Abstract
Hypertrophic cardiomyopathy (HCM) affects the right ventricle (RV) because of the anatomically hypertrophied septum and plausibly by extension of the myopathic process to the RV. We sought to investigate RV strain in patients with left ventricular hypertrophy secondary to either HCM or hypertension (H-LVH). Our cross-sectional study included 32 patients with HCM, 21 patients with H-LVH, and 11 healthy subjects, who were evaluated with transthoracic echocardiography. Using a dedicated software package, bi-dimensional acquisitions were analyzed to measure segmental longitudinal strain in apical views. Right ventricular global longitudinal strain (GLS) was calculated by averaging septal and right free wall strains. The HCM and H-LVH groups were comparable for age and demographic characteristics. Right ventricular tricuspid annular plane systolic excursion was not significantly different between HCM and H-LVH subjects. Moreover, RV GLS, septal and lateral RV myocardial strain were significantly impaired in patients with HCM (all p < 0.001). Regional and global RV strain parameters were not significantly impaired in H-LVH compared to healthy controls An RV GLS cut-off value of >14.9 % differentiated HCM and H-LVH with a 90 % sensitivity and a 95 % specificity (p < 0.001). RV strain parameters are impaired in patients with HCM. Assessment of two-dimensional RV strain parameters could help differentiate between HCM and H-LVH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypertrophic cardiomyopathy (HCM) is a chronic heritable disorder characterized histologically by myocyte disarray and regional or global ‘left’ ventricular hypertrophy (LVH) [1]. Nonetheless, recent studies suggest that morphopathologic and functional abnormalities in HCM are not limited to the left ventricle but affect the right ventricle as well, ostensibly from an extension of the cardiomyopathic process [2–4].
Although reports on structural and functional abnormalities of the right ventricle (RV) in patients with HCM are sparse, a growing body of evidence suggests that RV involvement is not uncommon, manifesting mainly as right ventricular hypertrophy, increased right ventricular myocardial mass as well as systolic and diastolic dysfunction [4–6].
The advent of novel echocardiographic imaging modalities such as tissue Doppler imaging (TDI) and 2D strain or speckle tracking imaging(STI) has substantively advanced our understanding of left ventricular deformational mechanics [7, 8]. Although the differentiation of physiologic left ventricular hypertrophy (LVH) from pathologic LVH can be greatly facilitated using these techniques [9–13], overlapping patterns in variant forms of pathologic LVH often present challenges that limit the discriminatory utility of these novel imaging modalities.
Data on RV strain profiles in patients with HCM are limited [5, 14, 15]. More specifically, to our knowledge, no prior head-to-head comparative study characterizing RV strain patterns in HCM with patients hypertensive LVH exists in the literature.
Accordingly, in this cross-sectional study, we sought to: (a) characterize regional and global RV strain in a cohort of patients with known HCM using 2D strain imaging (2DS), (b) compare strain patterns of RV involvement in HCM patients to those of patients with hypertensive LVH (H-LVH) and healthy controls and (c) explore the potential clinical utility of RV strain to more established functional parameters of RV systolic function for differentiating HCM from H-LVH.
Methods
We studied a total of 64 individuals who presented to our outpatient Cardiology clinic from 2013 to 2014 and fulfilled the inclusion criteria, including 32 consecutive patients with established HCM, 21 patients with hypertensive left ventricular hypertrophy (H-LVH) and 11 healthy controls. Another five patients (two with HCM and 3 with H-LVH were excluded from our analysis due to incomplete RV strain data. All patients were in sinus rhythm with preserved regional and global (left ventricular ejection fraction, EF > 55 %) and without pulmonary hypertension. The diagnosis of HCM was based on known familial history of HCM and/or conventional echocardiographic demonstration of unexplained LVH in the absence of an identifiable cardiac or systemic cause, exhibiting a septal wall thickness >15 mm and septal–posterior wall thickness ratio >1.3 [16]; asymptomatic hypertensive patients (with a diastolic blood pressure >90 mm Hg before treatment) and exhibiting at least moderate left ventricular hypertrophy on conventional echocardiography were included. Additional exclusion criteria were coronary artery disease, severe tricuspid, mitral or pulmonary valvular disease. The Institutional Review Board of the University approved the protocol in compliance with the Health Insurance Portability and Accountability Act (HIPAA) prior to data utilization.
Patient charts were retrospectively reviewed for clinical and demographic variables. All subjects underwent standard echocardiography as requested by the physician for various indications using a standard commercial ultrasound machine (Vivid E9, GE Vingmed, Horten, Norway) with the 4 V multiphased array probe and the images were digitally stored for offline analysis. Standard apical four-chamber, three-chamber and two-chamber views were obtained at frame rates of 60–100 frames/s. Three cardiac cycles were captured to allow for optimal cycle selection in the post processing period and subsequent analysis. Right ventricular fractional area shortening was calculated from end-systolic and end-diastolic areas traced from the 4-chamber view. Right ventricular thickness was evaluated by 2D measurement of RV free wall in the subcostal 4-chamber view and the RV basal dimension from the apical 4-chamber view from end-diastolic frames. All patients were evaluated with color-coded TDI from the apical four chamber view to determine the RV annular velocities, including systolic (S′) and early (E′) and late (A′) diastolic velocities, similarly TAPSE data were obtained in standard manner, in accordance with American Society of Echocardiography (ASE) guidelines [17].
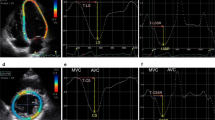
A commercially available speckle-tracking echocardiographic software program (EchoInsight, Epsilon, Ann Arbor, MI) was used to obtain 2D strain data from DICOM-format images offline for all included patients. EchoInsight software derives strain values using natural strain algorithms. Right ventricular longitudinal strain data were prospectively extracted in a blinded manner from images acquired in the four (4C) chamber view. A point-and-click approach was utilized to identify 3 RV anchor points (both annuli and RV apex) enabling the software to track the endocardial contour automatically. Subsequently, tracking was visually inspected throughout systole to ensure adequate border detection and the endocardial contours adjusted manually as necessary to further optimize tracking. Segments identified as inadequate for strain analysis by the software were noted. Longitudinal strains for each individual segment were measured and averaged to obtain RV septal (basal mid and apical walls) and lateral RV free wall (basal, mid and apical) strain. RV global longitudinal strain (GLS) was estimated by averaging septal and lateral right ventricular segmental strains (Fig. 1).
All statistical analyses were performed with SPSS (version 21.0; SPSS, Chicago, IL). All variables were tested for normal data distribution. Normally distributed data were expressed as mean ± standard deviation (SD). Non-normally distributed data were presented as the median with the interquartile range. For categorical variables, the Chi Square or Fisher exact test were used to compare the distributions for the two randomized groups. Non-paired Student’s t tests were used for comparisons of continuous variables. Receiver operating characteristic (ROC) curve analysis was performed to select optimal cut-off values of echocardiographic measurements. We applied the Youden index, which uses the maximum of vertical distance of ROC curve from the point (x, y) on diagonal line (chance line) and maximizes the difference between sensitivity and 1-specificity (Youden Index = sensitivity + specificity-1). Thus, by maximizing sensitivity and specificity aross various cut-off points, the optimal cut- off point is calculated. All significance tests were two tailed and conducted at the 5 % significance level. To assess reproducibility, strain parameters were independently measured by two blinded observers on 15 randomly selected image sets. Intraclass correlation coefficients (ICCs) and Bland–Altman plots were used for agreement. Statistical significance was accepted at a p value of <0.05.
Results
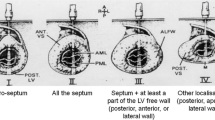
Demographics of the patients with HCM, H-LVH, and the control subjects are detailed in Table 1. There were no statistically significant differences between the H-LVH and HCM with regard to age, gender, body surface area across groups. No differences in LV ejection fraction, left atrial size or RV thickness was observed between groups, although the end-diastolic diameter was significantly increased in controls compared to HCM and H-LVH patients (<0.05 for both). None of the patients had evidence or right ventricular cavitary or left ventricular outflow obstruction at rest (defined as a resting late peaking LVOT gradient ≥30 mm Hg). Most frequently involved territories exhibiting prominent LVH in HCM patients were septal (81.2 %), followed by apical (15 %) and concentric LVH (3.8 %).
No significant differences in fractional area change (%) and RV systolic pressure among studied groups were found. RV fractional area change was comparable in all 3 groups, while right ventricular tricuspid annulus systolic excursion was not significantly different in patients with HCM compared with hypertensive subjects Table I). Right ventricular annular tissue Doppler S′ and E′ peak velocities were decreased in patients with HCM compared to all other groups. Adequate tracking and strain quantification was possible in 94 % of analyzed RV segments. Septal strain was attenuated in H-LVH compared to controls. RV global longitudinal strain (GLS), (as well as septal and lateral RV myocardial strain values) were significantly impaired in patients with HCM compared to patients with H-LVH, and controls (p < 0.001 for all comparisons) (Table 2).
To explore the discriminatory ability of various echo parameters to discern HCM from LVH, receiver–operator characteristic (ROC) curve analysis was performed. Optimal cut-off values for RV strain and echocardiographic parameters presented on Table 2, were determined by using the ROC curve. Global longitudinal strain provided the highest area under the curve (0.96 ± 0.02) for discriminating HCM. An RV GLS cut-off value of 14.9 % differentiated HCM and H-LVH with 90 % sensitivity and 95 % specificity (p < 0.001). 90.6 % of cases in the HCM had GLS less than the cutoff. None of the patient with HCM had normal GLS values. Regional and global RV strain parameters were not significantly different between H-LVH patients, and controls (Table 2).
Inter-observer and intra-observer agreement for strain parameter measurements was excellent for GLS. Intra-observer correlation coefficient was 0.97 [95 % confidence intervals (CI) 0.92–0.99, p < 0.001] and inter-observer correlation coefficient was 0.98 (95 % CI 0.97–0.99, p < 0.001). Bland–Altman analysis showed mean bias ± 2 SD of 0.29 ± 1.073 with 95 % limits of agreement −1.81 to 2.395 and 0.052 ± 0.77 (−1.45 to 1.56) respectively (Figs. 2a, b, 3).
Discussion
This is a hypothesis generating study directed at exploring the potential role of RV global longitudinal strain as a surrogate measure of right ventricular involvement and performance in patients with HCM. The salient findings of our study may be summarized as follows: (1) RV global systolic strain is attenuated in patients with HCM reflecting structural alterations from RV involvement in the myopathic process, (2) RV global strain was superior to more conventional functional parameters of RV systolic function for distinguishing HCM from H-LVH. (3) A global RV strain cutoff value of 14.9 %, offered the best discriminatory value for differentiating HCM from H-LVH.
Our observations extend and complement previously published data suggesting that the right ventricle is frequently involved in patients with HCM. Although the phenotypic expression of RV involvement is not echocardiographically apparent and cannot be relied on alone, to diagnose HCM, our findings suggest significant impairment of RV axial systolic function in these patients, despite the absence of manifest systolic dysfunction as assessed by more traditional parameters of systolic performance such as fractional area shortening, TAPSE and tissue Doppler velocities. These observations are further supported by findings of a cardiovascular magnetic resonance imaging (cMRI) phenotypic study that reported morphologic RV abnormalities including hypertrophy/increase RV mass index in only a third of patients with HCM with the advantage of tomographic visualization and superior spatial resolution associated with cMRI compared to 2D echocardiography [4].
Data on right ventricular involvement and systolic performance in patients with HCM are sparse [14, 18]. A recent ultrasound speckle two dimensional strain report compared regional and global longitudinal (GLS) RV strain in athletes against patients with HCM [14]. In accord with our findings, these investigators reported significant reductions in GLS and regional RV strain in the HCM group compared to athletes, without a difference in TAPSE values between groups. An RV GLS cutoff value of 16 % differentiated HCM from athletes with an 86 % sensitivity and 92 % specificity.
Previous cross-sectional studies using pulsed Doppler techniques have shown that despite the absence of RV systolic dysfunction, most patients with HCM have signs of abnormal filling patterns. Efthimiadis et al. [3] showed that the RV E/A ratio was lower and the RV isovolumetric relaxation time and RV deceleration time were significantly prolonged in patients with HCM compared to controls. In a larger prospective study, Pagourelias et al. [19] found that the RV E/E′ ratio was a strong predictor of deaths due to heart failure and sudden cardiac death in patients with HCM. Similarly, a recent study demonstrated that RV dysfunction assessed with the RV myocardial performance index is more common than RV dysfunction using the TAPSE or RV fractional area change, however only TAPSE was an independent predictor of mortality [5].
Although caution was exercised to track the RV septal endocardium, the assessment of global RV deformation with two-dimensional strain software is limited by the fact that it cannot accurately separate LV components from RV components of the septum and thus global strain indices for either are obligatorily affected by septal function. However, our study shows that RV free wall regional strain parameters are also impaired in patients with HCM, with global average longitudinal strain providing the best discriminatory value likely because of the larger overall number of RV segments analyzed. In aggregate, it is clear from the aforementioned evidence as well as our own observations that the RV in patients with HCM manifests contractile abnormalities that may not be readily detectable by more traditional echocardiographic measures of RV function.
Abnormal GLS is an independent predictor of adverse outcomes in HCM patients [20] and can be used to differentiate various HCM subtypes [21]. Thematically, differentiating between variant forms of pathologic LVH based on morphologic criteria alone is often challenging. Indeed, prior studies, including one by our group (using 2D strain) have suggested that left ventricular strain mapping can assist with the differentiation of hypertrophic cardiomyopathy from hypertensive LV [10, 22]. Nonetheless, despite suggested discriminatory cutoffs for global LV strain, overlapping strain values can often lead to ambiguity and present diagnostic challenges. Our data underscore the potential utility of 2D strain for ascertaining the presence and extent of RV involvement in patients with HCM while further raising the prospect of discerning HCM from hypertensive LVH.
A few study limitations have to be acknowledged. Only patients with preserved ejection fraction, free from pulmonary hypertension in sinus rhythm were studied and accordingly results should not be extrapolated to individuals with reduced ejection fraction, pulmonary hypertension or atrial flutter/fibrillation. The other limitations of our study include a small sample size and the limited assessment of RV longitudinal strain from the apical four-chamber view only. Analyzing RV strain from the subcostal and RV inflow views may permit a more comprehensive appraisal of the extent of RV involvement in patients with HCM.
Lack of standardization of strain software between vendors and the lack of widely accepted normative RV strain values in the literature may somewhat limit the ‘real world’ applicability of these data, nevertheless, we studied healthy controls in an attempt to mitigate this drawback; of note, efforts to standardize deformational imaging are well underway [23]. Despite the limitations of our study we believe our exploratory analysis strongly supports our conclusions.
Further studies are warranted to better characterize the diagnostic utility of RV strain and to prospectively correlate this measure with cardiovascular outcomes.
References
Maron BJ (1997) Hypertrophic cardiomyopathy. Lancet 350(9071):127–133
D’Andrea A, Caso P, Severino S, Sarubbi B, Forni A, Cice G et al (2003) Different involvement of right ventricular myocardial function in either physiologic or pathologic left ventricular hypertrophy: a Doppler tissue study. J Am Soc Echocardiogr 16(2):154–161
Efthimiadis GK, Parharidis GE, Karvounis HI, Gemitzis KD, Styliadis IH, Louridas GE (2002) Doppler echocardiographic evaluation of right ventricular diastolic function in hypertrophic cardiomyopathy. Eur J Echocardiogr 3(2):143–148
Maron MS, Hauser TH, Dubrow E, Horst TA, Kissinger KV, Udelson JE et al (2007) Right ventricular involvement in hypertrophic cardiomyopathy. Am J Cardiol 100(8):1293–1298
Morner S, Lindqvist P, Waldenstrom A, Kazzam E (2008) Right ventricular dysfunction in hypertrophic cardiomyopathy as evidenced by the myocardial performance index. Int J Cardiol 124(1):57–63
McKenna WJ, Kleinebenne A, Nihoyannopoulos P, Foale R (1988) Echocardiographic measurement of right ventricular wall thickness in hypertrophic cardiomyopathy: relation to clinical and prognostic features. J Am Coll Cardiol 11(2):351–358
Sengupta PP, Mehta V, Mohan JC, Arora R, Khandheria BK (2004) Regional myocardial function in an arrhythmogenic milieu: tissue velocity and strain rate imaging in a patient who had hypertrophic cardiomyopathy with recurrent ventricular tachycardia. Eur J Echocardiogr. 5(6):438–442
Sun JP, Stewart WJ, Yang XS, Donnell RO, Leon AR, Felner JM et al (2009) Differentiation of hypertrophic cardiomyopathy and cardiac amyloidosis from other causes of ventricular wall thickening by two-dimensional strain imaging echocardiography. Am J Cardiol 103(3):411–415
Serri K, Reant P, Lafitte M, Berhouet M, Le Bouffos V, Roudaut R et al (2006) Global and regional myocardial function quantification by two-dimensional strain: application in hypertrophic cardiomyopathy. J Am Coll Cardiol 47(6):1175–1181
Kato TS, Noda A, Izawa H, Yamada A, Obata K, Nagata K et al (2004) Discrimination of nonobstructive hypertrophic cardiomyopathy from hypertensive left ventricular hypertrophy on the basis of strain rate imaging by tissue Doppler ultrasonography. Circulation 110(25):3808–3814
Yang H, Sun JP, Lever HM, Popovic ZB, Drinko JK, Greenberg NL et al (2003) Use of strain imaging in detecting segmental dysfunction in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr 16(3):233–239
Richand V, Lafitte S, Reant P, Serri K, Lafitte M, Brette S et al (2007) An ultrasound speckle tracking (two-dimensional strain) analysis of myocardial deformation in professional soccer players compared with healthy subjects and hypertrophic cardiomyopathy. Am J Cardiol 100(1):128–132
Saghir M, Areces M, Makan M (2007) Strain rate imaging differentiates hypertensive cardiac hypertrophy from physiologic cardiac hypertrophy (athlete’s heart). J Am Soc Echocardiogr 20(2):151–157
D’Andrea A, Caso P, Bossone E, Scarafile R, Riegler L, Di Salvo G et al (2010) Right ventricular myocardial involvement in either physiological or pathological left ventricular hypertrophy: an ultrasound speckle-tracking two-dimensional strain analysis. Eur J Echocardiogr. 11(6):492–500
Zemanek D, Tomasov P, Prichystalova P, Linhartova K, Veselka J (2010) Evaluation of the right ventricular function in hypertrophic obstructive cardiomyopathy: a strain and tissue Doppler study. Physiol Res 59(5):697–702
Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS et al (2011) 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 58(25):e212–e260
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K et al (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23(7):685–713 (quiz 86–88)
Severino S, Caso P, Cicala S, Galderisi M, de Simone L, D’Andrea A et al (2000) Involvement of right ventricle in left ventricular hypertrophic cardiomyopathy: analysis by pulsed Doppler tissue imaging. Eur J Echocardiogr 1(4):281–288
Pagourelias ED, Efthimiadis GK, Parcharidou DG, Gossios TD, Kamperidis V, Karoulas T et al (2011) Prognostic value of right ventricular diastolic function indices in hypertrophic cardiomyopathy. Eur J Echocardiogr 12(11):809–817
Hartlage GR, Kim JH, Strickland PT, Cheng AC, Ghasemzadeh N, Pernetz MA et al (2015) The prognostic value of standardized reference values for speckle-tracking global longitudinal strain in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging 31(3):557–565
Reant P, Donal E, Schnell F, Reynaud A, Daudin M, Pillois X et al (2015) Clinical and imaging description of the Maron subtypes of hypertrophic cardiomyopathy. Int J Cardiovasc Imaging 31(1):47–55
Afonso L, Kondur A, Simegn M, Niraj A, Hari P, Kaur R et al (2012) Two-dimensional strain profiles in patients with physiological and pathological hypertrophy and preserved left ventricular systolic function: a comparative analyses. BMJ Open 2(4). doi:10.1136/bmjopen-2012-001390
Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R et al (2015) Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. J Am Soc Echocardiogr 28(2):183–193
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Afonso, L., Briasoulis, A., Mahajan, N. et al. Comparison of right ventricular contractile abnormalities in hypertrophic cardiomyopathy versus hypertensive heart disease using two dimensional strain imaging: a cross-sectional study. Int J Cardiovasc Imaging 31, 1503–1509 (2015). https://doi.org/10.1007/s10554-015-0722-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-015-0722-y