of mechanical aortic valves. Int J Cardiovasc Imaging 32 (Suppl 1), 63–71 (2016). https://doi.org/10.1007/s10554-015-0817-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-015-0817-5