A synthetic oil-soluble iron-based catalyst was studied experimentally. A physical model of the catalytic transformation of high-viscosity oil at 200°C was developed. The composition and physicochemical and rheological characteristics of the thermocatalysis products were studied. IR spectroscopy found that the compositions of individual fractions changed. It was shown that the fraction of high-molecular-mass components could be substantially reduced by using the synthetic catalyst in combination with a hydrogen donor. This reduced the viscosity and; therefore, increased the degree of oil extraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Extraction of heavy crudes is hindered mainly by their anomalously high viscosities due to significant contents of resins and asphaltenes. Various methods that reduce the viscosity in the formation and partially transform heavy constituents by aquathermolysis are used to extract heavy crudes [1,2,3,4,5]. The drawbacks of this method are the high cost and formation of free radicals from ruptured bonds. Free radicals can polymerize to form larger molecules that increase the viscosity. Various catalysts are used to inhibit free-radical formation and reduce the oil viscosity and thermal-treatment temperatures [6,7,8,9]. They are added as nano-sized particles or precursors that decompose directly in the formation to form the active catalytic species.
The search for new efficient catalysts produced from available feedstocks is critical for increasing the energy efficiency of thermal extraction of heavy crudes. However, knowledge of the mechanisms of processes occurring in the heavy-crude reservoir is needed for a successful solution of such problems.
Therefore, the goal of the present work was to study the potential of using synthetic oil-soluble precursors of Fe-based catalysts for hydrogenolysis, hydrolysis, and cracking that transform the oil inside the formation and improve its physicochemical and rheological characteristics with respect to increasing the energy efficiency of steam extraction of heavy crudes. Similar catalysts for transforming high-viscosity oil under laboratory conditions were demonstrated to be efficient [10,11,12,13].
High-viscosity oil from Ashal’chinskii field in the Republic of Tatarstan that was obtained via steamgravitational settling and its noncatalytic and catalytic aquathermolysis products were studied.
Aquathermolysis was modeled in the laboratory using a high-pressure stirred tank reactor (300 mL volume, Parr Instruments, USA) (Table 1).
When the process was finished, oil was initially separated from water by standing for 16 h and then by centrifugation on an Eppendorf 5804R laboratory centrifuge at 3,000 rpm for 1 h. The validity of using this separation method was checked by determining the water content in the oil using the Dean—Stark method given in GOST 2477-65. The results showed that the water content varied in the range 0.7-0.8 wt. % for all oil samples and allowed further research to be conducted.
After the water content was determined, the dynamic and kinematic viscosities and the density were measured using an SVM 3000 automated Stabinger viscometer-densitometer (Anton Paar, Austria).
The group composition was determined by SARA separation taking into account methodical recommendations of ASTM standard D4124-09 and GOST 32269-2013 in several steps. Before the SARA analysis, the content of volatile organic compounds (VOCs) boiling below 110°C was determined using the literature method [14].
FTIR spectroscopy in the range 2,000-400 cm–1 on a TENSOR 27 spectrometer (Bruker, Germany) was used to study high-molecular-mass resins included in the oil.
Pellets were pressed from anhydrous KBr powder before each measurement because the compound was highly hygroscopic. For example, resins were immobile at room temperature so they were heated to 40-50°C before spreading a drop over a pellet. Asphaltenes were ground and mixed with KBr in an agate mortar. The obtained pellets were placed on the instrument and analyzed.
The saturated fraction was studied on a GC-MS system including a Chromatec-Crystal 5000 GC with an ISQ mass-selective detector and Xcalibur data processing software. The chromatograph was equipped with a capillary column (30 m × 0.25 mm) and used He carrier gas at 1 mL/min and injector temperature 310°C. The thermostat was programmed for 100-300°C at 3°C/min followed by isothermal conditions until the end of the analysis. The ion-source potential was 70 eV; temperature, 250°C. Compounds were identified using the NIST electronic mass-spectra library and literature data.
The dynamic viscosities of the oil samples were measured at 20-50°C in 5°C intervals (Fig. 1). Figure 2 shows the measured densities and dynamic viscosities at 20°C as functions of the aquathermolysis conditions.
Figure 2 shows that the densities of all studied samples varied little. However, the viscosities were greater than that of starting oil for all aquathermolysis products except that using a hydrogen donor. Apparently, this phenomenon was due to the formation of a denser three-dimensional network structure because of the recombination of free radicals formed by the action of steam on resins and asphaltenes in the absence of free protons. The viscosity decreased more substantially by 11.3% as compared with the noncatalytic aquathermolysis if catalyst and hydrogen donor were added together. This was an acceptable parameter for transformation of the starting oil under these subcritical conditions and could be characterized as the combined effect of the used reagents.
However, studies of the temperature dependences of the viscosities were not entirely adequate for evaluating the efficiency of using catalysts. Redistribution of the light and high-molecular-mass oil constituents during steam treatment could give a more complete picture.
Oil feedstock is known to be a multi-component system dominated by hydrocarbons that includes a large number of chemical compounds with various compositions and structures. The chemical composition of oil can be successfully studied only by using reliable methods for separating them into groups of chemically similar compounds.
SARA analysis is a simple scheme that is widely used in foreign research for separating oil into components under laboratory conditions. Table 2 presents the results for SARA separation of the studied oils.
Table 2 shows that the quantitative group compositions correlated with the dynamic viscosities. Noncatalytic aquathermolysis increased the resin content and decreased insignificantly the asphaltene fraction. This could also be a reason for the increased oil viscosity. The experiment using a catalyst and hydrogen donor gave the greatest effect with respect to decreasing the fraction of high-molecular-mass resins and asphaltenes and increasing the contents of light fractions (saturated and aromatic hydrocarbons). Destructive hydrogenation catalyzed cracking and rupture of the weakest –C-–S-–C– bonds in resins and asphaltenes and prevented increases of molecular mass and compaction of the structure by saturating free radicals with hydrogen transferred from aromatic naphthenes with formic acid in the system.
IR spectroscopy was used next to analyze the structure and group composition of resins and asphaltenes from starting Ashal’chinskii oil and noncatalytic and catalytic aquathermolysis products.
The structure and group composition of the studied products were studied by calculating spectral coefficients defined as the ratio of optical densities at maxima of the corresponding absorption bands:
These parameters were determined from IR spectra of the compounds with the highest molecular masses, i.e., resins and asphaltenes, using the baseline in the spectral range 2,000-400 cm–1.
The spectral coefficients (Table 3) of resins from Ashal’chinskii field oils were calculated using the IR spectra shown in Fig. 3.
Hydrothermal transformations of oil increase the aromaticity and decrease the aliphaticity. IR spectroscopy showed that resins in oil with a hydrogen donor and catalyst had higher aromaticity coefficients than those in oils without catalysts. This could be seen from the increasing intensities of absorption bands at 1600 cm–1 and 900-730 cm–1. Also, the aliphaticity decreased as compared with starting oil and reached a maximum of 3.54 units in sample 2 because of the reduced aromaticity. It should be noted that the resin oxidation increased characteristically with a catalyst and hydrogen donor because carbon—heteroatom bonds were hydrolyzed to form mostly phenols and alcohols. The noticeable increase of the sulfation parameter, which characterized the –SO content, was considered just as important and resulted from oxidation of sulfides to sulfoxides. The decrease of the resin branching parameter with a catalyst and hydrogen donor in the oil was a consequence of the increased length of the alkyl chains as compared with the experiment without the additives.
Thus, the IR spectral studies indicated that the structures of resins and asphaltenes changed significantly in the presence of a catalyst and hydrogen donor.
Figure 4 shows chromatograms of the alkanes (saturated hydrocarbon fraction) for starting oil and the experimental products.
Figure 4 shows that the studied oil samples contained significant amounts of isoprenoid alkanes, in particular, biomarkers such as pristane (i-C19H40) and phytane (i-C20H42), the ratio of which was an important indicator characterizing the degree of thermal transformability of the oils. This ratio was 0.73 for starting oil and 0.72, 0.49, and 0.6 for samples 1, 2, and 3, respectively. The quantitative values of this parameter decreased insignificantly for steam treatment. This suggested that temperature and the use of catalysts had little effect on structural changes in the light oil fraction. This was confirmed by the data in Fig. 5, which shows portions of the chromatograms of the studied saturated hydrocarbon fractions in the range 18-36 min that corresponded to the retention times of n-alkanes. It can be seen that heat treatment of the starting oil did not change the contents of normal alkanes.
The research on thermal transformation of high-viscosity oil at 200°C found that the Fe-based catalyst destroyed selectively resins and asphaltenes. Loss of resin and asphaltene aliphatic substituents increased the fraction of saturated hydrocarbons. The resin oxidation parameter increased in the presence of a catalyst and hydrogen donor because carbon—heteroatom bonds ruptured to form mostly phenols and alcohols. The noticeable increase of the sulfation parameter, which characterized the –SO content, was just as important and resulted from oxidation of sulfides to sulfoxides.
The resin and asphaltene fractions could be decreased significantly by combining the catalyst and hydrogen donor. This reduced irreversibly the viscosity of the extracted high-viscosity oil.
References
J. B. Hyne, J. W. Greidanus, J. D. Tyrer, et al., in: 2nd Int. Conf. “The Future of Heavy Crude and Tar Sands,” Caracas, Venezuela, Feb. 7-17, 1982, McGraw Hill, New York, 1984, pp. 404-411.
B. P. Tumanyan, N. N. Petrukhina, G. P. Kayukova, et al., Usp. Khim., 84, No. 11, 1145-1175 (2015).
V. Balsamo, D. Nguyen, and J. Phan, J. Pet. Sci. Eng., 122, 331-345 (2014).
T. N. Yusupova, Y. M. Ganeeva, G. V. Romanov, et al., Pet. Chem., 57, No. 3, 198-202 (2017).
B. P. Tumanyan, G. V. Romanov, D. K. Nurgaliev, et al., Chem. Technol. Fuels Oils, 50, 185-188 (2014).
N. N. Petrukhina, G. P. Kayukova, G. V. Romanov, et al., Chem. Technol. Fuels Oils, No. 4, 30-37 (2014).
G. P. Kayukova, L. E. Foss, D. A. Feoktistov, et al., Pet. Chem., 57, No. 8, 657-665 (2017).
S. K. Maity, J. Ancheyta, and G. Marroquin, Energy Fuels, 24, 2809-2816 (2010).
S. Desouky, A. Al sabagh, M. Betiha, et al., Int. J. Chem., Nucl., Metall. Mater. Eng., 7, No. 8, 286-291 (2013).
A. V. Vakhin, V. P. Morozov, S. A. Sitnov, et al., Chem. Technol. Fuels Oils, 50, No. 6, 569-578 (2015).
S. A. Sitnov, M. S. Petrovnina, D. A. Feoktistov, et al., Neft. Khoz., No. 11, 106-108 (2016).
S. A. Sitnov, D. A. Feoktistov, G. P. Kayukova, et al., Int. J. Pharm. Technol., 8, No. 3, 14884-14892 (2016).
G. P. Kayukova, A. T. Gubaidullin, S. M. Petrov, et al., Energy Fuels, 30, No. 2, 773-783 (2016).
A. M. Kharrat, J. Zacharia, V. J. Cherian, and A. Anyatonwu, Energy Fuels, 21, No. 6, 3618-3621 (2007).
Acknowledgments
The work was sponsored by the RT Investment-Venture Foundation program for innovative projects “Ideya-1000”. The work was performed using a subsidy for state support of Kazan Federal University to increase its competitiveness among leading global science and education centers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 5, pp. 24-28, September-October, 2017.
Rights and permissions
About this article
Cite this article
Vakhin, A.V., Sitnov, S.A., Mukhamatdinov, I.I. et al. Aquathermolysis of High-Viscosity Oil in the Presence of an Oil-Soluble Iron-Based Catalyst. Chem Technol Fuels Oils 53, 666–674 (2017). https://doi.org/10.1007/s10553-017-0848-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-017-0848-9


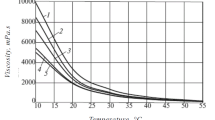

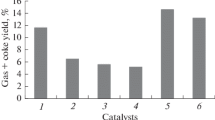


 at 20°C as functions of aquathermolysis conditions.
at 20°C as functions of aquathermolysis conditions.

