Abstract
Assessment of the left atrial appendage (LAA) for thrombus and anatomy is important prior to atrial fibrillation (AF) ablation and LAA exclusion. The use of cardiovascular CT (CCT) to detect LAA thrombus has been limited by the high incidence of pseudothrombus on single-pass studies. We evaluated the diagnostic accuracy of a two-phase protocol incorporating a limited low-dose delayed contrast-enhanced examination of the LAA, compared with a single-pass study for LAA morphological assessment, and transesophageal echocardiography (TEE) for the exclusion of thrombus. Consecutive patients (n = 122) undergoing left atrial interventions for AF were assessed. All had a two-phase CCT protocol (first-past scan plus a limited, 60-s delayed scan of the LAA) and TEE. Sensitivity, specificity, diagnostic accuracy, positive (PPV) and negative predictive values (NPV) were calculated for the detection of true thrombus on first-pass and delayed scans, using TEE as the gold standard. Overall, 20/122 (16.4 %) patients had filling defects on the first-pass study. All affected the full delineation of the LAA morphology; 17/20 (85 %) were confirmed as pseudo-filling defects. Three (15 %) were seen on late-pass and confirmed as true thrombi on TEE; a significant improvement in diagnostic performance relative to a single-pass scan (McNemar Chi-square 17, p < 0.001). The sensitivity, specificity, diagnostic accuracy, PPV and NPV was 100, 85.7, 86.1, 15.0 and 100 % respectively for first-pass scans, and 100 % for all parameters for the delayed scans. The median (range) additional radiation dose for the delayed scan was 0.4 (0.2–0.6) mSv. A low-dose delayed scan significantly improves the identification of true LAA anatomy and thrombus in patients undergoing LA intervention.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The advent of percutaneous AF ablation and subsequently left atrial appendage (LAA) closure procedures has led to an exponential growth in left atrial (LA) interventions [1, 2]. In patients with non-valvular AF, 90 % of the thrombi responsible for stroke are thought to originate in the LAA [3, 4] and all LA interventions are contraindicated in the presence of clot due to the high risk of subsequent thromboembolic events [5].
LAA exclusion is at least as effective, if not better than, warfarin in reducing AF sequelae such as stroke, death and mitral valve intervention [1]. Such procedures are undertaken in isolation or at the same time as ablation procedures to treat the underlying AF, and as with the latter, require the exclusion of thrombus prior to intervention, as well as accurate delineation of LAA anatomy for device selection [6, 7].
Currently, Doppler transesophageal echocardiography (TEE) is considered the gold standard technique for the detection of LAA thrombus [8–11]. However, TEE, when used purely to exclude thrombus at the time of AF ablation, is a time consuming, semi-invasive test which requires conscious patient sedation and special operator skills [12].
Prior to LAA exclusion or AF ablation, cardiovascular CT (CCT) images may be used to assess the LA, LAA, pulmonary veins and adjacent structures [2, 13]. Additionally, CCT may detect LAA thrombus which is demonstrated as a filling defect [2]. It can provide valuable information for guiding device selection, assessing early procedural success, and determining medium-to-long term outcomes [2, 14]. It is often performed prior to AF ablation to assess the number, location and size of the pulmonary veins, as well as the size and morphology of the LA, including any additional LAAs or diverticulae [15]. This data is often fused with electrophysiological maps using various electro-anatomic mapping systems, such as the Cartomerge™ Image Integration Module [16, 17].
It is well known that the absence of a LAA filling defect on CCT allows confident exclusion of thrombus with a sensitivity and negative predictive value of 100 %, thus obviating the need for further TEE assessment at the time of intervention [18, 19]. However, the converse is not true; apparent filling defects on CCT do not always correspond to thrombus but may represent ‘pseudo-thrombus’ due to circulatory stasis [20]. The addition of a delayed acquisition is known to reduce false positive rates and is a reliable alternative to TEE for the detection of LA/LAA thrombus, avoiding the discomfort and risks associated with TEE [21]. However, the high resultant doses of these dual-phase acquisitions have, to date, limited their use in routine clinical practice.
Poor delineation of the distal LAA may also potentially lead to problems when considering patients for LAA exclusion, both when determining the size of the device and the dimensions of the landing site for internally deployed devices, but equally for determining a patient’s suitability for an epicardial LAA closure procedure using the Lariat suture device (SentreHEART, California, USA) [22]. No previous studies have looked at the effect of pseudo-filling defects on the accurate visualisation of the LAA anatomy prior to LAA exclusion and the potential improvements using delayed image acquisitions.
The aim of this study was to assess the diagnostic performance relative to TEE and the additional radiation burden of a low-dose, dual-phase CCT protocol, incorporating a limited 60-s delayed scan (to allow equilibration of contrast and the blood pool), in an attempt to more accurately determine LAA anatomy and exclude true LAA thrombus.
Materials and methods
Patient selection
Consecutive patients with refractory AF referred for left atrial intervention underwent a clinically indicated TEE and a CCT for exclusion of LAA thrombus and for anatomical evaluation of the left atrium, pulmonary veins and LAA. All patients had a dual-phase contrast CCT protocol comprising a standard and a 60-s delayed scan.
This clinical data is usually integrated with the Cartomerge electrophysiology mapping system (Carto, Biosense Webster, California, USA) prior to AF ablation procedures. For patients undergoing LAA exclusion, the CT data is also used to assist in determining the most appropriate exclusion device (Watchman, Amplatz Cardiac Plug (ACP), Lariat etc.) and approach.
Informed consent was not required by our institutional review board as the dual-phase protocol introduced into routine clinical practice was deemed to be a service improvement that would improve diagnostic accuracy in clinically indicated routine CT studies with minimal increase in radiation burden. This was based on evidence from previously published data from similar studies in patients with previous stroke [23]. The study cohort consists of consecutive cases retrospectively reviewed after the change in practice.
CT image acquisition and interpretation
All patients were scanned using a Siemens Somatom Definition Flash Dual Source CT (Siemens, Forchheim, Germany). A prospective ECG-gating technique was used with full tube current applied at 70 % of R–R for stable heart rates ≤60 beats per minute, or at 60–80 % of R–R for unstable heart rates ≤60 beats per minute, or at 30–70 % of R–R for heart rates of 61–70 beats per minute. Exposure parameters were adjusted according to patient size. Scans were performed at 80 kV and 400 quality referenced mAs [for patients with a body mass index (BMI) < 20 kg/m2], 100 kV (300 mAs) or 120 kV (200 mAs) (for BMI > 30 kg/m2). Images were reconstructed using filtered back-projection at 0.75 mm slice width, 0.5 mm slice increment.
The median time between the CT and the TEE investigation was 2 days (IQR 1–3 days). Whole heart scanning was routinely performed, as the gross coronary anatomy is often deemed helpful to the referring clinician. A total of 90 ml of contrast (Ultravist 370, Bayer Healthcare, Whippany, New Jersey, USA) was administered at 6 ml/s. The standard scan was performed using a test bolus technique for scan timing. The delayed scan was performed 60 s following the beginning of the standard scan to allow contrast equilibration within the blood pool. The delayed scan was planned at the level of the carina and extended 4–8 cm caudally to include the LAA but not the whole heart, to minimise unnecessary radiation exposure.
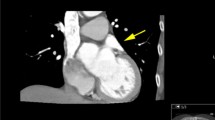
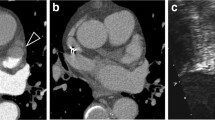
All CCT data sets were reviewed by two Society for Cardiovascular Computed Tomography level III accredited radiologists, blinded to patients’ cardiac rhythm and TEE data. Disagreements between observers were resolved by consensus. The LAA was qualitatively assessed for filling defects on both standard and delayed images. The LAA was categorized as having either no filling defect, a pseudo-filling defect (a filling defect seen on standard images but disappearing on delayed images) (Fig. 1) or a persistent filling defect (seen both on standard and delayed images) (Fig. 2). LAA morphology was classified into chicken-wing, windsock, cactus and cauliflower types using definitions previously described (Fig. 3) [2].
TEE image acquisition and interpretation
TEE was performed as part of the patient’s work up prior to LA intervention using a Philips iE33 echocardiography system and Omni III 5 MHz probe. The LAA was assessed for thrombus at the mid-oesophageal level using 0, 45, 90 and 135 degree views; spectral Doppler was used to quantify blood flow. Multiple standard images were recorded on digital video in real time for display and off-line evaluation. Of note, neither spontaneous echo contrast, nor low LAA velocity alone met the criteria for determination of thrombus on TEE. A diagnosis of LAA thrombus was made only after image review and agreement between the echo physician performing the TEE and a senior echo physician, blinded to CCT and other clinical data.
Statistical analysis
Categorical variables are expressed as frequencies and percentages, continuous variables as mean ± standard deviation or median with interquartile range (IQR). Categorical data were compared using Fisher’s exact or Chi-square tests as appropriate. Where relevant, 95 % confidence intervals for proportions were calculated using the continuity-corrected efficient-score method [24]. Paired comparisons of the performance of the single-phase to the delayed-phase scan were performed using McNemar’s test. Two-tailed values of p < 0.05 were considered statistically significant. All data were analysed using MedCalc Version 12.3.0 (Medcalc, Mariakerke, Belgium).
Results
A total of 122 consecutive studies were assessed (96 male, median age 60 years, range 32–88 years); 65 (53.2 %) patients were in AF at the time of the CCT scan. In all cases, image quality was technically adequate for clinical assessment.
The delayed-pass scan significantly improved diagnosis of thrombus relative to the single-pass study (McNemar Chi-square: 17, p < 0.001) (Table 1). Filling defects were detected in 20/122 (16.4 %) patients on the first-pass study. Of these, 17/20 (85 %) were confirmed as pseudo-filling defects and did not allow full delineation of the true LAA morphology (Fig. 4). The remaining 3/20 (15 %, or 2.5 % of total cohort) were also seen on late-pass imaging and confirmed as true thrombi on TEE. The sensitivity, specificity, diagnostic accuracy, PPV and NPV of CCT detection of true thrombus were 100, 85.7, 86.1, 15.0 and 100 % respectively for first-pass scans and 100 % for all parameters for the delayed scans. With regard to the latter parameters, the 95 % confidence intervals for sensitivity, specificity, diagnostic accuracy, positive and negative predictive values were: 31.0–100, 96.1–100, 96.1–100, 31.0–100, and 96.1–100 % respectively. In total, 16/17 (94 %) pseudo-filling defects were seen in patients who were in AF at the time of the CT scan versus 1 (6 %) in sinus rhythm (p < 0.01).
Left atrial pseudo-filling defect (a first-pass, e late-pass) causing a potentially erroneous assessment of LAA dimensions (b vs. f). Having calculated a landing zone (10–20 mm) for a Watchman device (c), measurements in the plane of implantation are significantly smaller in the first pass study (d) compared with the delayed scan (g)
The distributions of LAA morphologies for patients with and without filling defects on first pass imaging were significantly different (p < 0.001) and are summarised in Table 2. There was higher preponderance of windsock and cauliflower morphologies amongst those with filling defects and a lower prevalence of the chicken-wing and cactus conformations.
The median radiation dose (range) for the dual-phase protocol was 3.5 mSv (2.1–5.2 mSv), with a dose-length product (DLP) of 251 mGy cm (150–374). The median radiation dose (range) for the delayed scan only was 0.4 mSv (0.2–0.6 mSv) or DLP 32 mGy cm (19–45), which corresponds to an 11.4 % increase in radiation dose when compared to the standard scan alone.
Discussion
Our study demonstrates that in patients with a history of AF undergoing CCT for LA assessment before atrial intervention, a 60-s, low-dose, delayed CT acquisition, limited to the LAA, is able to more accurately delineate the LAA anatomy when compared with a single pass approach, and accurately excludes LAA thrombus when compared to the gold standard of TEE. The addition of this limited additional scan results in only a small additional radiation dose, and no additional contrast is required.
This study reaffirms that true LAA thrombus is a relatively uncommon finding just prior to LA intervention (2.5 %), as the majority of these patients are anticoagulated. However, false positives are not uncommon (13.9 %), particularly in patients who are in AF at the time of the study. It confirms the high diagnostic accuracy, sensitivity and negative predictive value of CCT. Several previous studies have demonstrated that standard single-phase CCT has a high sensitivity and negative predictive value (93–100 and 99–100 % respectively) for LAA thrombus detection, but the technique has historically been limited by a low specificity and positive predictive value (67–98 and 15–93 % respectively) [18, 19, 25–31], due to pseudo-filling defects.
It should be noted that in our study, pseudo-thrombus was much more common in patients who were in AF at the time of the scan. This is likely to be due to circulatory stasis and subsequent poor mixing of blood and contrast in the LAA [32]. LAA flow is known to be particularly poor in patients in AF and explains the predominance of this finding in those in AF at the time of CT acquisition [32]. This concurs with previously published data in stroke patients that also demonstrated that pseudo-thrombus is more common in AF patients undergoing CT angiography compared with patients in sinus rhythm [20]. We speculate that LAA pseudothrombus, analogous to low LAA flows on TEE, may be a harbinger of increased thromboembolic risk. There was a lower prevalence of the chicken-wing morphology amongst those with LAA pseudothrombus, in keeping with the findings of Di Biase et al. [33], who found this morphology to be associated with a lower incidence of stroke. There was a correspondingly higher prevalence of windsock and cauliflower types amongst those with pseudothrombus. These findings and their potential utility for thrombosis risk stratification in patients with AF require further study.
Previous studies [34, 35] have also demonstrated low radiation doses of 0.5–1.25 mSv for single-phase, but not delayed, prospectively-gated scans in stroke patients. Although the meta-analysis of Romero et al. [21] suggests that CCT with delayed imaging is a reliable alternative to TEE for the detection of LA/LAA thrombus as pseudo-filling defects disappear on the delayed scans, the associated radiation burden with older protocols has, to date, limited its value and uptake in clinical practice. No assessment of radiation dose was made in the large meta-analysis referenced above, however, the authors comment in their discussion that one of the major disadvantages of CCT was the high radiation burden required (9–15 mSv). One of the more recent, and lower dose studies, quoted within the meta-analysis, demonstrated a baseline radiation dose almost double that in our study (6.7 vs. 3.5 mSv) and their late-pass study resulted in an additional dose almost three times that demonstrated in our cohort (1.25 vs. 0.4 mSv) [35]. Our data demonstrate that using a limited late-pass scan acquired with prospective gating, fully diagnostic information can be obtained with minimal radiation dose penalty.
We have previously published evidence for the value of CT for the accurate delineation of the LAA when considering exclusion of the LAA [2]; however this study demonstrates the technical limitations and potential pitfalls if only a standard first-pass CT acquisition is performed. Accurate morphological classification, angles, and measurements are all important data prior to LAA intervention [2]. Pseudo-filling defects are important with internal occlusion devices such as Watchman and Amplatzer Cardiac Plug (ACP) devices as they may change the dimensions of the proposed landing site (Fig. 4d, g), and affect the size of the device selected by the operator. However, they are also critical when considering exclusion with the epicardial suture-based Lariat device as an inability to see the whole LAA may mean that the tip of the LAA is not fully visualised (Fig. 4a) and only when the late-pass study is performed can it be seen whether the tip of the LAA sits behind the pulmonary artery, or has additional lobes or architecture that result in a maximum width exceeding 45 mm. Either of these findings would make a lasso procedure impossible to perform [2].
No previous studies have looked at the visualisation of the LAA anatomy prior to LA exclusion using a combination of first-pass and delayed image acquisition. The low doses in our study suggest that this technique is suitable for routine clinical practice; however the doses could be further reduced, if desired, by limiting the initial contrast acquisition to the assessment of the atria only. Other groups such as Hur et al. [36] have used a two-phase injection protocol with a larger initial test bolus (50 ml) followed by a delayed second bolus of contrast (70 ml) after 3 min, but only a single scan at the 3 min time point to achieve similar results, but at the expense of a larger total volume of contrast. In our institution, the whole cardiac dataset is deemed sufficiently useful to referrers to warrant the first-pass study encompassing the whole heart in the scan range. The late-pass study is acquired without further contrast injection, thereby simplifying the protocol and minimising contrast load, and is confined to the LAA only, incurring minimal radiation dose penalty.
For patients who routinely undergo CCT prior to LA intervention, we believe the benefit of fully delineating the LA anatomy and excluding LAA thrombus with a delayed scan outweighs the drawback of a small increase in radiation dose when a delayed scan is performed using our technique. Our results suggest that the exclusion of LAA thrombus prior to LA atrial intervention for AF may be achieved with a limited, 60-s delayed CCT, performed in addition to the electrophysiologic mapping CT, with only a small increase in radiation dose. This would obviate exposing patients with detected thrombus to an unnecessary TEE, and save catheter laboratory time and patient inconvenience by avoiding unplanned procedure cancellation on the day of intervention.
Limitations
This is a single center study comparing the diagnostic performance of a dual-phase CCT against the first-pass study and TEE, the current gold standard imaging modality for LAA thrombus detection, however, without correlation with surgical or histopathological findings. Similar to previous studies [35, 37, 38], our study is limited by a very low prevalence of confirmed thrombi reflecting the fact that the majority of these patients are anti-coagulated at the time of examination. This resulted in relatively wide confidence intervals for both the sensitivity and positive predictive values. However, our study data represent 24 months of consecutive patients referred to our high volume center for this indication.
Finally, not all patients underwent atrial exclusion in this cohort and further outcome studies are required to confirm whether incomplete delineation of the LAA anatomy does indeed increase the potential for intervention or device failure in a prospective study.
Conclusion
A limited, reduced-dose, delayed contrast enhanced CCT scan of the LAA can reliably and fully delineate the LAA and exclude thrombus in AF patients referred for LA intervention. The addition of this low-dose, delayed scan may reduce the interventional failure rate, both for internal occluding devices and external lasso devices, and also obviate the need for pre-procedure TEE for thrombus exclusion in this patient group.
References
Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P, Investigators PA (2009) Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 374(9689):534–542. doi:10.1016/S0140-6736(09)61343-X
Ismail TF, Panikker S, Markides V, Foran JP, Padley S, Rubens MB, Wong T, Nicol E (2015) CT imaging for left atrial appendage closure: a review and pictorial essay. J Cardiovasc Comput Tomogr 9(2):89–102. doi:10.1016/j.jcct.2015.01.011
Holmes DR Jr, Lakkireddy DR, Whitlock RP, Waksman R, Mack MJ (2014) Left atrial appendage occlusion: opportunities and challenges. J Am Coll Cardiol 63(4):291–298. doi:10.1016/j.jacc.2013.08.1631
Stoddard MF, Dawkins PR, Prince CR, Ammash NM (1995) Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: a transesophageal echocardiographic study. J Am Coll Cardiol 25(2):452–459
Halperin JL, Hart RG (1988) Atrial fibrillation and stroke: new ideas, persisting dilemmas. Stroke 19(8):937–941
Panikker S, Virmani R, Sakakura K, Kolodgie F, Francis DP, Markides V, Walcott G, McElderry HT, Wong T (2015) Left atrial appendage electrical isolation and concomitant device occlusion: a safety and feasibility study with histologic characterization. Heart Rhythm 12(1):202–210. doi:10.1016/j.hrthm.2014.09.010
Badhwar N, Lakkireddy D, Kawamura M, Han FT, Iyer SK, Moyers BS, Dewland TA, Woods C, Ferrell R, Nath J, Earnest M, Lee RJ (2015) Sequential percutaneous LAA ligation and pulmonary vein isolation in patients with persistent AF: initial results of a feasibility study. J Cardiovasc Electrophysiol 26(6):608–614. doi:10.1111/jce.12655
Jaber WA, Prior DL, Thamilarasan M, Grimm RA, Thomas JD, Klein AL, Asher CR (2000) Efficacy of anticoagulation in resolving left atrial and left atrial appendage thrombi: a transesophageal echocardiographic study. Am Heart J 140(1):150–156. doi:10.1067/mhj.2000.106648
Mugge A, Kuhn H, Nikutta P, Grote J, Lopez JA, Daniel WG (1994) Assessment of left atrial appendage function by biplane transesophageal echocardiography in patients with non-rheumatic atrial fibrillation: identification of a subgroup of patients at increased embolic risk. J Am Coll Cardiol 23(3):599–607
Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, American College of Cardiology/American Heart Association Task Force on Practice G, European Society of Cardiology Committee for Practice G, European Heart Rhythm A, Heart Rhythm S (2006) ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 114(7):e257–e354. doi:10.1161/CIRCULATIONAHA.106.177292
European Heart Rhythm A, European Cardiac Arrhythmia S, American College of C, American Heart A, Society of Thoracic S, Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ, Jr., Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ (2007) HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 4(6):816–861. doi:10.1016/j.hrthm.2007.04.005
Schneider B, Stollberger C, Schneider B (2007) Diagnosis of left atrial appendage thrombi by multiplane transesophageal echocardiography: interlaboratory comparative study. Circ J 71(1):122–125
Kwong Y, Troupis J (2015) Cardiac CT imaging in the context of left atrial appendage occlusion. J Cardiovasc Comput Tomogr 9(1):13–18. doi:10.1016/j.jcct.2014.11.005
Qamruddin S, Shinbane J, Shriki J, Naqvi TZ (2010) Left atrial appendage: structure, function, imaging modalities and therapeutic options. Expert Rev Cardiovasc Ther 8(1):65–75. doi:10.1586/erc.09.161
Lazoura O, Reddy T, Shriharan M, Lindsay A, Nicol E, Rubens M, Padley S (2012) Prevalence of left atrial anatomical abnormalities in patients with recurrent atrial fibrillation compared with patients in sinus rhythm using multi-slice CT. J Cardiovasc Comput Tomogr 6(4):268–273. doi:10.1016/j.jcct.2012.02.004
Malchano ZJ, Neuzil P, Cury RC, Holmvang G, Weichet J, Schmidt EJ, Ruskin JN, Reddy VY (2006) Integration of cardiac CT/MR imaging with three-dimensional electroanatomical mapping to guide catheter manipulation in the left atrium: implications for catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 17(11):1221–1229. doi:10.1111/j.1540-8167.2006.00616.x
Tang K, Ma J, Zhang S, Zhang JY, Wei YD, Chen YQ, Yu XJ, Xu YW (2008) A randomized prospective comparison of CartoMerge and CartoXP to guide circumferential pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation. Chin Med J 121(6):508–512
Singh NK, Nallamothu N, Zuck VP, Issa ZF (2009) Left atrial appendage filling defects on 64-slice multidetector computed tomography in patients undergoing pulmonary vein isolation: predictors and comparison to transesophageal echocardiography. J Comput Assist Tomogr 33(6):946–951. doi:10.1097/RCT.0b013e31819cabc3
Martinez MW, Kirsch J, Williamson EE, Syed IS, Feng D, Ommen S, Packer DL, Brady PA (2009) Utility of nongated multidetector computed tomography for detection of left atrial thrombus in patients undergoing catheter ablation of atrial fibrillation. JACC Cardiovasc Imaging 2(1):69–76. doi:10.1016/j.jcmg.2008.09.011
Saremi F, Channual S, Gurudevan SV, Narula J, Abolhoda A (2008) Prevalence of left atrial appendage pseudothrombus filling defects in patients with atrial fibrillation undergoing coronary computed tomography angiography. J Cardiovasc Comput Tomogr 2(3):164–171. doi:10.1016/j.jcct.2008.02.012
Romero J, Husain SA, Kelesidis I, Sanz J, Medina HM, Garcia MJ (2013) Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circ Cardiovasc Imaging 6(2):185–194. doi:10.1161/CIRCIMAGING.112.000153
Bartus K, Morelli RL, Szczepanski W, Kapelak B, Sadowski J, Lee RJ (2014) Anatomic analysis of the left atrial appendage after closure with the LARIAT device. Circ Arrhythm Electrophysiol 7(4):764–767. doi:10.1161/CIRCEP.113.001084
Hur J, Kim YJ, Lee HJ, Ha JW, Heo JH, Choi EY, Shim CY, Kim TH, Nam JE, Choe KO, Choi BW (2009) Left atrial appendage thrombi in stroke patients: detection with two-phase cardiac CT angiography versus transesophageal echocardiography. Radiology 251(3):683–690. doi:10.1148/radiol.2513090794
Newcombe RG (1998) Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 17(8):857–872
Achenbach S, Sacher D, Ropers D, Pohle K, Nixdorff U, Hoffmann U, Muschiol G, Flachskampf FA, Daniel WG (2004) Electron beam computed tomography for the detection of left atrial thrombi in patients with atrial fibrillation. Heart 90(12):1477–1478. doi:10.1136/hrt.2003.027805
Feuchtner GM, Dichtl W, Bonatti JO, Jodocy D, Muller S, Hintringer F, Gradl J, Klauser A, Cury RC (2008) Diagnostic accuracy of cardiac 64-slice computed tomography in detecting atrial thrombi. Comparative study with transesophageal echocardiography and cardiac surgery. Invest Radiol 43(11):794–801. doi:10.1097/RLI.0b013e318184cd6c
Maltagliati A, Pontone G, Annoni A, Formenti A, Galli CA, Tamborini G, Alimento M, Andreini D, Tondo C, Pepi M (2011) Multidetector computed tomography vs multiplane transesophageal echocardiography in detecting atrial thrombi in patients candidate to radiofrequency ablation of atrial fibrillation. Int J Cardiol 152(2):251–254. doi:10.1016/j.ijcard.2011.07.086
Tang RB, Dong JZ, Zhang ZQ, Li ZA, Liu XP, Kang JP, Yu RH, de Long Y, Ma CS (2008) Comparison of contrast enhanced 64-slice computed tomography and transesophageal echocardiography in detection of left atrial thrombus in patients with atrial fibrillation. J Interv Card Electrophysiol 22(3):199–203. doi:10.1007/s10840-008-9243-0
Kim SC, Chun EJ, Choi SI, Lee SJ, Chang HJ, Han MK, Bae HJ, Park JH (2010) Differentiation between spontaneous echocardiographic contrast and left atrial appendage thrombus in patients with suspected embolic stroke using two-phase multidetector computed tomography. Am J Cardiol 106(8):1174–1181. doi:10.1016/j.amjcard.2010.06.033
Kim YY, Klein AL, Halliburton SS, Popovic ZB, Kuzmiak SA, Sola S, Garcia MJ, Schoenhagen P, Natale A, Desai MY (2007) Left atrial appendage filling defects identified by multidetector computed tomography in patients undergoing radiofrequency pulmonary vein antral isolation: a comparison with transesophageal echocardiography. Am Heart J 154(6):1199–1205. doi:10.1016/j.ahj.2007.08.004
Hur J, Kim YJ, Nam JE, Choe KO, Choi EY, Shim CY, Choi BW (2008) Thrombus in the left atrial appendage in stroke patients: detection with cardiac CT angiography—a preliminary report. Radiology 249(1):81–87. doi:10.1148/radiol.2491071544
Watson T, Shantsila E, Lip GY (2009) Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet 373(9658):155–166. doi:10.1016/S0140-6736(09)60040-4
Di Biase L, Santangeli P, Anselmino M, Mohanty P, Salvetti I, Gili S, Horton R, Sanchez JE, Bai R, Mohanty S, Pump A, Cereceda Brantes M, Gallinghouse GJ, Burkhardt JD, Cesarani F, Scaglione M, Natale A, Gaita F (2012) Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol 60(6):531–538. doi:10.1016/j.jacc.2012.04.032
Hur J, Kim YJ, Lee HJ, Ha JW, Heo JH, Choi EY, Shim CY, Kim TH, Nam JE, Choe KO, Choi BW (2009) Cardiac computed tomographic angiography for detection of cardiac sources of embolism in stroke patients. Stroke 40(6):2073–2078. doi:10.1161/STROKEAHA.108.537928
Sawit ST, Garcia-Alvarez A, Suri B, Gaztanaga J, Fernandez-Friera L, Mirelis JG, D’Anca M, Fuster V, Sanz J, Garcia MJ (2012) Usefulness of cardiac computed tomographic delayed contrast enhancement of the left atrial appendage before pulmonary vein ablation. Am J Cardiol 109(5):677–684. doi:10.1016/j.amjcard.2011.10.028
Hur J, Pak HN, Kim YJ, Lee HJ, Chang HJ, Hong YJ, Choi BW (2013) Dual-enhancement cardiac computed tomography for assessing left atrial thrombus and pulmonary veins before radiofrequency catheter ablation for atrial fibrillation. Am J Cardiol 112(2):238–244. doi:10.1016/j.amjcard.2013.03.018
Khan MN, Usmani A, Noor S, Elayi S, Ching CK, Di Biase L, Patel D, Burkhardt JD, Cummings J, Schweikert R, Saliba W, Natale A (2008) Low incidence of left atrial or left atrial appendage thrombus in patients with paroxysmal atrial fibrillation and normal EF who present for pulmonary vein antrum isolation procedure. J Cardiovasc Electrophysiol 19(4):356–358. doi:10.1111/j.1540-8167.2007.01070.x
Scherr D, Dalal D, Chilukuri K, Dong J, Spragg D, Henrikson CA, Nazarian S, Cheng A, Berger RD, Abraham TP, Calkins H, Marine JE (2009) Incidence and predictors of left atrial thrombus prior to catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 20(4):379–384. doi:10.1111/j.1540-8167.2008.01336.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Lazoura, O., Ismail, T.F., Pavitt, C. et al. A low-dose, dual-phase cardiovascular CT protocol to assess left atrial appendage anatomy and exclude thrombus prior to left atrial intervention. Int J Cardiovasc Imaging 32, 347–354 (2016). https://doi.org/10.1007/s10554-015-0776-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-015-0776-x