Abstract
Purpose
Low socioeconomic background (SB) has been associated with lower breast cancer (BC) incidence and higher BC mortality. One explanation of this paradox is the higher frequency of advanced BC observed in deprived women. However, it is still unclear if SB affects similarly BC incidence. This study investigated the link between SB and early/advanced BC incidence from Loire-Atlantique/Vendee Cancer registry data (France).
Materials and methods
Fourteen thousand three hundred fifty three women living in the geographic area covered by the registry and diagnosed with a primary BC in 2008–2015 were included. SB was approached by a combination of two ecological indexes (French European Deprivation Index and urban/rural residence place). Mixed effects logistic and Poisson regressions were used, respectively, to estimate the odds of advanced (stage ≥ II) BC and the ratio of incidence rates of early (stage 0–I) and advanced BC according to SB, overall and by age group (< 50, 50–74, ≥ 75).
Results
Compared to women living in affluent-urban areas, women living in deprived-urban and deprived-rural areas had a higher proportion of advanced BC [respectively, OR = 1.11 (1.01–1.22), OR = 1.60 (1.25–2.06)] and lower overall (from − 6 to − 15%) and early (from − 9 to − 31%) BC incidences rates Advanced BC incidence rates were not influenced by SB. These patterns were similar in women under 75 years, especially in women living in deprived-rural areas. In the elderly, no association between SB and BC frequency/incidence rates by stage was found.
Conclusion
Although advanced BC was more frequent in women living in deprived and rural areas, SB did not influence advanced BC incidence. Therefore, differences observed in overall BC incidence according to SB were only due to higher incidence of early BC in affluent and urban areas. Future research should confirm these results in other French areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lowering health-related social inequality is on the political agenda of many countries. In France, tackling socioeconomic and geographic inequalities has been one of the priorities of the successive national cancer plans since 2014 [1, 2].
Breast cancer (BC) remains by far the most common cancer and the leading cause of death from cancer in women, worldwide and in France [3, 4]. It has been previously reported that deprived and rural populations have a lower overall BC incidence but a higher proportion of advanced stage at diagnosis and a higher burden of BC mortality [5,6,7,8,9,10]. This paradox could in part reflect differences in exposure to BC risk factors and in the use of the healthcare system for cancer diagnosis/screening and treatment [11,12,13]. The higher mortality may also be related to the higher proportion of advanced BC at diagnosis in deprived populations [14,15,16], in line with lower participation to organized screening programs [11, 17, 18]. A higher mortality rate should be driven by a higher incidence of advanced stage (or other prognostic factors) at diagnosis and/or difference in BC management. Nevertheless, there is no clear evidence that the socioeconomic difference observed in terms of proportion would be found in terms of incidence rate of advanced BC. Although, incidence is the benchmark epidemiological indicator in public health and very useful to implement prevention actions, only a few studies focused on the relation between socioeconomic background (SB) and incidence of advanced BC [16, 19,20,21]. Two of them were performed in women over 50 years old and in the United States, where racial health disparities are important. Two studies were conducted in Europe but focused on limited age ranges (30–48 or ≥ 50 years old). In these studies, where definition of advanced BC changed between them, it was not consensual that incidence of advanced BC was higher in low socioeconomic groups. As the link between BC incidence, stage, and SB may vary according to age, country, and health system, further investigation is needed to better understand this relationship.
Thus, the aim of the study was to investigate stage-specific BC incidence according to SB from a large French population-based cancer registry.
Materials and methods
Study population
Fourteen thousand five hundred forty two women aged 15 and older who were newly diagnosed with a primary in situ or invasive breast carcinoma between 2008 and 2015 and living in Loire-Atlantique and Vendee (two affluent departments in western metropolitan France) at diagnosis were eligible for this study. Lymphomas and sarcomas were ineligible as well as women who presented prior in situ or invasive breast carcinoma.
Eligible cases were identified from Loire-Atlantique/Vendee Cancer Registry, with the following International Classification of Diseases for Oncology third edition (ICD-O-3) topography codes (C50.X) and malignant morphology codes (M8000-8575, 8980, 8982, 8983) with the exception of mammary Paget disease alone (M8540) (see Supplementary Material for the detailed list of exclusion codes).
The Loire-Atlantique/Vendee Cancer Registry registers all the incident cancer cases occurring in these two departments from different sources, including cytopathology laboratories, the medical information departments of public and private hospitals, the regional cancer network, health insurance organization departments, and general and specialist practitioners. The data quality and completeness of the Loire-Atlantique/Vendee Cancer Registry are certified every 5 years by the national Registries Evaluation Committee (CER). The last certification was obtained on 01/01/2021 for 5 years.
Data collection
In addition to data routinely collected in Loire-Atlantique/Vendee Cancer Registry, extensive information was collected from medical records: mode of detection, tumor characteristics at diagnosis (clinical and pathological TNM stages, Scarff-Bloom-Richardson (SBR) grade, estrogen (ER) and progesterone (PR) receptor status, human epidermal growth factor receptor-2 (HER2) status), and therapeutic management.
Demographic and urbanization information of each IRIS (“Ilots Regroupés pour l’Information Statistique,” smallest geographic unit for which French census data are available and corresponding to on average 2,000 individuals with relatively homogeneous social characteristics) was obtained from the French National Statistical Institute (INSEE).
BC organized screening participation rates for women targeted by the national program (50–74 y/o) were extracted by the French Public Health Agency (Santé Publique France) for each municipality.
Main outcome: early or advanced stage at diagnosis
According to the 7th TNM classification for malignant tumors, stage at diagnosis was defined from the pathological stage if surgery was the first treatment or from clinical stage in the case of neoadjuvant or non-surgical treatment [22]. For the purpose of the study, early BC included cancers at stages 0–I while advanced BC included cancers at stages II–IV cancers and minimum stage II cancers (i.e., BC that had not been calculated at an exact stage but with a tumor size > 2 cm (T > 1) and/or regional lymph nodes invasion (N > 0 except N1mi)).
Exposure: socioeconomic background (SB)
For each recorded cancer, the patient’s residence address at diagnosis was geolocalized using Geographic Information Systems (ArcGIS 10.2, ESRI Redlands, California, USA) and allocated to an IRIS [23, 24].
A socioeconomic deprivation score, assessed by the French version of the European Deprivation Index (F-EDI) based on the 2011 national census, was assigned to each IRIS by the ERISC/MapInMed platform (French national methodological platform for the study and reduction of health social inequalities in oncology) [25].
The EDI is a country-specific ecological deprivation index that best reflects individual experience of deprivation. It is based on individual data from the European Union Statistics on Income and Living Conditions survey (EU-SILC) and census data at the smallest available census unit. To be selected, variables must be available, phrased and coded in the same way at an individual level in the EU-SILC and at an ecological level in census population. Multivariate logistic regressions are performed to select and weight variables reflecting the best the deprivation. This methodology allows to construct an ecological deprivation index in a replicable way for each European country participating to EU-SILC. Variables and their weights changed according to their availability and country-specific features. This index filled up an important methodological gap and it was grounded on a solid theoretical framework, individual and aggregated variables, and on an annual Europe-wide survey allowing its replication over the time and in any European country [24]. In France, the calculation of the F-EDI score includes ten components best reflecting individual deprivation: overcrowding, no access to a system of central or electric heating, non-owner, unemployment, foreign nationality, no access to a car, unskilled worker or farmer worker, household with 6 or more persons, low level of education, and single parent education[23]. The categorical version (based on national quintiles) of the F-EDI was used to define the affluent (Q1, Q2, and Q3) and the deprived (Q4 and Q5) populations.
Regarding the urban/rural context, only IRIS within municipalities of more than 2,000 inhabitants and in which all buildings are interconnected by less than 200 m were considered to be “urban,” while all the others were considered to be “rural.”
To investigate SB and consider the intrinsic relationship between the urban/rural context and social deprivation, a composite variable was created using F-EDI and urban/rural residence to define four categories: affluent-urban, affluent-rural, deprived-urban, and deprived-rural. Urban and rural populations have not the same offer and access to healthcare; the composite variable offers a first approach of this component which is not included in the F-EDI [23].
Ethical statement
The Loire-Atlantique/Vendee Cancer Registry is approved by the French National Commission for Information Technologies and Liberties (CNIL) for the collection of nominal data on cancer patients without informed consent, for research purposes and in the strictest confidentiality. However, each cancer patient living in the geographic area covered by the registry is informed that their data may be recorded in the registry database and that they can oppose this registration. Only fully anonymized data are published.
Statistical analysis
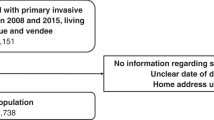
A total of 14,353 women (98.7% of eligible women) were finally included. Women with unknown data regarding early/advanced stage at diagnosis (n = 176) and residence address (n = 13) were excluded.
A two-step approach was implemented. (1) in terms of proportion of advanced BC: Mixed-effects logistic regressions were used to examine the likelihood (odds ratio, OR) of being diagnosed with advanced BC according to SB. (2) in terms of incidence: Early, advanced and all-stages age-standardized incidence rates (ASIR) were estimated according to SB and compared using Poisson regressions (incidence rates ratio, IRR).
The incidence rates were estimated using population data on 1st January from 2008 to 2015 provided by the National Institute of Statistics and Economic Studies (INSEE). Population estimates were given by IRIS, sex, year, and 5 year calendar age under 75 y/o (only a unique group over 75 y/o). Age-standardization was performed on the world standard population.
Assuming that BC characteristics and management may vary according to age and mode of detection, statistical analyses were performed overall and for 3 age groups: < 50, 50–74, and ≥ 75 years old. Age group definition was influenced by the French target population of the national organized screening program (50–74 y/o).
As age is a strong risk factor for breast cancer, logistic regression models were adjusted on age as a continuous variable. Since the annually reference populations were only available for 5 year age groups (only a unique group provided for ≥ 75 y/o), Poisson regression models were adjusted on 5 year age classes under 75 y/o. Regarding detection mode, logistic regression models were adjusted on detection mode recorded in the Registry. For Poisson regression models, for the 50–74 age group, IRR were secondly adjusted on the tertile of the municipality screening participation rate, defined as follows: < 58, 58–64, > 64%.
Each model included a random intercept at the IRIS level nested within a municipality to account for correlation among women within an IRIS and within a municipality. As screening participation rates were unavailable at the IRIS level, we assumed that all IRIS within a municipality had the same screening participation rate.
A set of sensitivity analyses were performed encompassing: (1) the removing of BC without exact stages but classified as advanced (i.e., minimum stage II according to our definition), (2) the removing of in situ (stage 0) BC, which could have a higher burden of deprivation and screening disparities, (3) the use of local EDI quintiles instead of national quintiles to increase the proportion of deprived women and the statistical power (Supplementary Table 1), and (4) the definition of advanced stage of BC as stage III/IV BC, as associations may be stronger for these latter stages. All statistical analyses were performed in R version 4.0.3 using the nlme package.
Results
The characteristics of the 14,353 women included in the study are presented in Table 1. Women were mostly diagnosed between 50 and 74 y/o (59.7%) (mean age: 61.1) and by screening (51.7%). Advanced BC (stage ≥ II) represented 45.2% of all cancers. Younger (< 50) and older (≥ 75) women had a greater proportion of advanced BC (51.3% and 64.4%, respectively, compared to 37.1% in the 50–74 age group) and of BC diagnosed on symptoms (66.0% and 73.1% compared to 27.7% in 50–74 group) (data not shown). No differences in SBR grade and phenotypic subtype were observed according to SB (Table 1).
One woman in five (20.7%) lived in deprived areas and almost the same proportion (20.3%) in rural municipalities. However, deprivation was mainly observed in urban areas (18.8% vs. 1.9% in deprived-rural areas). Regardless of urban/rural areas, women living in deprived areas were older than affluent ones. Women living in affluent-urban areas had the lowest proportion of advanced BC (43.5%). In contrast, women living in deprived areas, whether living in urban or rural areas, more often had an advanced BC (47.0% and 55.2%, respectively), which coincided with a lower proportion of BC detection by screening (49.6 and 44.4% vs. 53.6% in affluent-urban women). The proportion of advanced BC even surpassed that of early BC in deprived-rural areas (55.2% vs. 44.8%) (Table 1).
Logistic regressions found that the odds of developing advanced BC were significantly increased for women not living in affluent-urban areas, varying from 11% for women living in deprived-urban areas to 60% for women living in deprived-rural areas (Table 2). After stratification by age groups, only women aged under 75 years living in (affluent or deprived) rural areas had a significantly greater odds of advanced BC compared to women from affluent-urban areas [affluent-rural: < 50 y/o, OR = 1.3 95% CI (1.1–1.5); 50–74 y/o, OR = 1.2 95% CI (1.1–1.3)/deprived-rural: < 50 y/o, OR = 2.4 95% CI (1.3–4.4); 50–74 y/o, OR = 1.5 95% CI (1.1–2.1)). This difference was not observed in the elderly (≥ 75 y/o) (Table 2). After adjustment by mode of detection, the increased odds persisted only among deprived-rural women (Table 2).
Regarding BC incidence, the ASIR was estimated overall at 158.9 per 100,000 women (95% CI (155.6–162.2)) and was higher for early BC than for advanced BC (89.8 95% CI (87.4–92.3) vs. 68.9 95% CI (65.9–71.8) (Table 3). ASIR varied from 138.8 95% CI (117.7–159.9) in deprived-rural areas to 164.1 95% CI (159.6–168.5) in affluent-urban areas. Poisson models found significant lower incidence rates (from − 6 to − 15%) for women living in deprived and/or rural areas, compared to those living in affluent-urban areas. These lower IRR were only observed for early BC since none IRR of advanced BC according to SB was statistically significant (Table 3).
Results differed by age group (Table 3). In younger women (< 50 y/o), ASIR tended to be higher for advanced BC than for early BC, except in affluent-urban areas. Affluent-urban areas had the highest early-stage ASIR [39.9 95% CI (37.1–42.6)] while deprived-rural areas had the highest advanced-stage ASIR [42.7 95% CI (28.1–57.3)]. Poisson models found that overall and early BC incidences were significantly reduced in deprived and/or rural areas compared to affluent-urban areas (from − 11 to − 25% and from − 19% to − 48%, respectively).
The 50–74 age group was the only one in which early-stage ASIR were higher than advanced-stage ASIR, independently of SB. ASIR was the highest in affluent-urban areas for early BC [245.1 95% CI (240.3–249.9)] and in deprived-rural areas for advanced BC [157.6 95% CI (137.2–178.1)]. According to Poisson models, only early BC incidence was significantly reduced in deprived and/or rural areas compared to affluent-urban areas (from − 9 to − 26%). Results were unchanged after adjustment by screening municipality participation rate tertile (Table 4).
In the oldest women (≥ 75), early-stage ASIR were always lower than advanced-stage ASIR, in particular in deprived-rural areas (Table 3). Deprived-urban areas had the highest early- and advanced-stage ASIR [112.7 95% CI (111.2–114.3) and 204.4 95% CI (202.3–206.5), respectively], whereas deprived-rural areas had the lowest early- and advanced-stage ASIR [73.1 95% CI (69.4–76.9) and 164.6 95% CI (159.0–170.1), respectively). Nevertheless, no association between incidence rates and SB was detected in this age group.
Most importantly, for every age groups, the incidence rates of advanced BC were unaffected by SB.
In sensitivity analyses, neither the exclusion of minimum stage II BC (data not shown) or in situ BC (Supplementary Table 2–4) nor the use of local EDI quintiles (Supplementary Table 5–7) nor the definition of advanced BC as stage III/IV (Supplementary Table 8–10) changed the magnitude or direction of the estimated OR, ASIR or IRR.
Discussion
This population-based study showed that SB influenced BC incidence rates overall and according to stage at diagnosis. BC incidence was lower for women under 75 living in socioeconomically deprived and/or rural areas. However, this difference was only observed on early-stage BC incidence, while advanced-stage BC incidence remained unchanged between different SB.
In France, all individuals have universal free health insurance to minimize socioeconomic health inequalities. Since 2004, all women aged 50–74 years old are invited, free of charge, to a mammography every 2 years. Despite the specific features of the French healthcare system, the proportion of advanced BC was significantly higher among deprived and rural populations overall and in every age group, including in women aged 50–74 y/o, which is consistent with previous studies [8, 14, 15]. This result is explained in the scientific literature by a lower awareness of BC and lesser use of or access to the healthcare system (screening, medical services) in women living in deprived areas [26,27,28,29,30]. Indeed, socioeconomically deprived women are less likely to see a general practitioner or gynecologist during the last year before BC diagnosis [26,27,28]. In women targeted by organized screening, deprived women are more likely to not have repeat mammograms [26,27,28]. Some studies reported another possible explanation of a higher proportion of aggressive BC (triple negative, HER2 + , SBR grade 3) leading to a more advanced stage at diagnosis in deprived populations [31, 32]. However, no difference was found in tumor aggressiveness by SB in our study.
When focusing on incidence rate, our study showed higher BC incidence only in women living in affluent-urban areas, linked to higher early BC incidence. This finding could be the result of a more important exposition to BC risk factors combined with greater screening uptake in affluent populations.
On the one hand, BC is a multifactorial disease (partly related to reproductive/hormonal, lifestyle, and anthropometric risk factors): different prevalence in BC risk factors according to SB could explain higher/lower incidence [32, 33]. Some risk factors are more prevalent in affluent women (such as higher age at first parity [34, 35]), while other factors are more prevalent in deprived women (for example overweight, unhealthy lifestyle habits [36]). However, it seems difficult to compare the BC occurrence risks given the multiplicity and overlap of risk factors. On the other hand, another explanation of the higher all-stages and early BC incidence in women living in affluent-urban areas is a higher awareness and use of healthcare services, especially screening mammograms. In our study, screening detection was higher for women living in affluent-urban areas (53.6%) than for those living in deprived-rural areas (44.4%). We cannot exclude that excess of early BC incidence in women living in affluent-urban areas could in part be related to over-diagnosis. It has been estimated that over-diagnosis related to the organized screening program concerns 17% of in situ and 5.5% of invasive BC in France [37]. Over-diagnosis would concern both organized screening (50–74 years old women) and opportunistic screening, the latter being present in all age groups.
The impact of SB strongly varied across age groups. In particular, we did not identify socioeconomic disparities in the oldest subgroup in terms of advanced BC proportion nor in terms of incidence rates, probably due to small numbers and lack of statistical power. In women under 75 years old, we found that BC incidence varied according to SB. Young women (< 50) are particularly affected by opportunistic screening (30.9%), varied from 23.2% in deprived-rural areas to 32.7% in affluent-urban areas in our study, which could partly explain sociodemographic disparities in this subgroup. Women aged 50–74 years old, who were directly targeted by a screening program, were the only subgroup in which early BC incidence rates were clearly higher than advanced BC rates. In this subgroup the effect of SB was attenuated compared to younger women (< 50). We can therefore hypothesize that the organized screening program, concerning 82.0% of the screened diagnosed BC in this subgroup, could contribute to the reduction of socioeconomic BC inequalities.
Unexpectedly, our study did not show any impact of the SB on advanced stage BC incidence. Based on literature and clinical observations in the Loire-Atlantique Cancer Registry, we defined advanced BC as stage II-IV BC (i.e., BC with a tumor size > 2 cm or with node involvement or with metastasis) [16, 19,20,21]. However, in previous studies that looked at stage-specific BC incidence, the results cannot be agreed upon as the definition of advanced BC differed in each study. Advanced BC was defined as either stage II-III inclusive and stage IV alone, or regional ± distant (according to SEER staging) [16, 19,20,21]. One study was conducted in Europe on limited age group (30–48 years old) [20]. Distant BC incidence was higher in the lowest income bracket but was not associated with educational level, whereas regional BC incidence was higher in groups with a better education level and incomes [20]. Two studies were performed in women over 50 years old in the United States, where racial health disparities are a major factor in incidence rate differences; there were no differences in regional-stage BC incidence rates but distant-stage BC incidence was higher in lower socioeconomic groups [19, 21]. In our study, our definition of advanced BC was quite broad, because of the small number of cases, and may have hidden some existing associations. We also merged stage I and in situ in the definition of early BC. However, results were unchanged when removing in situ BC or BC without exact stage but classified as advanced (i.e., minimum stage II according to our definition) (data not shown) from the analyses. In addition, associations were unchanged when advanced BC was defined as stages III–IV.
Our study has some weaknesses. Except age and mode of detection, we were unable to include additional individual-level data in the regression models. Indeed, individual socioeconomic data such as marital status or socio-professional status are not recorded by the French Cancer Registries, making it necessary to use an ecological deprivation index rather. The absence of individual socioeconomic variables in cancer registries represents a limitation since ecological measurements may underestimate social inequalities compared to individual measurements [38]. However, by avoiding selection bias in socioeconomic individual data collection, ecological indexes are suitable to measure deprivation [39]. In addition, previous studies have shown consistent results whether socioeconomic status was measured by individual indices, such as educational attainment or income, or by ecological indicators based on the geographic area of residence for most cancer sites [10, 40,41,42,43,44,45]. Regarding our choice of the deprivation index, F-EDI allows to consider overall deprivation including objective and subjective poverty, respectively, assessed in the census and in the EU-SILC, a survey specifically designed to investigate the multidimensionality of deprivation at an individual level. F-EDI is also available for all smallest units on the French mainland and has been developed in several European countries to allow geographic comparisons [38].
Another point is that our results may not reflect the situation elsewhere in France because of the regional design of the study. Though Loire-Atlantique and Vendee are two affluent French departments, our results were unaffected when using the local F-EDI quintiles instead of the national F-EDI quintiles. Further research including other French departments could provide more detailed results and insights concerning the elderly age group (≥ 75 years old) and stage-specific BC incidence according to SB, using less aggregated stages. The inclusion of departments with different socioeconomic profiles would also increase the representativeness of the general French population.
Nevertheless, our study has several strengths. We used an original approach which combined analyses stratified by stage and age at diagnosis to study socioeconomic inequalities in BC incidence. Another strength is the data quality with few missing data and standardized information. The exhaustive record of all BC cases from the registry ensures that our sample is representative of the Loire-Atlantique/Vendee population and enables the description of incidence heterogeneity according to socioeconomic deprivation and urbanization. The inclusion of the urban/rural context, which is not present in the EDI, in the created composite variable of SB offered a first approach of access to health care. Indeed, access to health care is a multifactorial component and might be reflecting availability and accessibility to various healthcare professionals (doctors, midwives, nurses…) as well as access distance to hospitals and other healthcare facilities (radiology offices, medical practices…). Urban and rural populations have not the same offer and access to healthcare and further studies with specific methodologies are required to get more insights.
French healthcare features notwithstanding, our study provides new information regarding the simultaneous impact of socioeconomic environment and rural/urban environment on stage-specific BC incidence. The major result of this large population-based study is that the differences observed in BC incidence between affluent and deprived populations were due to higher incidence of early BC in affluent and urban women, mainly in women under 75 years old. The lowest BC incidence observed in more deprived women was largely explained by reduced incidence of early-stage BC. Further studies focused on access to medical care facilities and medical follow-up before BC diagnosis are needed to explain the influence of SB on incidence according to stage. They will be helpful for the implementation of targeted actions.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Stratégie décennale de lutte contre les cancers 2021–2030 [in French]
Touraine M (2014) Plan cancer 2014–2019: Guérir et prévenir les cancers: donnons les mêmes chances à tous, partout en France. Ministère des affaires sociales et de la santé, Paris
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Defossez G, Le Guyader-Peyrou S, Uhry Z, Grosclaude P, Remontet L, Colonna M, Dantony E, Delafosse P, Molinié F, Woronoff AS, Bouvier AM, Bossard N, Monnereau A (2019) Estimations nationales de l’incidence et de la mortalité par cancer en France métropolitaine entre 1990 et 2018. Étude à partir des registres des cancers du réseau Francim. Résultats préliminaires. Rapport. Santé Publique France, Saint-Maurice (Fra)
Bryere J, Tron L, Menvielle G, Launoy G, French Network of Cancer Registries (FRANCIM) (2019) The respective parts of incidence and lethality in socioeconomic differences in cancer mortality. An analysis of the French network Cancer registries (FRANCIM) data. Int J Equity Health 18:189. https://doi.org/10.1186/s12939-019-1087-y
Pollock AM, Vickers N (1997) Breast, lung and colorectal cancer incidence and survival in South Thames Region, 1987–1992: the effect of social deprivation. J Public Health Med 19:288–294. https://doi.org/10.1093/oxfordjournals.pubmed.a024632
Walsh PM, Byrne J, Kelly M, McDevitt J, Comber H (2014) Socioeconomic disparity in survival after breast cancer in Ireland: observational study. PLoS ONE 9:e111729. https://doi.org/10.1371/journal.pone.0111729
Nguyen-Pham S, Leung J, McLaughlin D (2014) Disparities in breast cancer stage at diagnosis in urban and rural adult women: a systematic review and meta-analysis. Ann Epidemiol 24:228–235. https://doi.org/10.1016/j.annepidem.2013.12.002
Hausauer AK, Keegan THM, Chang ET, Glaser SL, Howe H, Clarke CA (2009) Recent trends in breast cancer incidence in US white women by county-level urban/rural and poverty status. BMC Med 7:31. https://doi.org/10.1186/1741-7015-7-31
Carlsen K, Høybye MT, Dalton SO, Tjønneland A (2008) Social inequality and incidence of and survival from breast cancer in a population-based study in Denmark, 1994–2003. Eur J Cancer 44:1996–2002. https://doi.org/10.1016/j.ejca.2008.06.027
Deborde T, Chatignoux E, Quintin C, Beltzer N, Hamers FF, Rogel A (2018) Breast cancer screening programme participation and socioeconomic deprivation in France. Prev Med 115:53–60. https://doi.org/10.1016/j.ypmed.2018.08.006
Menvielle G, Rey G, Jougla E, Luce D (2013) Diverging trends in educational inequalities in cancer mortality between men and women in the 2000s in France. BMC Public Health 13:823. https://doi.org/10.1186/1471-2458-13-823
Ouédraogo S, Dabakuyo-Yonli TS, Roussot A, Pornet C, Sarlin N, Lunaud P, Desmidt P, Quantin C, Chauvin F, Dancourt V, Arveux P (2014) European transnational ecological deprivation index and participation in population-based breast cancer screening programmes in France. Prev Med 63:103–108. https://doi.org/10.1016/j.ypmed.2013.12.007
Lyratzopoulos G, Abel GA, Barbiere JM, Brown CH, Rous BA, Greenberg DC (2012) Variation in advanced stage at diagnosis of lung and female breast cancer in an English region 2006–2009. Br J Cancer 106:1068–1075. https://doi.org/10.1038/bjc.2012.30
Dos-Santos-Silva I, De Stavola BL, Renna NL, Nogueira MC, Aquino EML, Bustamante-Teixeira MT, Silva AEG (2019) Ethnoracial and social trends in breast cancer staging at diagnosis in Brazil, 2001–14: a case only analysis. Lancet Glob Health 7:e784–e797. https://doi.org/10.1016/S2214-109X(19)30151-2
de Munck L, Fracheboud J, de Bock GH, den Heeten GJ, Siesling S, Broeders MJM (2018) Is the incidence of advanced-stage breast cancer affected by whether women attend a steady-state screening program? Int J Cancer 143:842–850. https://doi.org/10.1002/ijc.31388
Pornet C, Dejardin O, Morlais F, Bouvier V, Launoy G (2010) Socioeconomic and healthcare supply statistical determinants of compliance to mammography screening programs: a multilevel analysis in Calvados, France. Cancer Epidemiol 34:309–315. https://doi.org/10.1016/j.canep.2010.03.010
Guillaume E, Launay L, Dejardin O, Bouvier V, Guittet L, Déan P, Notari A, De Mil R, Launoy G (2017) Could mobile mammography reduce social and geographic inequalities in breast cancer screening participation? Prev Med 100:84–88. https://doi.org/10.1016/j.ypmed.2017.04.006
Yabroff KR, Gordis L (2003) Does stage at diagnosis influence the observed relationship between socioeconomic status and breast cancer incidence, case-fatality, and mortality? Soc Sci Med 57:2265–2279. https://doi.org/10.1016/s0277-9536(03)00100-x
Trewin CB, Hjerkind KV, Johansson ALV, Strand BH, Kiserud CE, Ursin G (2020) Socioeconomic inequalities in stage-specific breast cancer incidence: a nationwide registry study of 1.1 million young women in Norway, 2000–2015. Acta Oncol 59:1284–1290. https://doi.org/10.1080/0284186X.2020.1753888
Kaur M, Joshu CE, Visvanathan K, Connor AE (2022) Trends in breast cancer incidence rates by race/ethnicity: patterns by stage, socioeconomic position, and geography in the United States, 1999–2017. Cancer 128:1015–1023. https://doi.org/10.1002/cncr.34008
Sobin LH, Gosporadowicz MK, Wittelkind C (2011) TNM classification of malignant tumors, 7th edn. Wiley, Hoboken
Pornet C, Delpierre C, Dejardin O, Grosclaude P, Launay L, Guittet L, Lang T, Launoy G (2012) Construction of an adaptable European transnational ecological deprivation index: the French version. J Epidemiol Community Health 66:982–989. https://doi.org/10.1136/jech-2011-200311
Guillaume E, Pornet C, Dejardin O, Launay L, Lillini R, Vercelli M, Marí-Dell’Olmo M, Fernández Fontelo A, Borrell C, Ribeiro AI, de Pina MF, Mayer A, Delpierre C, Rachet B, Launoy G (2016) Development of a cross-cultural deprivation index in five European countries. J Epidemiol Community Health 70:493–499. https://doi.org/10.1136/jech-2015-205729
Cancers and Preventions—UMR 1086 UCN.Caen. Plate-forme méthodologique nationale pour l’étude et la réduction des inégalités sociales en cancérologie (ERISC). http://cancerspreventions.fr/inegalites-sociales/plateforme-2/. Accessed 12 Dec 2021
Sicsic J, Franc C (2014) Obstacles to the uptake of breast, cervical, and colorectal cancer screenings: what remains to be achieved by French national programmes? BMC Health Serv Res 14:465. https://doi.org/10.1186/1472-6963-14-465
Duport N (2012) Characteristics of women using organized or opportunistic breast cancer screening in France. Analysis of the 2006 French Health, health care and insurance survey. Rev Epidemiol Sante Publique 60:421–430. https://doi.org/10.1016/j.respe.2012.05.006
Ferrat E, Le Breton J, Djassibel M, Veerabudun K, Brixi Z, Attali C, Renard V (2013) Understanding barriers to organized breast cancer screening in France: women’s perceptions, attitudes and knowledge. Fam Pract 30:445–451. https://doi.org/10.1093/fampra/cmt004
Aarts MJ, Hamelinck VC, Bastiaannet E, Coebergh JWW, Liefers GJ, Voogd AC, van der Sangen M, Louwman WJ (2012) Small but significant socioeconomic inequalities in axillary staging and treatment of breast cancer in the Netherlands. Br J Cancer 107:12–17. https://doi.org/10.1038/bjc.2012.205
Thomson CS, Hole DJ, Twelves CJ, Brewster DH, Black RJ, Network SCT (2001) Prognostic factors in women with breast cancer: distribution by socioeconomic status and effect on differences in survival. J Epidemiol Community Health 55:308–315. https://doi.org/10.1136/jech.55.5.308
Taylor A, Cheng KK (2003) Social deprivation and breast cancer. J Public Health Med 25:228–233. https://doi.org/10.1093/pubmed/fdg072
Afshar N, English DR, Milne RL (2021) Factors explaining socio-economic inequalities in cancer survival: a systematic review. Cancer Control 28:10732748211011956. https://doi.org/10.1177/10732748211011956
Menvielle G, Kunst AE, van Gils CH, Peeters PH, Boshuizen H, Overvad K, Olsen A, Tjonneland A, Hermann S, Kaaks R, Bergmann MM, Illner A-K, Lagiou P, Trichopoulos D, Trichopoulou A, Palli D, Berrino F, Mattiello A, Tumino R, Sacerdote C, May A, Monninkhof E, Braaten T, Lund E, Quirós JR, Duell EJ, Sánchez MJ, Navarro C, Ardanaz E, Borgquist S, Manjer J, Khaw KT, Allen NE, Reeves GK, Chajes V, Rinaldi S, Slimani N, Gallo V, Vineis P, Riboli E, Bueno-de-Mesquita HB (2011) The contribution of risk factors to the higher incidence of invasive and in situ breast cancers in women with higher levels of education in the European prospective investigation into cancer and nutrition. Am J Epidemiol 173:26–37. https://doi.org/10.1093/aje/kwq319
Heer E, Harper A, Escandor N, Sung H, McCormack V, Fidler-Benaoudia MM (2020) Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health 8:e1027–e1037. https://doi.org/10.1016/S2214-109X(20)30215-1
Porter P (2008) “Westernizing” women’s risks? Breast cancer in lower-income countries. N Engl J Med 358:213–216. https://doi.org/10.1056/NEJMp0708307
Youlden DR, Cramb SM, Dunn NAM, Muller JM, Pyke CM, Baade PD (2012) The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol 36:237–248. https://doi.org/10.1016/j.canep.2012.02.007
Seigneurin A, Labarère J, François O, Exbrayat C, Dupouy M, Filippi M, Colonna M (2016) Overdiagnosis and overtreatment associated with breast cancer mammography screening: a simulation study with calibration to population-based data. Breast 28:60–66. https://doi.org/10.1016/j.breast.2016.04.013
Lamy S, Molinié F, Daubisse-Marliac L, Cowppli-Bony A, Ayrault-Piault S, Fournier E, Woronoff AS, Delpierre C, Grosclaude P (2019) Using ecological socioeconomic position (SEP) measures to deal with sample bias introduced by incomplete individual-level measures: inequalities in breast cancer stage at diagnosis as an example. BMC Public Health 19:857. https://doi.org/10.1186/s12889-019-7220-4
Bryere J, Pornet C, Copin N, Launay L, Gusto G, Grosclaude P, Delpierre C, Lang T, Lantieri O, Dejardin O, Launoy G (2017) Assessment of the ecological bias of seven aggregate social deprivation indices. BMC Public Health 17:86. https://doi.org/10.1186/s12889-016-4007-8
Lundqvist A, Andersson E, Ahlberg I, Nilbert M, Gerdtham U (2016) Socioeconomic inequalities in breast cancer incidence and mortality in Europe-a systematic review and meta-analysis. Eur J Public Health 26:804–813. https://doi.org/10.1093/eurpub/ckw070
Rosskamp M, Verbeeck J, Gadeyne S, Verdoodt F, De Schutter H (2021) Socio-economic position, cancer incidence and stage at diagnosis: a nationwide cohort study in Belgium. Cancers 13:933. https://doi.org/10.3390/cancers13050933
Hoebel J, Kroll LE, Fiebig J, Lampert T, Katalinic A, Barnes B, Kraywinkel K (2018) Socioeconomic inequalities in total and site-specific cancer incidence in Germany: a population-based registry study. Front Oncol 8:402. https://doi.org/10.3389/fonc.2018.00402
Shack L, Jordan C, Thomson CS, Mak V, Møller H, UK Association of Cancer Registries (2008) Variation in incidence of breast, lung and cervical cancer and malignant melanoma of skin by socioeconomic group in England. BMC Cancer 8:271. https://doi.org/10.1186/1471-2407-8-271
Redondo-Sánchez D, Marcos-Gragera R, Carulla M, Lopez de Munain A, Sabater Gregori C, Jimenez Chillarón R, Guevara M, Nuñez O, Fernández-Navarro P, Sánchez MJ, Luque-Fernandez MA (2021) Lung, breast and colorectal cancer incidence by socioeconomic status in Spain: a population-based multilevel study. Cancers 13:2820. https://doi.org/10.3390/cancers13112820
Tweed EJ, Allardice GM, McLoone P, Morrison DS (2018) Socio-economic inequalities in the incidence of four common cancers: a population-based registry study. Public Health 154:1–10. https://doi.org/10.1016/j.puhe.2017.10.005
Acknowledgments
We would like to thank those who provided data to the registry: pathologists (CHD Vendée, CH Saint-Nazaire, CHU Nantes, IHP group, Kerlo-Morin laboratory, CRLCC Nantes-Angers, CHU Angers, laboratories from Ille-et-Vilaine and Nouvelle-Aquitaine), oncologists, public and private hospital medical data processing departments, and medical practitioners as well as the medical departments of the national health insurance programs, the screening coordination center and Cancer Regional Network. We would like to thank ERISC/MapInMed platform (French national methodological platform for the study and reduction of health social inequalities in oncology) for the attribution of F-EDI value.
Funding
This work was prepared within the framework of the SIRIC ILIAD program supported by the French National Cancer Institute (INCa), the French Ministry of Health, and the Institute of Health and Medical Research (INSERM); SIRIC ILIAD INCa-DGOS-Inserm_12558 grant. The Loire-Atlantique / Vendee Cancer Registry is supported for the routine collection of data by the French National Cancer Institute (INCa), Santé Publique France, Direction Générale de l’Offre de Soins (DGOS), local institutions (Conseil Régional des Pays de la Loire, ARS des Pays de la Loire, CHU Nantes, CHD Vendée, Institut de Cancérologie de l’Ouest) and the League Against Cancer (comités de Loire-Atlantique et de Vendée).
Author information
Authors and Affiliations
Contributions
FM, ACB had the idea of the study. CD, JMA, SD, SAY, ACB, FM contributed to the conception and design of the analysis. KM, AB, MM collected the data. JMA, GB performed the analysis. CD, JMA, SD, SAY, ACM, FM contributed to the interpretation. The first draft of the work was written by CD and JMA; all authors commented on previous versions of the manuscript. All authors revised it critically for important intellectual content and approved the final version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no competing interests.
Ethical approval
This study is based on data from the Loire-Atlantique/Vendee Cancer Registry, a member of the French network of cancer registries (FRANCIM). It has received the approval of the French regulatory authorities for the collection and analysis of medical data: the Comité Consultatif sur le Traitement de l’Information en matière de Recherche dans le Domaine de la Santé (ethical approval) and the Commission Nationale Informatique et Libertés (legal framework and data protection).
Informed consent
In conformity with French law, patients were informed individually of the nature of the information provided, the purpose of data processing, their right of access, rectification and objection. The ethics committee, in accordance with French law, did not request informed consent.
Research involving in human and animal participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or a comparable ethical standard. All methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Delacôte, C., Ariza, J.M., Delacour-Billon, S. et al. Socioeconomic and geographic disparities of breast cancer incidence according to stage at diagnosis in France. Cancer Causes Control 35, 241–251 (2024). https://doi.org/10.1007/s10552-023-01779-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-023-01779-8