Abstract
Background
Obesity is known to stimulate the mammalian target of rapamycin (mTOR) signaling pathway and both obesity and the mTOR signaling pathway are implicated in breast carcinogenesis. We investigated potential gene-environment interactions between mTOR pathway genes and obesity in relation to breast cancer risk among Black women.
Methods
The study included 1,655 Black women (821 incident breast cancer cases and 834 controls) from the Women’s Circle of Health Study (WCHS). Obesity measures including body mass index (BMI); central obesity i.e., waist circumference (WC) and waist/hip ratio (WHR); and body fat distribution (fat mass, fat mass index and percent body fat) were obtained by trained research staff. We examined the associations of 43 candidate single-nucleotide polymorphisms (SNPs) in 20 mTOR pathway genes with breast cancer risk using multivariable logistic regression. We next examined interactions between these SNPs and measures of obesity using Wald test with 2-way interaction term.
Results
The variant allele of BRAF (rs114729114 C > T) was associated with an increase in overall breast cancer risk [odds ratio (OR) = 1.81, 95% confidence interval (CI) 1.10–2.99, for each copy of the T allele] and the risk of estrogen receptor (ER)-defined subtypes (ER+ tumors: OR = 1.83, 95% CI 1.04,3.29, for each copy of the T allele; ER- tumors OR = 2.14, 95% CI 1.03,4.45, for each copy of the T allele). Genetic variants in AKT, AKT1, PGF, PRKAG2, RAPTOR, TSC2 showed suggestive associations with overall breast cancer risk and the risk of, ER+ and ER– tumors (range of p-values = 0.040–0.097). We also found interactions of several of the SNPs with BMI, WHR, WC, fat mass, fat mass index and percent body fat in relation to breast cancer risk. These associations and interactions, however, became nonsignificant after correction for multiple testing (FDR-adjusted p-value > 0.05).
Conclusion
We found associations between mTOR genetic variants and breast cancer risk as well as gene and body fatness interactions in relation to breast cancer risk. However, these associations and interactions became nonsignificant after correction for multiple testing. Future studies with larger sample sizes are required to confirm and validate these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity plays an important role in breast carcinogenesis [1,2,3,4,5,6]. In the US, Black women have the highest rates of obesity compared to other racial and ethnic groups [7]. In addition, epidemiological data have shown that there are differences in breast cancer risk in women with different patterns of body fat distribution; these associations also differ by estrogen receptor (ER)– defined breast cancer subtypes and menopausal status [8, 9]. In postmenopausal Black women, BMI ≥ 30 kg/m2 is associated with an increased risk of ER-positive (ER+) tumors [7, 10,11,12] and a decreased risk of ER-negative (ER–) and triple-negative breast cancer risk [8]. Evidence from the African American Breast Cancer Epidemiology and Risk (AMBER) consortium is similar; however, central obesity, measured by waist circumference (WC) or waist-to-hip ratio (WHR), is associated with an increased risk of ER+ tumors in premenopausal women and a suggestive increased risk in postmenopausal Black women [8]. WHR is also associated with a suggestively increased risk of ER- and triple-negative breast cancer in premenopausal and postmenopausal Black women [8, 9]. Taken together, the epidemiologic evidence suggests the complexity of body fatness and its influence on breast cancer subtypes. The mechanism commonly thought to affect breast cancer in postmenopausal women is the estrogen synthesized by adipose tissues [13]. However, the association between central obesity and ER– tumors in both pre- and post-menopausal breast cancer suggests that mechanisms other than estrogen such as insulin resistance may explain this relationship [8, 9]. Overactivation of the mammalian target of rapamycin (mTOR) pathway has been implicated as one of the underlying mechanisms of breast cancer [14], as its activity is influenced by nutrients, growth factors (including insulin like growth factors), and hormones to promote cell proliferation and resistance to apoptosis [15]. mTOR is a part of phosphatidylinositol 3-kinase (PI3K) pathway generally involved in cell growth, differentiation, and survival [14, 16]. To date, a few studies have investigated the associations between genetic variants in mTOR pathway and breast cancer risk [17,18,19,20,21,22,23,24,25,26,27]. Specifically, data from the AMBER consortium has delineated some important mTOR and IGF-related genes associated with breast cancer risk [21, 28], including TSC2, BRAF, PGF, MAPK3, RPS6KB2; and BAIAP2, CALM2, CSNK2A1, BAD, MAPK3 [21, 28]. Genetic variants in mTOR signaling pathway have also been found to be associated with other cancer types [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. Given that the mTOR pathway may play a vital role in breast cancer etiology, examining genetic variants in the mTOR pathway may help explain the etiology of breast cancer as well as the impact of obesity on breast cancer risk.
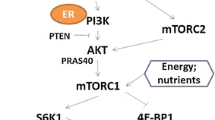
At the cellular level, it is unclear how obesity impacts breast cancer risk. Several mechanisms have been hypothesized including hormonal signaling, inflammation, and insulin resistance, which involves promoting the IGF-PI3K-mTOR axis (Fig. 1) as mTOR pathway is linked to the maintenance of cellular homeostasis through cellular bioenergetics and nutrient availability. Obesity enhances activation of the mTOR signaling pathway which may subsequently promote breast cancer risk [49]. As shown in preclinical data, adipocyte-derived conditioned media activated the mTOR pathway and enhanced the proliferation and migration of breast cancer cells [49]. How obesity influences the mTOR pathway in humans is not fully understood. Joint effects of genetic variants in the mTOR pathway and energy balance on increased bladder cancer risk have been reported [50]. An interaction between obesity and genetic variants in the mTOR pathway in relation to breast cancer risk has been previously reported in White women [20]. A recent study reported an association between genetic variants in the mTOR signaling pathway and breast cancer risk in Black women but did not elucidate how obesity may modify this association [21]. Thus, the evidence on the interaction between obesity and the mTOR pathway remains very limited.
The primary objective of this study was to investigate whether the selected candidate polymorphisms in the mTOR pathway were associated with breast cancer risk in Black women. We examined the association with overall breast cancer risk, ER+ and ER− breast cancer risk separately due to potential differences in the etiology of these subtypes related to obesity [8, 51]. We hypothesized that the selected candidate polymorphisms in the mTOR signaling pathway are associated with breast cancer risk. We further evaluated gene-environment interactions between mTOR pathway candidate genes and obesity measures (body size and body composition) on breast cancer risk overall and by ER-defined subtypes. We hypothesized that the associations of mTOR candidate polymorphisms with breast cancer risk were stronger in women with higher body size and body fat composition than in women with lower body size and body fat composition.
Methods
Study population
Women for these analyses were selected from participants of the Women’s Circle of Health Study (WCHS), described in detail elsewhere [52, 53]. WCHS is a case–control study comprised of two recruitment bases, a hospital-based case ascertainment in New York City and a population-based case ascertainment in New Jersey [52]. The hospital-based case ascertainment in New York City started in 2003 and included women who were between the ages of 20 and 75 years old, with no previous history of cancer other than nonmelanoma skin cancer, who were diagnosed within 9 months with primary, histologically confirmed invasive breast cancer or ductal carcinoma in situ and were English-speaking. In the population-based case ascertainment in New Jersey, cases were identified through rapid case ascertainment by the New Jersey State Cancer Registry. Black women who were less than 75 years of age, diagnosed within 9 months with primary, histologically confirmed invasive breast cancer or ductal carcinoma in situ were eligible for participation. Control eligibility and identification was similar for New York City and New Jersey study bases as women who were between the ages of 20 and 75 years without a history of any cancer diagnosis other than non-melanoma skin cancer were eligible to be controls. Random digit dialing was used to generate controls in New York City while community-based recruitment was used to supplement random digit dialing for sampling controls in New Jersey [53]. Controls were frequency matched to cases by 5-year age groups and race. The in-person interview comprised of the informed consent procedure, an in-depth in-person interview, administration of many behavioral questionnaires, collection of biospecimens and anthropometric measurements. Requests for medical records release, pathology data and tumor tissue release were obtained from cases. Information on ER status was obtained from pathology reports. The current study included 1,655 Black women (821 cases and 834 controls) with available questionnaire, anthropometric, and genetic data. The WCHS protocol was approved by the Institutional Review Boards at Roswell Park Cancer Institute, the Rutgers Cancer Institute of New Jersey, Mount Sinai School of Medicine, and participating hospitals in New York. Signed informed consent was obtained from each participant prior to interview and biospecimen collection. The current study was approved by the University of Florida’s institutional review board.
Anthropometric data collection
Anthropometric measurements were taken at the end of the interview by trained research staff using standardized protocols [50]; participants were asked to wear light clothing. Weight was measured in pounds while standing height was measured once to the nearest 0.1 cm. Body mass index (BMI) was calculated as the measured weight (kg) divided by height (m)2. To minimize measurement error, waist and hip circumferences were measured twice to the nearest 0.1 cm; a third measurement was taken if the difference between the first and second measurement was > 2 cm. The 2 (or 3) measurements were averaged for analyses. Body composition was measured by bioelectrical impedance analysis using a Tanita® TBF-300A scale, and the data were transformed to fat mass in kg, fat mass index, and percent body fat.
SNP selection
We performed a computerized literature search of the PubMed database (2000–2021) and Google search engine to identify all the relevant studies of mTOR candidate polymorphisms and any cancer risk. The search strategy included the following key words: “Candidate polymorphisms in the mTOR pathway and breast cancer risk”, “mTOR genetic variants and breast cancer”, “mTOR genetic variants and breast cancer risk”, “mTOR genetic variants and cancer”, “Candidate polymorphisms in the mTOR pathway and cancer”. The studies selected were required to meet the following criteria: (1) evaluate the associations between mTOR genetic variants and breast cancer risk in Black and/or Non-Black populations; (2) evaluate the associations between mTOR genetic variants and risk of other cancer types in Black and/or Non-Black populations. Data were extracted from all eligible publications and the following information was extracted from each of the included publications: the first author’s name, publication date, gene/SNP name, sample population, cancer type, study type and sample size. We identified 86 SNPs in 38 genes in the mTOR pathway that were significantly associated with breast cancer risk, as well as the risk of other cancer types from the literature. (Supplemental Table 1). Candidate SNPs in this present study are defined as SNPs that were statistically significantly associated with breast cancer risk and other cancers. Out of 86 identified SNPs, we selected a total of 43 candidate SNPs in the 20 mTOR pathway genes (43 SNPs in 20 genes) that were previously genotyped in WCHS for statistical analyses (Supplemental Table 2). WCHS is a subset of AMBER consortium and genotyping procedures have been previously reported [21, 54,55,56,57].
Statistical analyses
Characteristics of cases and controls were compared with t-tests for continuous variables and chi-square tests for categorical variables. Pearson’s correlation coefficients were used to examine correlations between body size and body fat composition measurements. Multivariable logistic regression was used to test the associations between selected candidate SNPs and breast cancer risk while adjusting for age group (18–39, 40–49, 50–59 [as reference] and 60–79 years), BMI (< 25 [as reference], 25– < 30, and ≥ 30 kg/m2), geographic location (New Jersey [as reference] and New York City, DNA source (blood [as reference], mouth wash and saliva) and principal components (PC) of the genotypes (PC5, PC6 and PC8). The largest or normative category was chosen as the reference group for these covariates. These covariates were regarded as known or probable risk factors with the potential to confound the associations between mTOR genetic variants and breast cancer risk. Since menopausal status and education did not change the estimates, they were not included in the final models. The associations were presented as odds ratios (OR) and corresponding 95% confidence intervals (CI). The genetic association analysis tested for an additive model and the genetic variants, i.e., the independent variables, were modeled as 0, 1, or 2 alleles. We examined associations for all breast cancer cases combined as well as separately for ER+ and ER– tumors. Among cases with known ER status, case-only analyses were conducted comparing genetic variants between ER− tumors and ER+ tumors to determine the extent of etiologic heterogeneity in breast cancer cases and tumor subtypes. Statistical significance was defined as nominal p < 0.05 for selected candidate SNPs and all statistical tests were 2-sided. To control for the inflation of false-positive rates from multiple comparisons, we controlled the false discovery rate (FDR). The adjusted p-value with a significance threshold of 0.05 was applied [58].
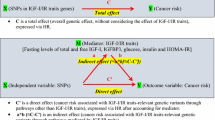
To explore whether associations between mTOR genetic variants and breast cancer were modified by obesity, we conducted stratified analyses by obesity measures defined as BMI; central obesity i.e., waist circumference (WC) and waist/hip ratio (WHR); and body fat distribution (fat mass, fat mass index and percent body fat). BMI was categorized as < 25 (underweight to normal), 25– < 30 (overweight), and ≥ 30 kg/m2 (obese), according to the World Health Organization (WHO) International Classification; WC was categorized as ≤ 88 (normal) and > 88 cm (abdominal obesity) while WHR was categorized as ≤ 0.85 (normal) and > 0.85 (abdominal obesity) [59]. Fat mass (≤ 25.40, > 25.40–≤ 33.80, > 33.80–≤ 44.20 and > 44.20 kg); fat mass index (≤ 9.41, > 9.41– ≤ 12.75, > 12.75–≤ 16.62 and > 16.62 kg/m2) and percent body fat (≤ 35.90, > 35.90–≤ 41.35, > 41.35–≤ 46.50 and > 46.50) were categorized into quartiles based on the distribution in the control group. The Wald test was used to evaluate effect modification, including a 2-way interaction term between the SNPs and effect modifiers (body size and body composition variables). We further conducted a stratification analysis by menopausal status for the gene-environment (body size and body composition variables) interactions in association with overall breast cancer risk. We calculated aggregated genetic risk scores only for mTOR candidate SNPs associated with overall breast cancer with nominal p-values (p < 0.05) and (p < 0.10) and evaluated whether their associations with overall breast cancer were modified by body size and body composition variables. The scores for risk alleles were modeled as 0, 1, or 2 alleles and imputed values were rounded up to the nearest whole number. The scores of all the SNPs were summed and the distribution of the total SNP score was divided into quartiles in multivariable logistic regressions. Statistical significance was defined as nominal p < 0.05 for selected candidate SNPs and all the statistical tests were 2-sided. We controlled the FDR at 0.05 for the multiple hypotheses corrections as before. Statistical analyses were performed using SAS 9.4 software (SAS Institute Inc.).
Results
Characteristics of study sample
Table 1 shows the descriptive characteristics of the study participants. Study participants included 821 cases and 834 controls. Among cases with known ER status, 20.10% were ER– and 52.98% were ER+ tumors. Cases were more likely to be older than controls (52.93, and 51.02 years, p = 0.002). The categories for body size and body composition measures as well as menopausal status did not differ by case–control status.
In our sample, we observed strong positive correlations of BMI with WC, fat mass, fat mass index and percent body fat (range of correlation coefficients, r = 0.81–0.97); WC with fat mass, fat mass index and percent body fat (r = 0.81–0.89) and fat mass with fat mass index and percent body fat (r = 0.99 and 0.91, respectively) (Supplemental Table 3). However, WHR was weakly correlated with BMI, fat mass, fat mass index and percent body fat (range of correlation coefficients, r = 0.25–0.30) while the correlation between WHR and WC was moderate (r = 0.54).
Associations of selected SNPs with breast cancer risk
Table 2 shows the significant associations that were found in the SNP-level association analyses for overall, ER+ and ER– breast cancer as well as in the case-only analysis. The variant allele of BRAF (rs114729114 C > T) was associated with an increase in overall breast cancer risk (OR = 1.81, 95% CI 1.10, 2.99, for each copy of the T allele) as well as the risk of ER + (OR = 1.83, 95% CI 1.04,3.29, for each copy of the T allele) and ER- tumors (OR = 2.14, 95% CI 1.03,4.45, for each copy of the T allele). In addition, some variant alleles showed suggestive associations with overall, ER+ and ER– breast cancer. These associations did not remain significant after correction for multiple testing (FDR-adjusted p-value > 0.05).
Interactions of obesity measures with selected SNPs
Table 3 provides the summary of nominally significant gene-environment (gene-BMI) interactions showing the results of the associations of mTOR candidate SNPs with breast cancer risk stratified by BMI. PRKAG2 (rs2727572 C > T) was associated with an increased risk of overall breast cancer among obese women (OR = 1.51, 95% CI 1.08, 2.11, for each copy of the T allele, p-interaction = 0.046) but not in normal weight and overweight women. For AKT1 (rs1130214 C > A), AKT1 (rs10138227 C > T) and TSC2 (rs181088346 G > A), there was a decreased risk of breast cancer among normal-weight women but not among overweight and obese women (p-interaction = 0.046, 0.030 and 0.035). In contrast, STRADB (rs16837635 A > G) was associated with an increased risk of ER + breast cancer among obese women (OR = 1.56, 95% CI 1.06, 2.30, for each copy of the G allele, p-interaction = 0.035) but not in overweight and normal-weight women. These interactions did not remain significant after correction for multiple testing (FDR-adjusted p-value > 0.05).
Table 4 provides the summary of nominally significant gene-environment (gene-WC) interactions, showing the results of the associations of mTOR candidate SNPs with breast cancer risk stratified by WC. The variant PRKAG2 (rs1104897 C > T) was associated with an increased risk of overall breast cancer among normal WC women (OR = 1.51, 95% CI 1.02, 2.22, for each copy of the T allele, p-interaction = 0.010) but not abdominal obese women. The variant PRKAG2 (rs9632641 A > C) was associated with an increased risk of ER- breast cancer among abdominal obese women (OR = 1.63, 95% CI 1.18, 2.24, for each copy of the T allele, p-interaction = 0.004) but not normal WC women. The variant AKT1 (rs10138227 C > T) was associated with a decreased risk of ER– breast cancer among normal WC women (OR = 0.42, 95% CI 0.21, 0.83, for each copy of the T allele, p-interaction = 0.0256) but not abdominal obese women. These interactions did not remain significant after correction for multiple testing (FDR-adjusted p-value > 0.05).
Table 5 provides the summary of nominally significant gene-environment (gene-WHR) interactions, showing the results of the associations of mTOR candidate SNPs with breast cancer risk stratified by WHR. WHR was an effect modifier for the association of PRKAG2 (rs6464156 A > G) with overall breast cancer (p-interaction = 0.032). The variant PRKAG2 (rs6464156 A > G) was associated with an increased risk of overall breast cancer among abdominal obese women (OR = 1.23, 95% CI 1.01, 1.49, for each copy of the G allele, p-interaction = 0.032) but not normal WHR women. The variant PRKAG2 (rs2727572 C > T) was associated with an increased risk of ER+ breast cancer among abdominal obese women (OR = 1.47, 95% CI 1.02, 2.12, for each copy of the T allele, p-interaction = 0.023) but not normal WHR women.
FRAP1 (rs1057079 C > T) was associated with an increased risk of overall and ER- breast cancer among abdominal obese women (OR = 1.24, 95% CI 0.97, 1.59, for each copy of the C allele, p-interaction = 0.038 and OR = 1.42, 95% CI 0.94, 2.13, for each copy of the C allele, p-interaction = 0.034, respectively) but not normal WHR women. TSC2 (rs2073636 A > G) was associated with an increased risk of overall and ER+ breast cancer among abdominal obese women (OR = 1.28, 95% CI 1.00, 1.64, for each copy of the A allele, p-interaction = 0.084 and OR = 1.61, 95% CI 1.18, 2.20, for each copy of the A allele, p-interaction = 0.015, respectively) but not normal WHR women. PGF (rs11542848 C > T) was associated with an increased risk of ER- breast cancer among abdominal obese women (OR = 1.99, 95% CI 1.21, 3.27, for each copy of the T allele, p-interaction = 0.024) but not normal WHR women. These interactions did not remain significant after correction for multiple testing (FDR-adjusted p-value > 0.05).
Table 6 provides the summary of nominally significant gene-environment (gene-fat mass) interactions, showing the results of the associations of mTOR candidate SNPs with breast cancer risk stratified by fat mass. PI3KCA (rs7651265 A > G) was associated with an increased risk of overall breast cancer among women in Q4 (OR = 1.61, 95% CI 1.00, 2.58, for each copy of the G allele) but not among women in other strata. AKT1 (rs1130214 C > A) was associated with a decreased risk of overall and ER+ breast cancer among women in Q1 (OR = 0.62, 95% CI 0.46, 0.84, for each copy of the A allele and OR = 0.59, 95% CI 0.40, 0.88, for each copy of the A allele, respectively, p-interaction < 0.05) but not among women in other strata. AKT1 (rs2494752 A > G) and AKT1 (rs10138227 C > T) were associated with a decreased risk of ER- breast cancer among women in Q1 (OR = 0.40, 95% CI 0.24, 0.67, for each copy of the A allele, p-interaction = 0.035) but not among women in other strata (p-interaction = 0.035 and 0.029, respectively). These interactions did not remain significant after correction for multiple testing (FDR-adjusted p-value > 0.05).
Supplemental Table 4 provides the summary of nominally significant gene-environment (gene-fat mass index) interactions, showing the results of the associations of mTOR candidate SNPs with breast cancer risk stratified by fat mass index. TSC2 (rs181088346 G > A) was associated with a decreased risk of overall and ER+ breast cancer among women in Q1 (OR = 0.31, 95% CI 0.15, 0.65, for each copy of the A allele, p-interaction = 0.012 and OR = 0.35, 95% CI 0.14, 0.87, for each copy of the A allele, p-interaction = 0.016, respectively) but not among women in other strata. PRKAG2 (rs1104897 C > T) was associated with an increased risk of ER- breast cancer among women in Q1 (OR = 2.06, 95% CI 1.11, 3.82, for each copy of the T allele) and a decreased risk of ER- breast cancer among women in Q3 (OR = 0.51, 95% CI 0.24, 1.11, for each copy of the T allele, p-interaction = 0.0214). These interactions did not remain significant after correction for multiple testing (FDR-adjusted p-value > 0.05).
Supplemental Table 5 provides the summary of nominally significant gene-environment (gene-percent body fat) interactions, showing the results of the associations of mTOR candidate SNPs with breast cancer risk stratified by percent body fat. TSC2 (rs181088346 G > A) was associated with a decreased risk of overall and ER+ breast cancer among women in Q1 (OR = 0.35, 95% CI 0.18, 0.70, for each copy of the A allele, p-interaction = 0.017 and OR = 0.37, 95% CI 0.15, 0.89, for each copy of the A allele, respectively) but not among women in other strata. PRKAG2 (rs7784818 A > G) was associated with a decreased risk of overall breast cancer among women in Q2 (OR = 0.60, 95% CI 0.44, 0.82, for each copy of the G allele) and an increased risk of overall breast cancer among women in Q3 (OR = 1.28, 95% CI 0.96, 1.72, for each copy of the G allele, p-interaction = 0.004). PRKAG2 (rs7784818 A > G) was associated with a decreased risk of ER+ breast cancer among women in Q2 (OR = 0.63, 95% CI 0.43, 0.92, for each copy of the G allele, p-interaction = 0.016) but not among women in other strata. These interactions did not remain significant after correction for multiple testing (FDR-adjusted p-value > 0.05).
Supplemental Table 6 provides the aggregated genetic risk score estimation for the gene-environment (gene-body size and body fat composition) interactions showing the associations of mTOR candidate SNPs with overall breast cancer risk stratified by body size and body fat composition measures. Interactions were observed for BMI, fat mass index and percent body fat. Q4 and Q3 vs Q1 of the aggregated genetic risk score was associated with an increased risk of overall breast cancer among normal weight women (Q4, OR = 2.57, 95% CI 1.21, 5.47; Q3, OR = 1.92, 95% CI 1.04, 3.55) and overweight women (Q4, OR = 3.14, 95% CI 1.66, 5.94; Q3, OR = 1.97, 95% CI 1.21, 3.21, p-interaction = 0.065) but not in obese women. Q4, Q3 and Q2 vs Q1 of the aggregated genetic risk score was associated with an increased risk of overall breast cancer among women in Q2 of fat mass index (Q4, OR = 3.89, 95% CI 1.91, 7.95; Q3, OR = 1.97, 95% CI 1.13, 3.45 and Q2, OR = 2.05, 95% CI 1.21, 3.46, respectively, p-interaction = 0.072) but not in Q3 and Q4 of fat mass index. Q4, Q3 and Q2 vs Q1 of the aggregated genetic risk score was associated with an increased risk of overall breast cancer among women in Q2 of percent body fat (Q4, OR = 4.37, 95% CI 2.05, 9.34; Q3, OR = 3.10, 95% CI 1.76, 5.56 and Q2, OR = 2.25, 95% CI 1.32, 3.85, respectively, p-interaction = 0.019) but not in Q3 and Q4 of percent body fat.
Supplemental Tables 7–12 provides the results of the stratification analysis by menopausal status for the gene-environment (body size and body composition) interactions in association with overall breast cancer risk. PRKAG2 rs1104897 (C > T) was associated with an increased overall breast cancer risk in premenopausal women with normal weight, normal WC and in Q1 of fat mass but not in postmenopausal women. PRKAG2 rs7784818 (A > G) was associated with a decreased risk of overall breast cancer risk in premenopausal women in Q2 of fat mass index and percent body fat but not in postmenopausal women. AKT1 rs10138227 (C > T) was associated with a decreased overall breast cancer risk in postmenopausal women with normal weight but not in premenopausal women. PI3KCA rs7640662 (C > G) was associated with a decreased overall breast cancer risk in postmenopausal women with abdominal WC and in Q4 of percent body fat but not in premenopausal women. These interactions did not remain significant after correction for multiple testing (FDR-adjusted p-value > 0.05).
Discussion
In this sample of Black women enrolled in the WCHS, we found significant associations of several SNPs within mTOR pathway with the risk of breast cancer, overall and in ER+ and ER– tumors. Some of these associations were limited to specific tumor subtypes only. We also found significant interactions of several of the SNPs with BMI, WHR, WC, fat mass, fat mass index and percent body fat in relation to breast cancer risk. These associations and interactions did not remain statistically significant after correction for multiple testing and thus should be interpreted with caution.
Epidemiological literature on the associations of mTOR pathway SNPs and breast cancer in Black population is limited. Our finding that BRAF rs114729114 was significantly associated with an increase in overall, ER+ and ER– breast cancer risk is consistent with the previously reported results. We have previously shown that BRAF rs114729114 was associated with an increase in overall, ER+ and ER– breast cancer, among Black women in the AMBER consortium of 3663 cases and 4687 controls, inclusive of WCHS participants [21]. The BRAF gene encodes a protein in the RAF family of serine/threonine protein kinases that regulates the MAP kinase (MAPK)/ERK signaling pathway which influences cell growth, division and differentiation [60]. Activated MAPK pathway has been linked to breast cancer [60]. Activated MAPK in wild-type MCF-7 breast cancer cells have been shown to be hypersensitive to the proliferative effects of estradiol after long-term deprivation of estrogen, highlighting the importance of interactions between the MAPK and ER pathways in mediating cell proliferation [61]. MAPK signaling is activated in response to both growth factors and cellular stress and have had a long-standing implication in endocrine and chemotherapy resistance in breast cancer [61]. Intronic SNPs PGF rs11542848 and PGF rs61759375 were associated with increased risk of ER-tumors in AMBER [21]. We found that PGF rs61759375 was associated with a suggestive increased risk of ER- breast cancer in our current study. Note, however, that the study participants in our current study were a subset of AMBER consortium, so this comparison should be interpreted cautiously. However, associations of mTOR pathway SNPs and breast cancer in other populations have been extensively reported [17,18,19,20, 23,24,25,26, 62,63,64]. There are complex reasons responsible for the potential differences in the associations between genetic variants in mTOR pathway and breast cancer risk in Black women when compared to associations in women of other racial groups from previous studies. Minor allele frequencies and linkage disequilibrium (LD) structures differ by racial groups [20, 21]. Racial difference in obesity phenotype and breast cancer subtypes is also implicated. Evidence shows that Black women have the highest rates of obesity compared to other races in the US [7]. Given that mTOR pathway is activated by positive energy imbalance- an obesity-related phenomenon [65], associations between mTOR genetic variants and breast cancer risk may differ among Black women and women of other racial groups. Black women have a higher percentage of ER- tumors than other races. Therefore, research is needed to investigate gene-environment interactions involving obesity phenotypes and breast cancer subtypes to further understand the influence of mTOR pathway genes on breast cancer risk.
Gene-environment interaction
In this study, we found interactions of BMI, WC, WHR, fat mass, fat mass index and percent body fat with genetic variants of mTOR pathway related genes including AKT, AKT1, FRAP1, MAPK3, MTOR, PRKAG2, PI3KCA, PIK3CA, PGF, RAPTOR, STRADB, and TSC2 in relation to breast cancer risk in Black women.
Data on gene-environment interactions involving the interactions of mTOR pathway SNPs and obesity in relation to breast cancer risk are very limited. Previous analysis of WCHS data observed no effect modification of BMI and WHR with mTOR genetic variants in association with breast cancer in Black women but effect modification of BMI was observed for FRAP1 rs12125777 in White women only [20]. The study had a smaller sample size compared to our current study.
In our study, PRKAG2 rs2727572 interacted with BMI, WC and WHR. The stratified results generally showed an increased risk for overall breast cancer and ER+ breast cancer in obese women. PRKAG2 rs2727572 is an intronic coding gene located in a region with strong transcriptional enhancers in mammary epithelial cells [66]. PRKAG2 rs7784818, an intronic SNP interacted with fat mass index and percent body fat with a decreased risk of overall and ER+ breast cancer in normal weight women and an increased risk of overall breast cancer in obese women, findings that supported our study hypothesis. The regulatory function of PRKAG2 rs7784818 is unclear. These genetic variants in PRKAG2 were found to be associated with an increased risk of colon and rectal cancer [45]. PRKAG2 rs9632641 and PRKAG2 rs6464156, intronic SNPs interacted with WC and WHR respectively with an increased risk of ER- and overall breast cancer in obese women, findings that supported our hypothesis. PRKAG2 rs9632641 was associated with increased colon cancer risk [45]. The genetic variants in PRKAG2 gene have not been reported in studies of breast cancer risk and warrant replication.
In the present study, AKT1 rs1130214 interacted with BMI, fat mass and fat mass index with decreased risk of overall breast cancer and ER+ breast cancer in normal weight women, which is in the same direction with our hypothesis. Based on ENCODE data, AKT1 rs1130214 is a 5’-UTR genetic variant found in regions with weak transcriptional enhancers in mammary epithelial cells and DNase hypersensitivity sites in mammary gland (adenocarcinoma) [66]. AKT1 rs1130214 has also been mapped in regions with active transcriptional enhancers in breast myoepithelial cells [66]. AKT1 rs1130214 was associated with HER2-positive breast cancer in Sri-Lankan women [19]. AKT1 rs10138227 interacted with BMI, WC and fat mass with decreased risk of overall breast cancer and ER- breast cancer in normal weight women, which supports our study hypothesis. Based on ENCODE data, AKT1 rs10138227 is a 5’-UTR genetic variant found in regions with weak transcriptional enhancers in mammary epithelial cells and DNase hypersensitivity sites in mammary gland (adenocarcinoma) [66]. AKT1 rs10138227 has also been mapped in regions with active transcriptional enhancers in breast myoepithelial cells [66]. AKT1 rs2494752 showed interactions with WC and fat mass with decreased risk in ER- breast cancer in normal weight women, which also supports our hypothesis. The functional annotation for AKT1 rs2494752 is unknown but the SNP is found in regions with weak transcriptional enhancers in breast myoepithelial cells and DNase hypersensitivity sites in mammary gland (adenocarcinoma) [66]. AKT1 rs2494752 was associated with an increased risk of breast cancer in a Chinese population [26].
In our study, we observed that STRADB (rs16837635), an intronic SNP interacted with BMI with increased risk of ER+ breast cancer in obese women which supports our hypothesis. Our finding is consistent with the literature. In the ROOT consortium study, STRADB rs16837635 was associated with an increased risk of ER+ breast cancer in women of African Ancestry [22]. PGF rs11542848 interacted with WHR with an increased risk of ER- breast cancer in obese women, which supports our hypothesis. We have previously shown that intronic SNP PGF rs11542848 was associated with increased risk of ER-tumors in Black women in the AMBER consortium, which also included our current study participants [21]. Based on ENCODE data, PGF rs11542848 is a 5'-UTR SNP located in regions with active transcriptional promoters in mammary epithelial cells and hepatocellular carcinoma. It has been mapped in regions with active TSS in breast myoepithelial cells [66].
We observed that higher versus lower category of aggregated genetic risk score for the mTOR candidate genes generally were associated with increased risk of overall breast cancer among women who are normal weight and overweight as well as in women in lower quartiles of body fat composition measures, which did not entirely support our study hypothesis. The exact reason for the directions of association in the different strata of body size and body composition measures is unclear. A hypothesis is that there is potential attenuation of predictive performance of aggregated genetic risk scores in populations with African ancestry [67].
Our stratification analysis by menopausal status showed that the directions of association between body size and body fat composition measures with overall breast cancer differed in pre- and postmenopausal women, implying potential differences in disease etiology. AKT1 rs10138227 (C > T) was associated with a decreased overall breast cancer risk in post-menopausal women with normal weight, which supports our hypothesis. PRKAG2 rs1104897 (C > T) was associated with an increased overall breast cancer risk in premenopausal women with normal weight, normal WC and in Q1 of fat mass while PI3KCA rs7640662 (C > G) was associated with a decreased overall breast cancer risk in post-menopausal women with abdominal WC and in Q4 of percent body fat, which were not in the direction of our study hypothesis. These study findings should however be interpreted with caution, given that the current stratification analysis by menopausal status may lack adequate statistical power. Taken together, the epidemiologic evidence suggests the complexity of body fatness and its interplay with menopausal status and overall breast cancer risk. Our study had several strengths. To our knowledge, this is the first study to comprehensively evaluate gene-environment interactions involving the interactions of mTOR pathway candidate genes and obesity measured as body size and body compositions on breast cancer risk in Black women, a population with the highest rate of obesity. It had a relatively large sample size of Black women which enabled analysis of risk for overall breast cancer, as well as for ER+ and ER− cancer separately. The information on breast cancer subtypes aided a better understanding of breast cancer etiology and contributed to the limited knowledge for the population of Black women. The study analyzed the effect of obesity phenotype on breast cancer risk in Black women using comprehensive measurements of body size and body fat composition. Most anthropometric measurements were taken by trained staff and were thus less prone to measurement error than self-report. The large panel of genes used in the study covered a detailed mTOR signaling pathway and genes were available for the selected candidate SNPs that were reported in the literature.
The study has a few limitations. First, our findings require validation, as the SNP-level associations and gene-environment interactions were not significant after correction for multiple tests. Thus, interpretation of the study findings with caution is warranted. Second, there is lack of generalization of study findings to other racial and ethnic groups as the study only used Black women participants. Third, there is still a possibility for residual confounding potentially due to unmeasured variables despite the study aiming to adjust for important confounders in the statistical analysis. We did not have information on comorbidities, but these variables may not have a significant effect on the result estimates. Another potential limitation is that the anthropometric measurements occurred after diagnosis and participants were asked about their weight 1 year prior to diagnosis. We, however, observed good correlation between BMI determined by self-report and BMI measured by study staff. This suggests that body size was relatively stable before and after diagnosis for this study. The difference in ascertainment of controls in New York City and New Jersey may raise concerns regarding bias due to systematic over-enumeration of controls in New Jersey but the sampled controls were representative of the same populations from which the cases were derived [53].
In conclusion, we observed associations of several genetic variants within mTOR pathway with risk of breast cancer, overall, and ER+ and ER– tumors. We additionally found interactions of several of the SNPs with BMI, WHR, WC, fat mass, fat mass index and percent body fat in relation to breast cancer risk. The study findings suggest that mTOR genetic variants may impact breast cancer risk in Black women. Body size and body composition may modify the associations between mTOR genetic variants and breast cancer risk in Black women. Therefore, there may be an interplay between mTOR genetic variants, body size and body composition on breast cancer risk in Black women. Validations for these observed associations and effect modifications are required. Our findings may be clinically meaningful because it will inform changes in lifestyle factors especially in Black women with existing germline encoded polymorphisms in the mTOR pathway, which would to a greater extent reduce breast cancer risk. Studies with larger sample size of black women are needed to validate our findings. Future studies should examine the functional consequence of the mTOR genetic variants.
Data availability
Data will be made available on request.
References
Ramos-Nino ME (2013) The role of chronic inflammation in obesity-associated cancers. ISRN Oncol 2013(697521):1–25
Simpson ER, Brown KA (2013) Obesity and breast cancer: Role of inflammation and aromatase. J Mol Endocrinol 51:3
Simone V, D’Avenia M, Argentiero A, Felici C, Rizzo FM, De PG et al (2016) Obesity and breast cancer: Molecular interconnections and potential clinical applications. Oncologist 21:404–417
Macciò A, Madeddu C (2011) Obesity, inflammation, and postmenopausal breast cancer: Therapeutic implications. ScientificWorldJournal 11:2020–2036
Iyengar NM, Hudis CA, Dannenberg AJ (2013) Obesity and inflammation: New insights into breast cancer development and progression. Am Soc Clin Oncol Educ B 33:46–51
Ervin RB (2009) Prevalence of metabolic syndrome among adults 20 years of age and over by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Report 13:2003–2006
Center Office of Minority Health Resource. (2020) Obesity and African Americans. Available from: https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=25. Accessed 18 Feb 2021
Bandera EV, Chandran U, Hong C-C, Troester MA, Bethea TN, Adams-Campbell LL et al (2015) Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER consortium. Breast Cancer Res Treat 150(3):655–666
Houghton SC, Eliassen H, Tamimi RM, Willett WC, Rosner BA, Hankinson SE (2021) Central adiposity and subsequent risk of breast cancer by menopause status. JNCI J Natl Cancer Inst 113(7):900–908
Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study : cohort study. BMJ 335(7630):1134.
Kolb R, Sutterwala FS, Zhang W (2016) Obesity and cancer: inflammation bridges the two. Curr Opin Pharmacol 29(1):77–89
Rose DP, Vona-Davis L (2009) Influence of obesity on breast cancer receptor status and prognosis. Expert Rev Anticancer Ther 9(8):1091–1101
Bhardwaj P, Au CC, Benito-martin A, Ladumor H, Moges R, Brown KA et al (2020) Estrogens and breast cancer: mechanisms involved in obesity- related development, growth and progression. J Steroid Biochem Mol Biol 189(646):161–170
Liu P, Cheng H, Roberts TM, Zhao JJ (2011) Targeting the phosphoinositide 3-kinase (PI3K) pathway in cancer. Nat Rev Drug Discov 8(8):627–644
Weijenberg MP, Hughes LAE (2013) The mTOR pathway and the role of energy balance throughout life in colorectal cancer etiology and prognosis: Unravelling mechanisms through a multidimensional molecular epidemiologic approach. Curr Nutr Rep 2:19–26
Altomare DA, Khaled AR (2012) Homeostasis and the importance for a balance between AKT/mTOR activity and intracellular signaling. Curr Med Chem 19(22):3748–3762
De AME, Erhart G, Buck K, Mu E, Hubalek M, Fiegl H et al (2013) Polymorphisms in the gene regions of the adaptor complex LAMTOR2/LAMTOR3 and their association with breast cancer risk. PLoS ONE 8(1):1–8
Mehta MS, Vazquez A, Kulkarni DA, Kerrigan JE, Atwal G, Metsugi S et al (2013) Polymorphic variants in TSC1 and TSC2 and their association with breast cancer phenotypes. Breast Cancer Res Treat 125(3):1–15
Sirisena ND, Adeyemo A, Kuruppu AI, Samaranayake N, Dissanayake VHW (2018) Genetic variants associated with clinicopathological profiles in sporadic breast cancer in Sri Lankan women. J Breast Cancer 21(2):165–172
Cheng TYD, Shankar J, Zirpoli G, Roberts MR, Hong CC, Bandera EV et al (2017) Genetic variants in the mTOR pathway and interaction with body size and weight gain on breast cancer risk in African-American and European-American women. Cancer Causes Control 27(8):965–976
Cheng TD, Ambrosone CB, Hong C, Lunetta KL, Liu S, Hu Q et al (2015) Genetic variants in the mTOR pathway and breast cancer risk in African American women. Carcinogenesis 00(2):1–7
Wang S, Huo D, Ogundiran TO, Ojengbede O, Nathanson KL, Nemesure B et al (2017) Association of breast cancer risk and the mTOR pathway in women of African ancestry in ‘The Root ’ Consortium. Carcinogenesis 38(8):789–796
Campa D, Claus R, Dostal L, Stein A, Karina JC, Benetou V et al (2011) Variation in genes coding for AMP-activated protein kinase (AMPK) and breast cancer risk in the European Prospective Investigation on Cancer (EPIC). Breast Cancer Res Treat 127:761–767
Zhao Y, Diao Y, Wang X, Lin S, Wang M, Kang H (2016) Impacts of the mTOR gene polymorphisms rs2536 and rs2295080 on breast cancer risk in the Chinese population. Oncotarget 7(36):58174–58180
Ren H, Wang X, Lin S (2014) Associations between C1772T Polymorphism in Hypoxia-Inducible Factor-1 a Gene and Breast Cancer: A Meta-Analysis. Med Sci Monit 20:2578–2583
Li X, Zhang R, Liu Z, Li S, Xu H (2017) The genetic variants in the PTEN/PI3K/AKT pathway predict susceptibility and CE(A)F chemotherapy response to breast cancer and clinical outcomes. Oncotarget 8(12):20252–20265
Wang Y, Zhang H, Lin M, Wang Y (2018) Association of FGFR2 and PI3KCA genetic variants with the risk of breast cancer in a Chinese population. Cancer Manag Res 10:1305–1311
Ruiz-narváez EA, Lunetta KL, Hong C, Haddad S, Yao S, Cheng TD et al (2016) Genetic variation in the insulin, insulin-like growth factor, growth hormone, and leptin pathways in relation to breast cancer in African-American women: the AMBER consortium. npj Breast Cancer 2:16034
Wang LE, Ma H, Hale KS, Yin M, Meyer LA, Liu H et al (2012) Roles of genetic variants in the PI3K and RAS/RAF pathways in susceptibility to endometrial cancer and clinical outcomes. J Cancer Res Clin Oncol 138(3):377–385
Lin J, Wang J, Greisinger AJ, Grossman HB, Forman MR, Dinney CP et al (2010) Energy balance, the PI3K-AKT-mTOR pathway genes, and the risk of bladder cancer. Cancer Prev Res 3(4):505–517
Ge Y, Liu H, Qiu X, Ma G, Wang H, Du M et al (2018) Genetic variants in PI3K/Akt/mTOR pathway genes contribute to gastric cancer risk. Gene 670:130–135. https://doi.org/10.1016/j.gene.2018.05.093
Xue W, Wang M, Zhang L, Gu J, Zhu X, Wang Y et al (2018) Genetic variants within MTORC1 genes predict gastric cancer prognosis in Chinese populations. J Cancer 9(8):1448–1454
Piao Y, Li Y, Xu Q, Liu JW, Xing CZ, Xie XD et al (2015) Association of mTOR and AKT gene polymorphisms with susceptibility and survival of gastric cancer. PLoS ONE 10(8):1–19
Xu M, Tao G, Kang M, Gao Y, Zhu H, Gong W et al (2013) A polymorphism (rs2295080) in mTOR promoter region and its association with gastric cancer in a Chinese population. PLoS ONE 8(3):e60080
Chen J, Shao P, Cao Q, Li P, Li J, Cai H et al (2012) Genetic variations in a PTEN/AKT/mTOR axis and prostate cancer risk in a Chinese population. PLoS ONE 7(7):1–8
Li Q, Gu C, Zhu Y, Wang M, Yang Y, Wang J et al (2013) Polymorphisms in the mTOR gene and risk of sporadic prostate cancer in an Eastern Chinese Population. PLoS ONE 8(8):e71968
Crezee T, Petrulea M, Piciu D, Jaeger M, Smit JWA, Plantinga TS et al (2020) AKT1 genetic variants confer increased susceptibility to thyroid cancer. Endocr Connect 9(11):1065–1074
Xu X, Qian D, Liu H, Cruz D, Luo S, Walsh KM et al (2019) Genetic variants in the liver kinase B1-AMP-activated protein kinase pathway genes and pancreatic cancer risk. Mol Carcinog 58(8):1338–1348
Zhu M, Yu H, Shi T, He J, Wang M, Li Q et al (2013) Polymorphisms in mTORC1 genes modulate risk of esophageal squamous cell carcinoma in Eastern. JTO Acquis 8(6):788–795. https://doi.org/10.1097/JTO.0b013e31828916c6
Wang Z, Fu H, Li W (2018) Association between AKT rs2494752 single nucleotide polymorphism and the risk of metastasis in hepatocellular carcinoma. Oncol Lett 16(3):3699–3705
Chen M, Cassidy A, Gu J, Delclos GL, Zhen F, Yang H et al (2009) Genetic variations in PI3K-AKT-mTOR pathway and bladder cancer risk. Carcinogenesis 30(12):2047–2052
Bizhani F, Hashemi M, Danesh H, Nouralizadeh A, Narouie B, Bahari G et al (2018) Association between single nucleotide polymorphisms in the PI3K/ AKT/ MTOR pathway and bladder cancer risk in a sample of IRANIAN population. EXCLI J 17:3–13
Lee SJ, Kang BW, Chae YS, Kim HJ, Park SY, Park JS et al (2014) Genetic variations in STK11, PRKAA1, and TSC1 associated with prognosis for patients with colorectal cancer. Ann Surg Oncol 21(4):634–639
Allam L, Arrouchi H, Ghrifi F, El KA, Kandoussi I, Bendahou MA et al (2020) AKT1 polymorphism (rs10138227) and risk of colorectal cancer in Moroccan population: A case control study. Asian Pacific J Cancer Prev 21(11):3165–3170
Slattery ML, Herrick JS, Lundgreen A, Fitzpatrick FA, Curtin K, Wolff RK (2010) Genetic variation in a metabolic signaling pathway and colon and rectal cancer risk: mTOR, PTEN, STK11, RPKAA1, PRKAG2, TSC1, TSC2, PI3K and Akt1. Carcinogenesis 31(9):1604–1611
Cao Q, Ju X, Li P, Meng X, Shao P, Cai H et al (2012) A functional variant in the MTOR promoter modulates its expression and is associated with renal cell cancer risk. PLoS ONE 7(11):1–8
Qi L, Sun K, Zhuang Y, Yang J, Chen J (2017) Study on the association between PI3K/AKT/mTOR signaling pathway gene polymorphism and susceptibility to gastric cancer. J BUON 22(6):1488–1493
Zheng W, Wu C, Wu X, Cai Y, Liu B, Wang C (2021) Genetic variants of autophagy-related genes in the PI3K/Akt/mTOR pathway and risk of gastric cancer in the Chinese population. Gene. https://doi.org/10.1016/j.gene.2020.145190
Park J, Kang S, Ahn KS, Um J, Yang WM, Yun M (2020) Inhibition of the PI3K-AKT-mTOR pathway suppresses the adipocyte-mediated proliferation and migration of breast cancer cells. J Cancer 11(9):2552–2559
Lin J, Wang J, Greisinger AJ, Grossman HB, Michele R, Dinney CP et al (2011) Energy balance, the PI3K-AKT-mTOR pathway genes and the risk of bladder cancer. Cancer Prev Res (Phila) 3(4):505–517
Bandera EV, Chandran U, Zirpoli G, Gong Z, Mccann SE, Hong C et al (2013) Body fatness and breast cancer risk in women of African ancestry. BMC Cancer 13:475
Ambrosone CB, Ciupak GL, Bandera EV, Jandorf L, Bovbjerg DH, Zirpoli G et al (2009) Conducting molecular epidemiological research in the age of HIPAA: A multi-institutional case-control study of breast cancer in African-American and European-American women. J Oncol 2009:871250
Bandera EV, Chandran U, Zirpoli G, Mccann SE, Ciupak G, Ambrosone CB (2013) Rethinking sources of representative controls for the conduct of case – control studies in minority populations. BMC Med Res Methodol 13(1):1
Sanderson M, Lipworth L, Shen-miller D, Nechuta S (2015) Energy-related indicators and breast cancer risk among White and Black women. PLoS ONE 10(4):8–9
Stolk L, Perry JR, Chasman DI, He C, Mangino M, Sulem P, Barbalic M, Broer L, Byrne EM, Ernst F, Esko T, Franceschini N, Gudbjartsson DF, Hottenga JJ, Kraft P, McArdle PF, Porcu E, Shin SY, Smith AV, van Wingerden S, Zhai G, Zhuang WV, Albrecht E, Alizade LK (2012) Meta-analyses identify 13 novel loci associated with age at menopause and highlights DNA repair and immune pathways. Nat Genet 44(3):260–268
Elks CE, Perry JR, Sulem P, Chasman DI, Franceschini N, He C, Lunetta KL, Visser JA, Byrne EM, Cousminer DL, Gudbjartsson DF, Esko T, Feenstra B, Hottenga JJ, Koller DL, Kutalik Z, Lin P, Mangino M, Marongiu M, McArdle PF, Smith AV, Stolk L, van Wingerden MA (2011) Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat Genet 42(12):1077–1085
Patterson N, Price AL, Reich D (2006) Population structure and eigenanalysis. PLoS Genet. https://doi.org/10.1371/journal.pgen.0020190
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate : A practical and powerful approach to multiple testing. Publi J R Stat Soc 57(1):289–300
World Health Organization.(2011) Waist circumference and waist–hip ratio: Report of a WHO expert consultation. Geneva 8–11; 2011. Available from: https://www.who.int/publications/i/item/9789241501491
National Library of Medicine. BRAF B-Raf proto-oncogene, serine/threonine kinase [ Homo sapiens (human). Available from: https://www.ncbi.nlm.nih.gov/gene/673. Accessed 5 Aug 2022
Yue WEI, Wang J, Conaway M, Masamura S, Li Y, Santen RJ et al (2002) Activation of the MAPK pathway enhances sensitivity of MCF-7 breast cancer cells to the mitogenic effect of estradiol. Endocrinology 143(9):3221–3229
Yan Q, Chen P, Wang S, Liu N, Zhao P, Gu A (2014) Association between HIF-1 α C1772T / G1790A polymorphisms and cancer susceptibility: an updated systematic review and meta-analysis based on 40 case-control studies. BMC Cancer 14(950):1–16
López-cortés A, Leone PE, Freire-paspuel B, Arcos-villacís N, Guevara-ramírez P, Rosales F et al (2018) Mutational analysis of oncogenic AKT1 gene associated with breast cancer risk in the high altitude Ecuadorian Mestizo population. Biomed Res Int. https://doi.org/10.1155/2018/7463832
Li Y, Li C, Shi H, Lou L, Liu P (2015) The association between the rs11549465 polymorphism in the hif-1α gene and cancer risk : a meta-analysis. Int J Clin Exp Med 8(2):1561–1574
Cheng TD, Omilian AR, Yao S, Sanchez PV, Polk LZ, Zhang W et al (2020) Body fatness and mTOR pathway activation of breast cancer in the Women’s Circle of Health Study. npj Breast Cancer 6(45):1–9
Broad Institute. HaploReg v3 [Internet]. Available from: https://pubs.broadinstitute.org/mammals/haploreg/haploreg_v3.php Accessed 9 May 2022
Shen H, Gelaye B, Meijsen J, Ressler K, Feldman M, Peterson R et al (2019) Analysis of polygenic risk score usage and performance in diverse human populations. Nat Commun 10:3328. https://doi.org/10.1038/s41467-019-11112-0
Funding
This work was supported by grants from the US National Institutes of Health, The National Cancer Institute (Grant Numbers P01 CA151135 J.R.P. and C.B.A, R01CA098663 to J.R.P.; R01 CA100598 to C.B.A. and E.V.B; R01 CA185623, P30 CA016056, P30 CA072720, K07 CA201334, R37 CA248371); the Breast Cancer Research Foundation (C.B.A., C-CH).
Author information
Authors and Affiliations
Contributions
Study conception and design: MNI, TYC. Data acquisition: JRP, SY, CCH, EVB, CBA. Writing—initial draft: MNI. Data analysis: MNI. Data interpretation: MNI, TYC, LY. Contributed to the statistical methods: KLL, TYC, LY, SD, JZ. Revised the paper: MNI, TYC, LY, SD, JZ, GZ, EVB, SY. Writing—final review and approval: all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The WCHS protocol was approved by the Institutional Review Boards at Roswell Park Cancer Institute, the Rutgers Cancer Institute of New Jersey, Mount Sinai School of Medicine, and participating hospitals in New York. The current study was approved by the University of Florida’s institutional review board.
Consent to participate
Signed informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ilozumba, M.N., Yaghjyan, L., Datta, S. et al. mTOR pathway candidate genes and obesity interaction on breast cancer risk in black women from the Women’s Circle of Health Study. Cancer Causes Control 34, 431–447 (2023). https://doi.org/10.1007/s10552-022-01657-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-022-01657-9