Abstract
Objectives
To test whether multidetector computed tomography (MDCT) could completely replace transoesophageal echocardiography (TEE) to detect left atrial appendage (LAA) thrombi in atrial fibrillation (AF) patients using a large sample size.
Methods
783 patients with AF who underwent MDCT and TEE before catheter ablation were retrospectively included. Demographic data were obtained. Two radiologists blinded to clinical data made the imaging diagnosis.
Results
Most of the patients (96.2 %) had a CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥ 75 years old (doubled), diabetes, stroke/transient ischaemic attack/thromboembolism (doubled), vascular disease, age 65–74 years, female sex) ≤ 3. Eight thrombi were identified by TEE, all of which were detected by MDCT; no thrombus was observed with TEE without the observation of filling defects by late-phase MDCT scanning in any of the patients. Using TEE as reference standard, the sensitivity, specificity, positive predictive value and negative predictive value of MDCT for thrombus detection were 100 %, 95.74 % (95 % CI 94.33 %–97.15 %), 19.51 % (95 % CI 16.73 %–22.29 %) and 100 %, respectively.
Conclusions
For AF patients with low risk of stroke, when MDCT images showed no filling defect in the late phase, TEE prior to catheter ablation can be avoided.
Key Points
• MDCT can help detect the presence of LAA thrombus.
• TEE can be avoided when late-phase MDCT shows no filling defect.
• TEE is required in patients whose MDCT images indicate thrombus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Catheter ablation (CA) to isolate pulmonary veins is commonly used as a therapeutic option for atrial fibrillation (AF) [1]. The presence of thrombus in the left atrial appendage (LAA) is considered to be an absolute contraindication to CA since the navigation of catheters inside the left atrium may dislodge the thrombus and result in a thromboembolic complication [1]. Therefore, transoesophageal echocardiography (TEE) is recommended to exclude the presence of a thrombus prior to the procedure.
Although TEE is currently considered the gold standard for the detection of an LAA thrombus, as a semi-invasive approach, it has several disadvantages, such as physical discomfort and potential life-threatening complications [2]. Additionally, there are also some contraindications for TEE, such as an oesophageal diverticulum. Therefore, an effective and comfortable modality that can identify an LAA thrombus with diagnostic accuracy similar to TEE would be of significant value in clinical practice.
Multidetector computed tomography (MDCT) has a well-defined role in the assessment of pulmonary veins and left atrial anatomy before CA in patients with AF and may have the ability to reliably detect thrombi in the LAA [3, 4]. Over the past few decades, several studies have investigated the use of MDCT as an alternative to TEE [5,6,7,8,9,10,11,12,13,14,15,16,17,18], and meta-analyses have concluded that MDCT may replace TEE [19, 20]. However, the sample size of the meta-analyses was not sufficiently large. Therefore, the aim of this study was to further test the diagnostic value of MDCT in the detection of an LAA thrombus and compare the results with TEE in a larger patient cohort.
Materials and methods
Patient population
A total of 783 consecutive patients (55 ± 11 years old; 72.3 % male) with AF between May 2009 and January 2016 who underwent dual-phase MDCT and TEE examinations before CA were included, and were divided into a thrombus group and a control group based on their TEE diagnosis. This study was approved by our institutional review board. As shown in Table 1, compared with patients in the control group, patients with a thrombus had lower left ventricular ejection fraction (LVEF) (63.25 % ± 7.38 % vs. 53.20 % ± 11.69 %, p < .001) and larger left atrial dimension (LAD) (38.63 mm ± 6.4 mm vs. 48.87 mm ± 5.06 mm, p = .001). Most of the patients had a low CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥ 75 years (doubled), diabetes previous stroke/transient ischaemic attack/thromboembolism (doubled), vascular disease, age 65–74 years, and sex category (female)); 96.2 % had a CHA2DS2-VASc score ≤ 3. Additionally, 22 (2.8 %) patients had a CHA2DS2-VASc score of 4, and six (0.8 %) and two (0.3 %) patients had CHA2DS2-VASc scores of 5 and 7, respectively. Baseline characteristics of the population are shown in Table 1.
Pre-procedure imaging
All the patients underwent electrocardiogram (ECG)-gated MDCT and TEE prior to operation. The imaging diagnosis was made in a blinded manner by two experienced radiologists. Two readers disagreed on the diagnosis of ten cases, but a consensus was reached during a joint reading. The interval between MDCT and TEE was analysed.
Multidetector computed tomography (MDCT)
ECG-gated MDCT was performed using a 64-slice spiral scanner (Lightspeed Volume CT, GE Healthcare, Little Chalfont, UK) within a single breath-hold (respiratory exercises were required prior to examination); the scanning range for early-phase scanning was from the diaphragm to the aortic arch, and to reduce the total radiation dose, the range for late-phase scanning was from the aortic arch to the middle of the left ventricle, mainly aiming at the LAA area. Detector collimation was 64 × 0.625 mm and the gantry rotation time was 350 ms. The tube settings for the early- and late-phase scans were the same: the tube voltage was 120 kv and the tube current was 300 mA. ECG-based tube current modulation was applied to obtain high-quality images and to reduce the radiation dose. A bolus of contrast media (50 - 60 ml) was injected via the antecubital vein at an infusion rate of 5 ml/s followed by an injection of saline (40 ml, 5 ml/s). Early-phase scanning started after a time delay determined by the bolus-tracking technique: 6 s after the threshold of 100 HU was reached in the region of interest. Late-phase scanning began 15 s after the end of early-phase scanning. In patients whose heart rate was > 70 beats per minute before the scanning procedure, a beta-adrenergic blocking agent was administered (5-15 ml metoprolol). If beta-adrenergic blocker treatment was not sufficient for heart rate control, intravenous amiodarone was used according to indications under close supervision, especially in patients with persistent AF. Retrospective ECG gating was used to obtain the helical scan data. Based on the dose-length product (250 - 400 mGy·cm), the total radiation dose was estimated to be approximately 4 - 7 mSv, but the actual radiation exposure may vary and may be higher.
The early-phase and late-phase imaging results were classified as normal filling, poor filling or filling defect, and an MDCT scoring system was established to better describe and analyse the results: 0 points were given for normal filling, 1 point was given for poor filling and 3 points were given for a filling defect; then the final MDCT score was calculated.
Transoesophageal echocardiography (TEE)
TEE was performed with a 5.0-mHZ, 128-element, multiplane probe (Phillips, Bothell, WA, USA). Examinations of the LAA were performed by rotating the imaging sector from 0° to 80° to optimize the visualization of the entire LAA, with a focus on detecting thrombus, spontaneous echo contrast (SEC) or other abnormalities. Thrombus was defined as an intracardiac echo-dense mass that was distinct from the adjacent normal tissue and SEC that appeared as a slow swirling, smoke-like echo.
The TEE imaging results were categorized into four classes: normal, SEC, thrombus, and SEC and thrombus. A TEE scoring system was generated to better describe and analyse the results: 0 points were given for a normal result (no thrombus and no SEC), 1 point was given for SEC and 2 points were given for thrombus; the final score was then obtained.
Statistical analysis
All statistical analyses were performed with SPSS software, version 20.0 (Statistical Package for the Social Sciences, Chicago, IL, USA). Continuous variables were expressed as the mean and standard deviation, and categorical data were expressed as a percentage of the frequency. Student’s t-test or analysis of variance was used to compare continuous variables between groups, and Pearson χ2 test or Fisher’s exact test was used for categorical variables. Using TEE as the reference standard, the sensitivity, specificity, positive predictive value and negative predictive value were calculated, including the 95 % confidence interval based on a binomial distribution. A two-tailed p value < .05 was considered to be statistically significant.
Results
Timing of pre-procedure imaging
All the intervals between MDCT and TEE were < 5 days; the percentage of intervals spanning 0 days, 1 day, 2 days and 3 days was 29.9 %, 35.9 %, 11.3 % and 12.1 %, respectively.
Results of left atrial appendage (LAA) images by MDCT
As shown in Table 2, the MDCT LAA imaging results were as follows: (1) 85.7 % (MDCT score of 0, 671/783) of patients did not exhibit filling defects in either the early phase or the late phase; (2) 5.24 % (MDCT score of 6, 41/783) exhibited filling defects in both phases; (3) 0.89 % (MDCT score of 4, 7/783) exhibited a filling defect in the early phase and poor filling in the late phase; (4) 1.53 % (MDCT score of 3, 12/783) exhibited a filling defect in the early phase but normal filling in the late phase; (5) 3.32 % (MDCT score of 2, 26/783) exhibited poor filling in both phases; and (6) 3.32 % (MDCT score of 1, 26/783) exhibited poor filling in the early phase but normal filling in the late phase.
Compared to patients with paroxysmal AF, patients with persistent AF were more likely to exhibit filling defects not only in the early phase but also in the late phase (13.3 % vs. 5.6 %, p = .001 and 9.0 % vs. 3.8 %, p = .006, respectively); this is shown in Table 3. Additionally, as shown in Table 4, the LVEF of patients with filling defects in the early phase or the late phase was lower than that of patients without a filling defect in the early phase or patients without a filling defect in the late phase (59.05 % ± 10.10 % vs. 63.50 % ± 7.13 %, p < .001 and 60.16 % ± 9.99 % vs. 63.33 % ± 7.29 %, p = .008, respectively), and the LADs of these patients were also larger (44.92 mm ± 8.84 mm vs. 38.29 mm ± 11.08 mm, p < .001 and 46.15 mm ± 6.71 mm vs. 38.39 mm ± 6.24 mm, p < .001, respectively).
Results of LAA imaging by TEE
The results of TEE were reported as normal (without thrombus or SEC) in 96.81 % (758/783) of patients, having a thrombus in 1.03 % (8/783) of patients and as having SEC in 2.68 % (21/783) of patients, among which four patients had a thrombus and SEC at the same time.
As shown in Table 3, the percentages of patients with thrombus or SEC in the persistent AF group were both higher than those in the paroxysmal AF group, but the difference in the percentage of patients with thrombus was not statistically significant (1.9 % vs. 0.7 %, p = .221 and 5.2 % vs. 1.7 %, p = .021, respectively). Additionally, patients with SEC and patients with a thrombus had a lower LVEF (53.20 % ± 11.69 % vs. 63.25 % ± 5.78 %, p < .001 and 57.51 % ± 10.16 % vs. 63.32 % ± 7.34 %, p < .001, respectively) and a larger LAD than patients without SEC or a thrombus (48.87 mm ± 5.06 mm vs. 38.63 mm ± 6.41 mm, p = .001 and 47.95 mm ± 7.27 mm vs. 38.55 mm ± 6.29 mm, p < .001, respectively), as shown in Table 4.
Agreement between MDCT and TEE
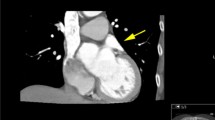
As shown in Table 2, a total of eight thrombi and 21 SECs were detected by TEE. As depicted by MDCT, all patients with a thrombus observed by TEE exhibited filling defects in both phases. Among the 17 patients with SEC alone, the MDCT images revealed filling defects in both phases in seven patients (41.2 %), early-phase filling defect in two patients and poor filling in one patient; the MDCT images of the remaining seven (41.2 %) patients were normal (Figs. 1, 2 and 3).
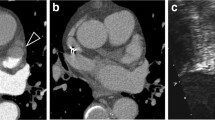
Multidetector computed tomography (MDCT) and transoesophageal echocardiography (TEE) images in a 43-year-old male patient without thrombus or spontaneous echo contrast (SEC). (a) Axial CT map shows no filling defect in the late phase in the left atrial appendage (LAA). (b) TEE image shows no thrombus or SEC in the LAA
Performance of MDCT in the detection of LAA thrombus in the whole population
TEE was used as the reference standard in our study and there were three conditions. When an early filling defect observed at LAA was considered a thrombus, the sensitivity, specificity, positive predictive value and negative predictive value of MDCT for thrombus detection were 100 %, 93.19 % (95 % CI 91.43 % – 94.95 %), 15.38 % (95 % CI 12.85 % – 17.91 %) and 100 %, respectively. When a filling defect observed in the late phase was regarded as a thrombus, the values were 100 %, 95.74 % (95 % CI 94.33 % – 97.15 %), 19.51 % (95 % CI 16.73 % – 22.29 %) and 100 %, respectively. When filling defects in both the early phase and late phase were considered a thrombus, the values were 100 %, 95.74 % (95 % CI 94.33 % – 97.15 %), 19.51 % (95 % CI 16.73 % – 22.29 %) and 100 %, respectively, which are similar to the values for the second condition.
Performance of MDCT in patients with high or low CHA2DS2-VASc scores
Seven hundred fifty-five patients (96.17 %) in our study had a low CHA2DS2-VASc score (≤ 3); therefore, we divided our patients into a low CHA2DS2-VASc score group (≤ 3) and a high CHA2DS2-VASc score group (≥ 4), and considered a filling defect in the late phase to indicate the presence of a thrombus according to the above results. The sensitivity, specificity, positive predictive value and negative predictive value of MDCT in the low CHA2DS2-VASc score group were 100 %, 95.38 % (95 % CI 93.91 % – 96.85 %), 15.38 % (95 % CI 12.85 % – 17.91 %) and 100 %, respectively. The MDCT values in the high CHA2DS2-VASc score group were all 100 %.
Discussion
The main findings of our study were that late-phase scanning with MDCT was sufficient for the detection of LAA thrombus and the performance of MDCT was much better among patients with a high CHA2DS2-VASc score than among patients with a low CHA2DS2-VASc score. Additionally, the specificity and positive predictive value of MDCT in patients with low CHA2DS2-VASc scores were relatively low, indicating that MDCT can not completely replace TEE in these patients. Therefore, TEE may not be required before CA for patients with a high CHA2DS2-VASc score and patients with a low CHA2DS2-VASc score without a filling defect in the late-phase MDCT image; however, TEE is still required to confirm the presence of a thrombus in other cases.
As shown in our study, MDCT imaging of the whole population exhibited good sensitivity and negative predictive value, especially in the late phase. In 2013, Romero et al. [20] reported that the mean sensitivity of MDCT was 96 % and the mean negative predictive value was 99 %. Additionally, the pooled sensitivity and negative likelihood rate reported by Zou et al. [19] was 95.7 % and 0.06, respectively. In this respect, our results were consistent with the conclusions of the two prior meta-analyses, exhibiting high sensitivity and negative predictive values for thrombus detection using MDCT.
Additionally, we found that when only late-phase images were considered, the diagnostic values of MDCT were the same as those when both phases were considered but better than those when only the early phase was considered. Romero et al. [20] also reported that the specificity of MDCT improved from 92 % to 99 % in their subanalysis of studies considering the delayed phase; moreover, the positive predictive value increased from 41 % to 92 %. Thus, this finding from our study was consistent with the findings from other previous studies [9, 10, 21, 22], further confirming that late-phase MDCT scanning is sufficient for the detection of LAA thrombus.
The positive predictive value in our study within the wide positive predictive value range (12 % –100 %) through the previous studies but was inferior to the mean values reported by the studies included in the two meta-analyses [6, 11,12,13, 16, 19, 20, 22]. The potential reasons for the false-positive results from MDCT have already been discussed by several previous studies [6, 10, 16], including: (1) the inadequate mixing of the contrast agent and the blood due to the low blood flow velocity in the LAA, which is observed as a filling defect by MDCT; and (2) the bulky pectinate muscles of the LAA might interfere with the filling of the contrast or even mimic a thrombus. Additionally, unlike images obtained by TEE, the images obtained by MDCT are static and lag behind the injection of the contrast, and as shown in our results, filling defects are associated with persistent AF, lower LVEF and a larger LAD. A sufficient long delay before late-phase scanning enables the complete mixture of contrast and blood in the LAA, thus allowing for better distinction of the thrombus from stagnant blood flow and decreasing the false-positive rate of MDCT. It should be noted that the late-phase scanning in our study was performed 15 s after the end of early-phase scanning, which was less than the 30-s delay in the two studies by Hur et al. [9, 10], and 60-s delay in the study by Lazoura et al. [18]. The positive predictive values reported by Hur et al. [9, 10] were 93 % and 100 %, which were much higher than the positive value reported by the present study. Additionally, Lazoura et al. [18] demonstrated that a 60-s delay improved the diagnosis of thrombus compared to the single standard scan, which may indicate that the high false-positive rate observed for late-phase scanning in our study may partially be attributed to the short delay time.
Of note, most of our patients (753) had a CHA2DS2-VASc score ≤ 3, indicating that they had a low risk of stroke, which was an important characteristic of our study. In our subanalysis, the performance of MDCT in patients with a low CHA2DS2-VASc score was not different from its performance in the whole population; both had a low positive predictive value, and the sensitivity and negative predictive values were both 100 %. Only 30 patients in our study had a CHA2DS2-VASc score higher than 3 and the predictive values of MDCT in these patients were all 100 %. As we discussed above, the false-positive results for MDCT were partially associated with the type of AF, slow blood flow in the LAA, larger LAD and bulky pectinate muscles, but these factors are not included in the CHA2DS2-VASc scoring stratification system [23]. Although the performance of MDCT in patients with a high CHA2DS2-VASc score was satisfactory in our study, additional studies on a larger sample size are required to confirm this finding.
Additionally, another concern about MDCT is the radiation burden on patients, but the radiation dose is associated with scan protocols and the radiation dose of the delayed MDCT scan could be significantly lower than that of the early-phase scan [24,25,26]. Although we used the ECG-based tube current modulation technique and minimized the scan range for the late-phase scan to reduce radiation exposure, the relatively old protocol of our study resulted in a relatively high radiation dose of approximately 4 - 7 mSv. In 2016, Lazoura et al. [18] used a low-dose, dual-phase CT protocol to detect LAA thrombus and reported that the total radiation dose was 2.1 - 5.2 mSv, which was much lower than the estimated dose in our study; moreover, the radiation dose for the delayed scan was only 0.2 - 0.6 mSv, which was consistent with the findings of several previous studies [9, 10, 22]. This demonstrated that novel CT techniques, modified scan parameters for different patients and modified scanning phases could help reduce the radiation dose, especially for the delayed scan, thereby improving the application value of MDCT in the detection of LAA thrombus.
SEC is caused by the stasis of blood in the LAA and is associated with an increased risk of thromboembolism in patients [27]. Established definitions of SEC using MDCT are still not available, but as reported by Kim et al. [22], filling defects observed only on early-phase MDCT images were more likely to be SEC, with high sensitivity and specificity when compared with TEE; however, as opposed to the results reported by Kim et al. [22], as depicted by MDCT in our study, about 41 % (7/17) of patients with SEC alone detected by TEE exhibited normal fillings in both phases, 41 % (7/17) of patients exhibited filling defects in both phases, and only three patients exhibited early-phase filling defect or poor filling, which indicated that MDCT alone cannot reliably diagnose SECs in patients with AF. It should be noted that the poor performance of MDCT in the detection and differentiation of SEC does not affect its potential usage in the detection of an LAA thrombus prior to CA since SEC is not a contraindication for CA [28].
Therefore, based on the findings of the present study, the following is recommended. First, whether MDCT can completely replace TEE for the detection of LAA thrombus prior CA in patients with AF depends on different situations. If the results of MDCT do not indicate a thrombus, especially in patients with a low risk of stroke, it is reasonable to avoid TEE, whereas if MDCT images show a filling defect in the late phase, TEE is still required to confirm the presence of a thrombus. Additionally, we found that the performance of MDCT is better among patients with a high risk of stroke than among patients with a low risk of stroke, which requires more attention in clinical practice. Second, late-phase MDCT scanning performed as well as dual-phase examination, which indicates that the late-phase scanning is sufficient to detect LAA thrombus in patients with AF and may be preferable as the radiation dose of the late-phase scan is lower and could be reduced further by modifying the scan protocol. Additionally, although MDCT could not definitively identify SEC, it may still be an alternative to TEE before CA of AF.
The main limitation of our study was the low incidence of thrombus in our population; almost all of the included patients were referred for CA and had taken anticoagulants regularly, potentially reducing the incidence of LAA thrombus below that in the general population. In addition, our study was limited by its retrospective design. As a result, the dimension of the thrombus, the morphology of the LAA, the blood flow velocity in the LAA, CT values and the radiation dose of each patient were not obtained. Additionally, the MDCT examination protocol used in our study was not the most recent, and the scanning parameters were not modified based on the specific conditions of each patient, such as body mass index, which prevented the further reduction of radiation exposure to patients. Furthermore, the delay time for the late-phase scan was relatively short, which may have influenced the performance of MDCT. Although we found that MDCT performed much better in patients with a high risk of stroke, the number of these patients in our study was small and thus additional studies are required to confirm our findings.
In conclusion, for patients with AF, especially those with low risk of stroke, delayed MDCT could be used to detect LAA thrombus prior to CA, and if the images do not show a filling defect, TEE can be avoided. However, for patients whose MDCT images indicate a filling defect, TEE is still required to confirm the presence of a thrombus.
Abbreviations
- AF:
-
Atrial fibrillation
- CA:
-
Catheter ablation
- CHA2DS2-VASc:
-
Congestive heart failure, hypertension, age ≥ 75 years (doubled), diabetes previous stroke/transient ischaemic attack/thromboembolism (doubled), vascular disease, age 65–74 years, and sex category (female)
- CHF:
-
Congestive heart failure
- LAA:
-
Left atrial appendage
- LAD:
-
Left atrial dimension
- LVEDD:
-
Left ventricular end-diastolic dimension
- LVEF:
-
Left ventricular ejection fraction
- MDCT:
-
Multidetector computed tomography
- SEC:
-
Spontaneous echo contrast
- TEE:
-
Transoesophageal echocardiography
- TIA:
-
Transient ischaemic attack
- VASc:
-
Vascular diseases
References
European Heart Rhythm A, European Cardiac Arrhythmia S, American College of Cardiology et al (2007) HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 4:816–861
Hilberath JN, Oakes DA, Shernan SK, Bulwer BE, D'Ambra MN, Eltzschig HK (2010) Safety of transesophageal echocardiography. J Am Soc Echocardiogr 23:1115–1127
Chen J, Yang ZG, Xu HY, Shi K, Long QH, Guo YK (2017) Assessments of pulmonary vein and left atrial anatomical variants in atrial fibrillation patients for catheter ablation with cardiac CT. Eur Radiol 27:660–670
Boucebci S, Pambrun T, Velasco S, Duboe PO, Ingrand P, Tasu JP (2016) Assessment of normal left atrial appendage anatomy and function over gender and ages by dynamic cardiac CT. Eur Radiol 26:1512–1520
Budoff MJ, Shittu A, Hacioglu Y et al (2014) Comparison of transesophageal echocardiography versus computed tomography for detection of left atrial appendage filling defect (thrombus). Am J Cardiol 113:173–177
Dorenkamp M, Sohns C, Vollmann D et al (2013) Detection of left atrial thrombus during routine diagnostic work-up prior to pulmonary vein isolation for atrial fibrillation: role of transesophageal echocardiography and multidetector computed tomography. Int J Cardiol 163:26–33
Gottlieb I, Pinheiro A, Brinker JA et al (2008) Diagnostic accuracy of arterial phase 64-slice multidetector CT angiography for left atrial appendage thrombus in patients undergoing atrial fibrillation ablation. J Cardiovasc Electrophysiol 19:247–251
Gudrun M, Feuchtner M, Dichtl W et al (2008) Diagnostic accuracy of cardiac 64-slice computed tomography in detecting atrial thrombi: comparative study with transesophageal echocardiography and cardiac surgery. Invest Radiol 43:794–801
Hur J, Kim YJ, Lee HJ et al (2009) Cardiac computed tomographic angiography for detection of cardiac sources of embolism in stroke patients. Stroke 40:2073–2078
Hur J, Kim YJ, Ha JW et al (2009) Left atrial appendage thrombi in stroke patients: Detection with two-phase cardiac CT angiography versus transesophageal echocardiography. Radiology 251:683–690
Kim YY, Klein AL, Halliburton SS et al (2007) Left atrial appendage filling defects identified by multidetector computed tomography in patients undergoing radiofrequency pulmonary vein antral isolation: a comparison with transesophageal echocardiography. Am Heart J 154:1199–1205
Maltagliati A, Pontone G, Annoni A et al (2011) Multidetector computed tomography vs multiplane transesophageal echocardiography in detecting atrial thrombi in patients candidate to radiofrequency ablation of atrial fibrillation. Int J Cardiol 152:251–254
Martinez MW, Kirsch J, Williamson EE et al (2009) Utility of nongated multidetector computed tomography for detection of left atrial thrombus in patients undergoing catheter ablation of atrial fibrillation. JACC Cardiovasc Imaging 2:69–76
Patel A, Au E, Donegan K et al (2008) Multidetector row computed tomography for identification of left atrial appendage filling defects in patients undergoing pulmonary vein isolation for treatment of atrial fibrillation: comparison with transesophageal echocardiography. Heart Rhythm 5:253–260
Sawit ST, Garcia-Alvarez A, Suri B et al (2012) Usefulness of cardiac computed tomographic delayed contrast enhancement of the left atrial appendage before pulmonary vein ablation. Am J Cardiol 109:677–684
Tang RB, Dong JZ, Zhang ZQ et al (2008) Comparison of contrast enhanced 64-slice computed tomography and transesophageal echocardiography in detection of left atrial thrombus in patients with atrial fibrillation. J Interv Card Electrophysiol 22:199–203
Madan A, Yan W, Byrne P et al (2016) Significance of left atrial appendage filling defects on cardiac CT prior to pulmonary vein isolation for atrial fibrillation. Int J Cardiol 203:520–522
Lazoura O, Ismail TF, Pavitt C et al (2016) A low-dose, dual-phase cardiovascular CT protocol to assess left atrial appendage anatomy and exclude thrombus prior to left atrial intervention. Int J Cardiovasc Imaging 32:347–354
Zou H, Zhang Y, Tong J, Liu Z (2015) Multidetector computed tomography for detecting left atrial/left atrial appendage thrombus: a meta-analysis. Intern Med J 45:1044–1053
Romero J, Husain SA, Kelesidis I, Sanz J, Medina HM, Garcia MJ (2013) Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circ Cardiovasc Imaging 6:185–194
Martinez MW, Lin G, Williamson EE, Brady PA (2009) Dual source computed tomography with delayed imaging for left atrial appendage thrombus compared with transoesophageal echocardiography. Heart 95:460
Kim SC, Chun EJ, Choi SI et al (2010) Differentiation between spontaneous echocardiographic contrast and left atrial appendage thrombus in patients with suspected embolic stroke using two-phase multidetector computed tomography. Am J Cardiol 106:1174–1181
January CT, Wann LS, Alpert JS et al (2014) 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol 64:e1–76
Jakobs TF, Becker CR, Ohnesorge B et al (2002) Multislice helical CT of the heart with retrospective ECG gating: reduction of radiation exposure by ECG-controlled tube current modulation. Eur Radiol 12:1081–1086
Hur J, Kim YJ, Lee HJ et al (2011) Dual-enhanced cardiac CT for detection of left atrial appendage thrombus in patients with stroke: a prospective comparison study with transesophageal echocardiography. Stroke 42:2471–2477
Hur J, Kin YJ, Lee HJ et al (2012) Cardioembolic stroke: dual-energy cardiac CT for differentiation of left atrial appendage thrombus and circulatory stasis. Radiology 263:688–695
Sadanandan S, Sherrid MV (2000) Clinical and echocardiographic characteristics of left atrial spontaneous echo contrast in sinus rhythm. J Am Coll Cardiol 35:1932–1938
Calkins H, Kuck KH, Cappato R et al (2012) 2012 HRS/EHRA/ECAS Expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 14:528–606
Acknowledgements
The authors thank Lei Han for MDCT consulting services.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Min Tang.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic study
• performed at one institution
Rights and permissions
About this article
Cite this article
Zhai, Z., Tang, M., Zhang, S. et al. Transoesophageal echocardiography prior to catheter ablation could be avoided in atrial fibrillation patients with a low risk of stroke and without filling defects in the late-phase MDCT scan: A retrospective analysis of 783 patients. Eur Radiol 28, 1835–1843 (2018). https://doi.org/10.1007/s00330-017-5172-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5172-6