Abstract
Objective
To characterize the epidemiological trends and sociodemographic variation of pediatric and adolescent neurological cancers by histological subtypes over time in the USA.
Methods
A total of 16,511 patients aged 0–19 years diagnosed with neurological cancers between 2000 and 2018, including 13,024 with central nervous system (CNS) neoplasms and 3,487 with neuroblastomas, were identified from 18 registries of the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program. Incidence trends over time and incidence rate ratios by race/ethnicity, sex, and age were calculated for histological subtype. Age-standardized incidence rates (ASIR) by year of diagnosis and average annual percent changes (AAPC) were calculated to measure incidence rates. ASIR by race/ethnicity, sex, and age were calculated to examine the incidence variation by these factors.
Results
Overall, age-standardized annual incidence per 100,000 person-years increased from 2.20 in 2000 to 3.21 in 2018 with an AAPC of 1.4% (95% confidence interval or CI: 0.5% to 2.4%); however, that of Hispanic decreased from 2.93 in 2000 to 2.59 in 2018 with an AAPC of − 0.8% (95% CI: − 1.2% to − 0.3%). Non-Hispanic Black children and adolescents had a statistically significantly lower incidence than non-Hispanic White peers both for CNS neoplasms (incidence rate ratio or IRR: 0.67; 95% CI: 0.63 to 0.71) and neuroblastomas (IRR: 0.75; 95% CI: 0.68 to 0.83). Females generally had a lower incidence than males, especially among those with intracranial and intraspinal embryonal tumors (IRR: 0.69; 95% CI: 0.64 to 0.75). The highest incidence rate of neuroblastoma was among newborns aged less than 1 year, and the highest incidence rate of CNS neoplasms was among children aged 1–4 years.
Conclusion
The incidence of neurological cancers has increased among children and adolescents from 2000 to 2018, with wide variation across demographic groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Central nervous system (CNS) neoplasms and neuroblastoma are common pediatric neurological cancers worldwide [1]. In the USA (U.S.), CNS neoplasms are the second most common cancer following leukemia among children aged 0–14 years, and the most common cancer among adolescents aged 15–19 years, accounting for 21–26% of all pediatric and adolescent cancer cases [2]. Each year, approximately 3,100 US children and adolescents younger than age 20 years are diagnosed with neurological cancer in the brain, spinal cord, or other parts of the nervous system [3]. Moreover, CNS neoplasms are the leading cause of pediatric cancer death, with a mortality rate of approximately 0.7 per 100,000 children per year [4, 5].
Several studies have suggested the possibility of substantial variation in pediatric neurological cancer incidence by region, race/ethnicity, and sex [6,7,8]. However, the research about the incidence of neurological cancer across a long-time horizon and across the full spectrum of neurological cancer types among children and adolescents is not sufficient [9, 10]. A comprehensive investigation into the patterns of neurological cancer incidence in the pediatric and adolescent populations is crucial to better understand the incidence profiles of these diseases and to inform cancer control efforts in this vulnerable population.
This study used Surveillance, Epidemiology, and End Results (SEER) registry data from 2000 through 2018 to examine the incidence and trends of pediatric and adolescent malignant neurological tumors by race/ethnicity, sex, age group, and subtypes of cancer.
Methods
Data and sample
The SEER program is a collection of population-based cancer registries in the USA. SEER collects patients’ demographic and clinical characteristics, including date of cancer diagnosis, age at diagnosis, sex, race/ethnicity, primary tumor site, and histology. We used data from 18 SEER registries, which began collecting data on cancer cases diagnosed in 2000. Our dataset included all cancer cases from 2000 to 2018.
We identified 16,753 children and adolescents aged ≤ 19 years who were diagnosed with a first primary malignant neurological cancer. Cases confirmed by death certificate or autopsy were excluded (n = 54; 0.32%). After further excluding 188 cases (1.12%) with unknown race, 16,511 patients were included in our final analytic sample.
Cancer histological types were classified using the third edition of the International Classification of Childhood Cancer (ICCC-3) [11] and include CNS neoplasms and miscellaneous intracranial and intraspinal neoplasms, including the subtypes of (a) ependymomas and choroid plexus tumors, (b) astrocytomas, (c) intracranial and intraspinal embryonal tumors, (d) other gliomas, (e) other specified intracranial and intraspinal neoplasms, and (f) unspecified intracranial and intraspinal neoplasms and neuroblastoma and other peripheral nervous cell tumors (neuroblastoma), including the subtypes of (i) neuroblastoma and ganglioneuroblastoma and (ii) other peripheral nervous cell tumors.
Our study was conducted by analyzing the de-identified, publicly available SEER data. Therefore, our study does not constitute human subjects research and its institutional review board review was waived because there was no interaction with any individual and no identifiable private information was used.
Statistical analysis
We calculated age-standardized incidence rates by sex and race/ethnicity for each histology type, using the SEER 2000 US standard population. Age-standardized incidence rates were also calculated for five age groups (< 1 year, 1–4 years, 5–9 years, 10–14 years, and 15–19 years) [12] and for five race and ethnicity groups (non-Hispanic White, non-Hispanic Black, non-Hispanic American Indian/Alaska Native, non-Hispanic Asian/Pacific Islander, and Hispanic), both stratified by sex (male and female). Non-Hispanic American Indian/Alaska Native and Non-Hispanic Asian/Pacific Islander were combined when trend of neurological cancer was analyzed as sample sizes were too small for separate analysis. We estimated incidence rate ratios (IRRs) calculated with Poisson regression and 95% confidence intervals (CIs) and examined temporal trends in incidence rate. We estimated yearly age-standardized incidence rates from 2000 to 2018 and calculated the annual percent change (APC) and average annual percent change (AAPC) during the entire period, using the Joinpoint Regression Program with permutations on a logarithmic scale and a maximum of 4 joinpoints. Two-sided tests were used with a statistical significance level of 0.05.
Results
Sample characteristics
Among children and adolescents diagnosed with CNS neoplasms (n = 13,024) or neuroblastoma (n = 3,487), the majority were non-Hispanic White (57.71%), male (53.40%), and diagnosed at age 1–9 years (67.34%; Table 1). Most patients (78.88%) were diagnosed with CNS neoplasms, of which astrocytomas, intracranial and intraspinal embryonal tumors, and other gliomas accounted for 50.08%, 20.79%, and 17.23%, respectively. Neuroblastoma and ganglioneuroblastoma accounted for over 97.05% of all the neuroblastoma and other peripheral nervous cell tumors.
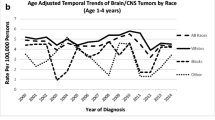
The distributions of sex and histological subtype were generally consistent across the years. From 2000 to 2018, the age-adjusted incidence rate of non-Hispanic White was continuously the highest among all race and ethnicity groups, while the age-adjusted incidence rates of other races and ethnicities were relatively close to each other despite some fluctuations. The proportion of non-Hispanic White patients decreased from 60.74% in 2000–2004 to 55.64% in 2015–2018, while the proportion of non-Hispanic American Indian/Alaska Native patients increased from 0.56% in 2000–2004 to 1.07% in 2015–2018 (Table 1).
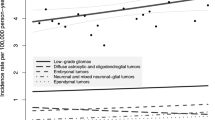
When stratified by race and ethnicity, the age-standardized incidence rates in non-Hispanic American Indian/Alaska Native and non-Hispanic Asian/Pacific Islander patients increased statistically significantly, from 2.20 in 2000 to 3.21 in 2018 (AAPC: 1.4%; 95% CI: 0.5% to 2.4%; Fig. 1A). While the age-standardized incidence per 100,000 person-years in Hispanic had a downward trend from 2.93 in 2000 to 2.59 in 2018 (APC: − 0.8%; 95% CI: − 1.2% to − 0.3%; Fig. 1A).
Trends in pediatric neurological cancer in the USA from 2000 to 2018. Trends were analyzed using the Joinpoint Regression Program, with a maximum of 4 Joinpoints (i.e., 5-line segments). APC Annual percent change, AAPC Average annual percent change, NHW non-Hispanic White, NHB non-Hispanic Black, NHAI/AN/API non-Hispanic American Indian, Alaska Native, Asian, and Pacific Islander. *Indicates that the Annual Percent Change is statistically significantly different from zero at the level of α = 0.05
Age-standardized incidence rate ratios by race, sex, and age group
Compared with non-Hispanic White children, non-Hispanic Black, non-Hispanic American Indian/Alaska Native, non-Hispanic Asian/Pacific Islander, and Hispanic children had statistically significantly lower incidence rates in both CNS neoplasms (IRR [95% CI]: 0.67 [0.63, 0.71], 0.64 [0.52, 0.77], 0.60 [0.56, 0.65], and 0.65 [0.62, 0.67], respectively; Fig. 2A) and neuroblastoma (IRR [95% CI]: 0.75 [0.68 to 0.83], 0.56 [0.36 to 0.83], 0.68 [0.60 to 0.78], and 0.54 [0.49 to 0.58], respectively; Fig. 2F). And conditions were observed rather consistently in both sexes (Fig. 2A, F) and across CNS neoplasm and neuroblastoma subtypes (Fig. 2B–E, G), but not always reaching statistical significance (Fig. 2).
Because of a small number of non-Hispanic American Indian or Alaska Native pediatric patients of neurological cancer in the registries, wider confidence intervals of the IRRs were obtained for these subgroups. Although there were not always significant statistical differences, non-Hispanic Black pediatric patients of neurological cancer had a relatively lower age-adjusted incidence rate than Hispanic pediatric patients with astrocytomas, other gliomas, or neuroblastoma and ganglioneuroblastoma (Fig. 2C–F; Table 2). Yet a higher age-adjusted incidence rate of Hispanic children with ependymomas and choroid plexus tumor, or with intracranial and intraspinal embryonal tumors, was discovered, compared to non-Hispanic Black children in both sex groups (Fig. 2B, D; Table 2). Similar patterns of non-Hispanic Black children were observed, when compared with non-Hispanic Asian/Pacific Islander children, except for the groups with Intracranial and intraspinal embryonal tumors (Fig. 2B–E, G; Table 2).
Female patients generally had a lower incidence rate than males for children and adolescents with CNS neoplasms (IRR [95% CI]: 0.90 [0.87–0.93]; Fig. 3A; Table 2) and in the patient group with intracranial and intraspinal embryonal tumors (IRR [95% CI]: 0.69 [0.64–0.75]; Fig. 3D; Table 2). For other subtypes such as ependymomas and choroid plexus tumor, the difference between males and female did not reach statistical significance (Fig. 3; Table 2).
Age-adjusted incidence rate ratios by cancer type and sex. 95% confidence intervals (CI) were obtained using F-intervals test. NHW non-Hispanic White, NHB non-Hispanic Black, NHAI/AN/API non-Hispanic American Indian, Alaska Native, Asian, and Pacific Islander. 95% CI bars for AI/API are not fully shown because of the wide ranges
In the age group comparison, while children aged 1–4 years had the highest incidence rate of CNS neoplasms and astrocytomas (Fig. 4A, C; Table 3), newborns (aged less than 1 year) had the highest rate of ependymomas and choroid plexus tumor, intracranial and intraspinal embryonal tumors, and neuroblastoma neoplasm (Fig. 4B–G; Table 3). In addition, the incidence rates of CNS neoplasm subtypes were the highest among newborns or those aged 1–4 years, decreasing gradually with increased age group (Fig. 4; Table 3). In contrast, the incidence rate for neuroblastomas was the highest among newborns, with a sharp decrease in those aged 1–4 years, then dropping to nearly 0 for the older age groups (Fig. 4F, G; Table 3).
Discussion
To our knowledge, this study provides the first updated US population-based estimates of the incidence and trends in neurological cancers among children and adolescents from 2000 to 2018 and by race/ethnicity, sex, and age group. We discovered that for children and adolescents aged 0–19 years, the adjusted incidence rate of neurological cancers was relatively stable during the study period. We also found that the incidence of neurological cancers was, on average, higher in non-Hispanic White children than in non-White children and higher among children aged ≤ 4 years, particularly newborns, than among older children and adolescents. With the largely unknown etiology of pediatric neurological cancer, these observed disparities in incidence suggest a role of environmental factors, perinatal exposures (e.g., unhealthy diet, physical inactivity, and radiation), and genetic-related factors [13].
We found that the incidence of malignant CNS cancer for most children and adolescents was stable from 2000 to 2018. Compared to the years before 2000, current health care systems in the USA bring more reliable cancer diagnosis and relative stable cancer detection [9]. The factors that contribute to this change include the improved cancer detection and diagnosis [14], the development of magnetic resonance imaging and computerized tomography, a wider range of diagnostic categories caused of enhanced imaging and accessibility [9, 15], the use of molecular markers to supplement morphological evidence [15], and the more frequent, accurate objective of monitoring, diagnosis, and reporting of tumor susceptibility syndrome by more radiotherapists and pathologists [15].
Consistent with previous studies [16, 17], we found males to be at a higher incidence rate of a neurological tumor diagnosis compared to females. There are sex differences in susceptibility to a wide range of brain diseases; for example, gene expression is sexually dimorphic during brain development. Early findings indicate that the epigenetic mechanism is partly responsible for differences in susceptibility between males and females and is an important feature of a series of neurological and psychiatric diseases [18]. The excess of male observed for medulloblastoma may be contributed by sex differences in neurological development and by microenvironment [19]. Biologic mechanisms, such as germline variation and gene expression on the X and autosomal chromosomes, immune responses, pubertal hormone profiles, and the corresponding growth rates may link to sex difference of pediatric neurological tumors as well [20, 21]. Further research is required to fully understand these differences.
Our analysis revealed that compared with children of other races and ethnicity, non-Hispanic White children had a significantly higher incidence rate for neurological cancers [22]. There are two potential explanations for these observed racial differences. First, compared to minority children, non-Hispanic White children may experience better access to healthcare [23, 24] and, therefore, may have been more likely to be diagnosed by the improved cancer detection technology [25]. Second, the genetic susceptibility of neurological cancers may vary by race. Genetic research has identified specific variation among individuals of European and African ancestry [26], and proportionally more deleterious genetic variation was found in European than in African populations [27]. Future research should investigate the root causes of the observed racial variation in pediatric and adolescent cancer incidence. An optimistic view that better understanding of the role of genetic variation in cancer incidence rate may potentially lead to more precise treatment for patients with different racial background is held by some researchers; however, some researchers hold not-so-optimistic view at the same time [28].
Compared with older children and adolescents, those under the age of 4 had a greater incidence for malignant neurological cancers. In particular, there was substantial variation in incidence between newborns and those aged 5–19 years, which may potentially be explained by two factors [29,30,31]. First, neuroblastoma and ganglioneuroblastoma are types of sympathetic embryonal carcinoma [32], which starts in very early forms of nerve cells, most often found in an embryo or fetus. These subtypes have a median onset age of 19 months [33] and are the most common tumors in the first year of life, with a very high incidence among those aged under 5 years [33]. Notably, this type of tumor could often resolve spontaneously in newborns, and the high level of medical vigilance, including screening, in developed countries may have led to diagnoses of tumors that would otherwise resolve. Individuals of high socioeconomic status receiving advanced medical services may have higher medical vigilance and are more likely to be over-diagnosed, thereby discovering tumors that would have subsided [33]. In the case of neuroblastoma where the fraction of spontaneously regressing tumors is comparable to the fraction of tumors clinically detectable through screening [33], it becomes hard to use variation in incidence by calendar period or race to suggest risk factors for this tumor.
Second, perinatal exposures to genotoxicants and carcinogens may increase the risk for certain childhood cancers [34]. In particular, prenatal exposures to acetaldehyde, butadiene, and toluene have been found to be positively associated with pediatric neurological cancers [13]. Radiation exposure from the increased use of electronic equipment may also affect the formation and differentiation of neural stem cells during embryonic development, potentially causing damage to the nervous system [35].
Our study has the following limitations. Despite a large sample overall, the sample sizes for specific racial minority groups and rare subtypes were relatively small, limiting the statistical power to detect differences. Due to a lack of adequate information collected by the SEER database, we were unable to measure the effect of immigration status, socioeconomic status, and other specific environmental and lifestyle exposures on neurological cancer incidence among children and adolescents.
Conclusions
Very few common risk factors are known, and the small variation in incidence over time is not suggestive of one or more overlooked important unknown predictors (including modifiable lifestyles) of neurological childhood cancer. This suggests that neurological childhood cancer is mainly a random phenomenon, helped along by genetic factors, maybe interaction with the environment. The distribution of neurological childhood cancer by sex and race suggests that further predictors of these cancers may be found, primarily involving genetic factors.
Data availability
Data are available from the public Surveillance, Epidemiology, and End Results (SEER) registry data.
Code availability
Codes are available from the corresponding author on reasonable request.
References
Mathew RK, O’Kane R, Parslow R et al (2014) Comparison of survival between the UK and US after surgery for most common pediatric CNS tumors. Neuro Oncol 16:1137–1145
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7–30
Huynh M, Marcu LG, Giles E, Short M, Matthews D, Bezak E (2018) Current status of proton therapy outcome for paediatric cancers of the central nervous system—analysis of the published literature. Cancer Treat Rev 70:272–288
Ward E, Jemal A, Cokkinides V et al (2004) Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin 54:78–93
Jaimes C, Poussaint TY (2019) Primary neoplasms of the pediatric brain. Radiol Clin North Am 57:1163–1175
Kong Z, Wang Y, Dai C, Yao Y, Ma W, Wang Y (2018) Central nervous system germ cell tumors: a review of the literature. J Child Neurol 33:610–620
Garcia CR, Slone SA, Dolecek TA, Huang B, Neltner JH, Villano JL (2019) Primary central nervous system tumor treatment and survival in the United States, 2004–2015. J Neurooncol 144:179–191
Tish S, Habboub G, Jones J et al (2019) The epidemiology of central and extraventricular neurocytoma in the United States between 2006 and 2014. J Neurooncol 143:123–127
Withrow DR, de Gonzalez AB, Lam CJK, Warren KE, Shiels MS (2019) Trends in pediatric central nervous system tumor incidence in the United States, 1998–2013. Cancer Epidemiol Biomark Prev 28:522–530
Linabery AM, Ross JA (2008) Trends in childhood cancer incidence in the U.S. (1992–2004). Cancer 112:416–432
Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P (2005) International classification of childhood cancer, third edition. Cancer. 103:1457–67
Institute NC (2021) Childhood cancers
von Ehrenstein OS, Heck JE, Park AS, Cockburn M, Escobedo L, Ritz B (2016) In utero and early-life exposure to ambient air toxics and childhood brain tumors: a population-based case-control study in California, USA. Environ Health Perspect 124:1093–1099
Kohler BA, Ward E, McCarthy BJ et al (2011) Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst 103:714–736
Gittleman HR, Ostrom QT, Rouse CD et al (2015) Trends in central nervous system tumor incidence relative to other common cancers in adults, adolescents, and children in the United States, 2000 to 2010. Cancer 121:102–112
Wong-Siegel JR, Johnson KJ, Gettinger K et al (2017) Congenital neurodevelopmental anomalies in pediatric and young adult cancer. Am J Med Genet A 173:2670–2679
Williams LA, Richardson M, Marcotte EL, Poynter JN, Spector LG (2019) Sex ratio among childhood cancers by single year of age. Pediatr Blood Cancer 66:e27620
Qureshi IA, Mehler MF (2010) Chapter 6—genetic and epigenetic underpinnings of sex differences in the brain and in neurological and psychiatric disease susceptibility. In: Savic I (ed) Progress in brain research. Elsevier, Amsterdam, pp 77–95
Ruigrok AN, Salimi-Khorshidi G, Lai MC et al (2014) A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev 39:34–50
Dunford A, Weinstock DM, Savova V et al (2017) Tumor-suppressor genes that escape from X-inactivation contribute to cancer sex bias. Nat Genet 49:10–16
Klein SL, Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol 16:626–638
Marcotte EL, Domingues AM, Sample JM, Richardson MR, Spector LG (2021) Racial and ethnic disparities in pediatric cancer incidence among children and young adults in the United States by single year of age. Cancer 127:3651–3663
Elster A, Jarosik J, VanGeest J, Fleming M (2003) Racial and ethnic disparities in health care for adolescents: a systematic review of the literature. Arch Pediatr Adolesc Med 157:867–874
Hambidge SJ, Emsermann CB, Federico S, Steiner JF (2007) Disparities in pediatric preventive care in the United States, 1993–2002. Arch Pediatr Adolesc Med 161:30–36
Friedrich P, Itriago E, Rodriguez-Galindo C, Ribeiro K (2017) Racial and ethnic disparities in the incidence of pediatric extracranial embryonal tumors. J Nat Cancer Inst. https://doi.org/10.1093/jnci/djx050
Tennessen JA, Bigham AW, O’Connor TD et al (2012) Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science (New York, N.Y.) 337:64–69
Lohmueller KE, Indap AR, Schmidt S et al (2008) Proportionally more deleterious genetic variation in European than in African populations. Nature 451:994–997
Joyner MJ, Paneth N, Ioannidis JP (2016) What happens when underperforming big ideas in research become entrenched? JAMA 316:1355–1356
Mazewski CM, Hudgins RJ, Reisner A, Geyer JR (1999) Neonatal brain tumors: a review. Semin Perinatol 23:286–298
Steliarova-Foucher E, Colombet M, Ries LAG et al (2017) International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol 18:719–731
Georgakis MK, Karalexi MA, Kalogirou EI et al (2017) Incidence, time trends and survival patterns of childhood pilocytic astrocytomas in Southern-Eastern Europe and SEER, US. J Neuro-Oncol 131:163–175
Tulla M, Berthold F, Graf N et al (2015) Incidence, trends, and survival of children with embryonal tumors. Pediatrics 136:e623–e632
Georgakis MK, Dessypris N, Baka M et al (2018) Neuroblastoma among children in Southern and Eastern European cancer registries: variations in incidence and temporal trends compared to US. Int J Cancer 142:1977–1985
Anderson LM (2006) Environmental genotoxicants/carcinogens and childhood cancer: bridgeable gaps in scientific knowledge. Mutation Res/Genet Toxicol Environ Mutagen 608:136–156
Kaplan S, Deniz OG, Önger ME et al (2016) Electromagnetic field and brain development. J Chem Neuroanat 75:52–61
Funding
National Nature Science Foundation of China (Grant No. 20003560 by YK) and Natural Science Foundation of Shandong Province (Grant No. ZR2020MH340 by YK).
Author information
Authors and Affiliations
Contributions
YK had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; concept and design: XH and BZ; acquisition, analysis, or interpretation of data: YK, XJ, XH, and BZ; drafting of the manuscript: YK and XJ; critical revision of the manuscript for important intellectual content: YK, XJ, XH, and BZ; statistical analysis: YK; administrative, technical, or material support: YK, XJ, XH, and BZ; and supervision: BZ and XH. The authors have no conflicts of interest to disclose.
Corresponding authors
Ethics declarations
Conflict of interest
None reported.
Ethical approval
This study was conducted by analyzing the de-identified, publicly available SEER data and does not constitute human subjects research. Its institutional review board review was, therefore, waived.
Consent for publication
All authors consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kong, Y., Ji, X., Han, X. et al. Pediatric neurological cancer incidence and trends in the United States, 2000–2018. Cancer Causes Control 33, 687–699 (2022). https://doi.org/10.1007/s10552-021-01535-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-021-01535-w