Abstract
Pilocytic astrocytomas (PA) comprise the most common childhood central nervous system (CNS) tumor. Exploiting registry-based data from Southern and Eastern Europe (SEE) and SEER, US, we opted to examine incidence, time trends, survival and tentative outcome disparities of childhood PA by sociodemographic and clinical features. Childhood PA were retrieved from 12 SEE registries (N = 552; 1983–2014) and SEER (N = 2723; 1973–2012). Age-standardized incidence rates (ASR) were estimated and survival was examined via Kaplan–Meier and Cox regression analysis. ASR of childhood PA during 1990–2012 in SEE was 4.2/106, doubling in the USA (8.2/106). Increasing trends, more prominent during earlier registration years, were recorded in both areas (SEE: +4.1 %, USA: +4.6 %, annually). Cerebellum comprised the most common location, apart from infants in whom supratentorial locations prevailed. Age at diagnosis was 1 year earlier in SEE, whereas 10-year survival was 87 % in SEE and 96 % in SEER, improving over time. Significant outcome predictors were age <1 year at diagnosis diagnosis (hazard ratio, HR [95% confidence intervals]: 3.96, [2.28–6.90]), female gender (HR: 1.38, [1.01–1.88]), residence in SEE (HR: 4.07, [2.95–5.61]) and rural areas (HR: 2.23, [1.53–3.27]), whereas non-cerebellar locations were associated with a 9- to 12-fold increase in risk of death. The first comprehensive overview of childhood PA epidemiology showed survival gains but also outcome discrepancies by geographical region and urbanization pointing to healthcare inequalities. The worse prognosis of infants and, possibly, females merits further consideration, as it might point to treatment adjustment needs, whereas expansion of systematic registration will allow interpretation of incidence variations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pilocytic astrocytomas (PA) represent 20 % of total central nervous system (CNS) tumors among children (0–14 years) [1], comprising the most common histological subtype in this age group [2, 3]. They are characterized by special histopathological features, and are classified as of borderline behavior tumors due to their considerably good prognosis (International Classification of Diseases for Oncology, third edition; ICD-O-3) [4].
Despite their high incidence, there is a paucity of population-based studies on the epidemiology of childhood PA. The latest annual age-standardized incidence rate (ASR) in the US is 9.3 per million children [2], whereas studies from the UK and Switzerland, report annual ASRs of 7.5 (1995–2003) and 8.3 (1980–1994) per million, respectively [5, 6]. Increasing incidence trends have been reported in the US during the last decades [7, 8], whereas studies in Europe, referring to astrocytomas in general, have also shown overall increasing patterns [9–11].
The excellent prognosis of childhood PA following surgical resection is reflected in a 10-year survival exceeding 90 % [2, 5, 12]. Despite their treatable nature, however, disparities in outcome have been recorded even between countries availing high health coverage and quality healthcare [13]. Tumor-specific characteristics, like location, percentage of resection, leptomeningeal dissemination and pathologic features have been reported to influence prognosis [14], whereas less is known on survival disparities by sociodemographic variables.
To this end, we opted to comprehensively explore the epidemiology of childhood PA using population-based data from an informal cancer registries network in Southern and Eastern Europe (SEE 1983–2014) and the Surveillance Epidemiology and End Results Program (SEER 1973–2012), US. Exploiting the largest to-date primary dataset on childhood PA, we sought to assess incidence, and temporal trends, as well as, to describe basic demographic (age, gender) and clinical (topography) characteristics. More importantly, we aimed to unveil potential predictors of disease outcome and seek for cross-country disparities, probably reflecting healthcare system inequalities.
Methods
Participating registries
The informal SEE network, established in the context of the EUROCOURSE project [11, 15–17], includes 14 childhood cancer registries (Belarus, Bulgaria, Croatia, Cyprus, Greece, Malta, Central Portugal, North Portugal, Romania-Cluj, Northeast Romania, Serbia, Slovenia, Ukraine, Turkey-Izmir) operating in 12 countries and coordinated by the Nationwide Registry of Childhood Hematological Malignancies and Brain Tumors (NARECHEM-BT) in Greece [11, 17]. Individual registries, provided primary data on incident CNS tumors diagnosed during variable registration periods expanding from 1983 to 2014.
Additionally, following signing of a Research Data Agreement, data on childhood CNS tumors were extracted from the SEER database, covering 18 cancer registries across US during 1973–2012 [18].
Pilocytic astrocytomas ascertainment
Morphology/behavior
All CNS tumors were codified by morphology and behavior using ICD-O-3 [19] and were classified according to the International Classification for Childhood Cancer, third edition (ICCC-3) [20]; PA cases (morphology code 9421) were thereafter extracted.
According to ICD-O-3, established in 2001, PA are classified as tumors of uncertain behavior, whereas precedent classifications considered them malignant [19]. Serbia and Cyprus, collecting solely malignant tumors, were excluded from analyses, whereas Ukraine starting registration in 2001 confirmed the non-systematic collection of PA and was, thus, excluded from incidence analysis; yet, Ukrainian data were retained in survival analysis given a random recording of PA cases. Bulgaria, despite pertaining to malignant tumors, confirmed that it maintained systematic collection of PA after the classification change. In SEER, PA were systematically collected as malignant tumors until 2001; although registration of non-malignant tumors officially started in 2004, an informal ongoing registration of PA was preserved in SEER during 2001–2004.
Registry definition of PA, might include either morphologically verified cases, or less usually cases clinically defined without available histological examination or cases defined solely by the death certificate. Nevertheless, non-histologically verified cases might definitely be missed in the NOS categories.
Topography
Topography was coded via the International Statistical Classification of Diseases-10th Edition (ICD-10) [21] and was classified to supratentorial site (C71.0–C71.5, C75.1–C75.3), cerebellum (C71.6), optic nerve (C72.3), brainstem (C71.7), spinal cord (C72.0), tumors in overlapping (C71.8) locations and brain PA of unspecified topography (C71.9).
Place of residence
SEE registries, except for Croatia, provided information on place of residence, classified as urban, semi-urban, rural. The classification was different for each country and was based on the respective guidelines of the National Statistical Services, which have already taken into account the special needs of each country’s population [22]. For comparability with SEER classification, a dichotomization to rural and urban place of residence (incorporating “urban” and “semi-urban” categories) was applied.
Follow-up data
Survival, as an endpoint, was assessed on the basis of date of diagnosis, date and status at last contact or lost to follow-up date. As all-cause mortality is negligible in children 0–14 years, the observed survival closely reflects the disease outcome. Death certificate only (DCO) and lost to follow-up cases were excluded from survival analysis.
Statistical analysis
Crude incidence rates (CIRs) by age group (0–4, 5–9, 10–14 years) and age-standardized incidence rates (ASR) per million children, using the World (Segi) standard population, were calculated for PA. Annual percent changes (APC) of incidence rates were estimated using Poisson regression analysis. Given the low numbers of PA cases in individual SEE registries and the consequent inadequacy to unveil temporal trends, incidence rates and time trends were also estimated for all participating SEE registries combined during the periods 1990–2012 and 2000–2012 [23], when the majority of registries were active; for SEER estimations pertained to the periods 1973–2012, 1990–2012, 2000–2012. Joinpoint regression analyses were performed to identify potential breaks in trends, whereas trends of astrocytomas NOS (ICD-O-3 coding: 9400) were also examined to explore tentative improvement of classification over time.
Consequently, Kaplan–Meier analysis was conducted for the overall sample, as well as stratified by age group, gender, topography, geographical region and diagnostic period and cumulative survivals for the 6-month, 1–3-, 5- and 10-year periods since diagnosis were calculated. Lastly, Cox proportional-hazard models were designed encompassing age, gender, diagnostic period and topography in a core model and subsequently, geographical region and place of residence interchangeably. SEE and SEER data were combined in the main analysis, as to increase statistical power, but due to the profound differences in survival between the two regions, stratified analyses were also performed. Cox analysis was repeated stratified by geographical region, excluding Ukranian data, and restricted to cases diagnosed after 1990 and after 2000.
SAS software (V9.4, SAS Institute Inc), Joinpoint Regression Program (V4.1.1, National Cancer Institute) and STATA (V13.0, StataCorp) were used for statistical analyses.
Results
Descriptive registry characteristics
SEE registries amounted 552 PA cases (1983–2014), whereas 2,723 PA cases were derived from the SEER database (1973–2012). Characteristics of the participating registries along with quality indicators [24] are presented in Supplemental Table S1. Out of the 12 SEE registries, seven have nationwide coverage, whereas SEER covers 29 % of childhood US population. No DCO cases were identified in SEE and only one in SEER and morphologically verified (MVs) cases comprised 97 % of the total in both areas. PA represented 41.5 % of astrocytomas in SEE and 55.0 % in SEER, accounting for 19.0 and 25.2 % of all childhood CNS tumors, respectively. By contrast, astrocytomas NOS cases represented 22.6 % of astrocytomas among SEE, with significant cross-registry disparities and 29.7 % in SEER. The vast majority of cases (98 %) were followed-up and therefore included in the survival analysis; mean follow-up duration was 8.8 years. Details on the registries included in each analysis are presented in Fig. 1.
Incidence rates and time trends
The combined ASR of PA (Table 1) in SEE in 1990–2012 was 4.2/106 children increasing to 5.1/106 during the most recent 2000–2012 period. In SEER, PA incidence was 7.1/106 during the entire 1973–2012 registration period and twice as high (8.4/106) compared to SEE during 1990–2012. Statistically significant increasing trends were recorded in both areas (annually, SEE: +4.1 %, 1990–2012; SEER: +4.6 %, 1973–2012); yet, the Joinpoint analysis revealed time-points when the rapid increase in incidence was smoothed. In particular, in SEER the 11.1 % annual increase until 1995 was followed by a smaller increase of 1.3 %, whereas in SEE registries a break in 1997 was noted, when the pronounced until then, annual rise of 17.8 % was substituted by a non-significant 1.1 % increasing pattern. Examination of the temporal trends in PA as compared to those of astrocytoma NOS (Supplemental Figure S1) revealed mirror temporal patterns in SEER (PA +4.6 %, astrocytomas NOS −4.3 %). On the other hand, the PA increase in SEER was accompanied by a rather stable incidence of astrocytomas NOS.
Demographic and clinical characteristics
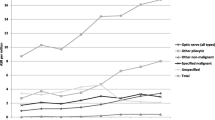
PA cases were evenly distributed by age group and gender (male-to-female ratio: 1.02; Table 2); compared to SEE, however, age at diagnosis was lower in SEER (6.8 years vs. 7.7 years, p < 0.001). Regarding tumor topography, PA were most commonly (36.5 %) located in cerebellum, followed by supratentorial locations (21.8 %). Brainstem PA represented 10.9 %, whereas tumors of the optic nerve and the spinal cord accounted for <10 % of cases. Brain PA of overlapping locations were more common in SEE. A differential topography pattern, however, emerged for infants (Fig. 2); particularly, a lower proportion of cerebellar (7.4 %) and brainstem (5.5 %) tumors was observed, as opposed to the preponderance of supratentorial (31.5 %) and optic nerve tumors (20.4 %); brain PA of unspecified topography were also more common in infants (30.2 %). No gender differences in tumor topography were noted (p = 0.82).
Survival analysis
Supplemental Table S2 shows the Kaplan–Meier derived survival in SEE and SEER during different time intervals. Although cumulative 10-year survival approached 95 % reflecting the rather curable nature of PA, significant disparities between SEE and SEER were noted with the former presenting poorer outcomes (10-year survival 87 % vs. 96 %, p < 0.001). Among SEE registries, only Belarus had a 10-year survival >90 %, whereas the highest 5-year survival (95.4 %) was recorded in Greece, which, however, availed data only for the most recent registration period (2009–2014).
PA presented significantly lower survival among infants in SEER (p < 0.001; Fig. 3a), as well as a marginally lower survival in SEE (p = 0.09; Fig. 3b), whereas no significant difference was found by gender (Fig. 3c, d). Tumors located in cerebellum had an excellent 10-year survival (99 %), which was significantly higher compared to non-cerebellar PA in both SEE and SEER (p < 0.001; Fig. 3e, f).
Improvements in PA outcome were evident for both SEE and SEER over registration periods (Supplemental Figure S2). In SEER the improvement in the already high 5-year survival was limited to the period until 1990, being stably >95 % thereafter. On the contrary, the increase in 5-year survival in SEE registries showed a significant increase from <80 % before 1995, to 94 % in the latest registration years. Improvements were restricted to non-cerebellar tumors.
The multivariate Cox regression analysis (Table 3) confirmed findings of the crude Kaplan–Meier analysis. Specifically, age at diagnosis <1 year, compared to 10–14 years (HR: 3.96, 95 % CI: 2.28–6.90) and female gender (HR: 1.38, 95 % CI: 1.01–1.88) were associated with higher risk of death, whereas occurrence of PA in any other location, compared to cerebellum, was associated with considerably worse outcome (9- to 12-fold increased risk of death). After introducing geographical region, children diagnosed in SEE, compared to the US, had a significantly fourfold increased risk of death. Irrespective of country, however, rural residence was sizably associated with worse outcome (HR: 2.23, 95 % CI: 1.53–3.27). The effects were similar in both SEE registries and SEER, except for an attenuation of the effect of age at diagnosis <1 year in SEE, possibly because of the low number of cases in this age group, as well as a non-significant effect of rural residency in the SEE registries. Restricting analyses to cases diagnosed after 1990 or after 2000, did not materially change the results, neither did stratification by SEE/SEER or exclusion of the Ukrainian data (data not shown).
Discussion
Epidemiologic features, including incidence, time trends and survival patterns of childhood PA were studied exploiting a dataset of 3,275 incident cases in SEE and SEER, US. A considerably higher incidence of PA was estimated for SEER compared to SEE registries, whereas the increasing trends during precedent decades seemed to stabilize in most recent periods. More than one-third of PA were located in cerebellum, followed by supratentorial locations, except for infants, among whom supratentorial and optic nerve tumors prevailed. Overall 10-year survival reached a high 95 %, increasing over registration period, whereas age <1 year at diagnosis, female gender, non-cerebellar location, rural place of residence and residence in SEE countries were associated with worse outcome from this, otherwise non-malignant, tumor.
There is a paucity of published data in Europe on the incidence of childhood PA; yet, studies from England (ASR: 7.5/106; 1995–2003) and Switzerland (8.3/106; 1980–1994) show higher rates approaching those of the US [5, 6]. In the current study the overall lower incidence in SEE (4.2/106 in 1990–2012; 5.1/106 after 2000) compared to SEER (8.4/106 in 1990–2012; 9.1/106 after 2000) could be attributed to underreporting and registration gaps; specifically, as PA comprise a treatable tumor, usually managed outside oncology departments, they could have slipped registration in SEE registries, which have been initiated most recently and may not avail an extensive network for complete registration. Furthermore, the recent behavior change in ICD-O-3 could have led to modification of registration policies adopted with variable delays. The younger-by 1 year- age at diagnosis in SEER may also indicate earlier tumor identification, possibly on account of better healthcare delivery system; in this context, some PA in SEE, despite their development during the conventional childhood period could have been diagnosed after 14 years leading to a phenomenal decrease of childhood incidence.
Increasing temporal trends, yet attenuated after 2000, were recorded in both SEE and SEER. Regarding SEER, the increasing trend was opposite to the decrease of astrocytomas NOS, indicating improvements in diagnostic classification of CNS tumors over time [8]. The rates of astrocytomas NOS in SEE registries remained, however, stable, possibly implying welcome improvements in registration processes [8, 25, 26]. Besides registry improvements, advances in neuroimaging modalities, especially the wide use of MRI, could be responsible for these trends. Indeed, the trends are in accordance with an overall temporal increase in childhood CNS tumor incidence in developed countries [9], and contrasted to the stable trends in countries of lower socioeconomic status [27, 28]; the diagnostic improvements have been suggested as the main contributors to these observations [29]. If this stipulation were genuine for PA, disease diagnosis at an earlier time due to use of imaging methods would have been expected to result in a more pronounced increase among younger children; such a trend has not been noted, though, in this study. It has lastly been suggested that the increase in childhood astrocytomas could be rather real due to exogenous environmental factors, not yet identified [26, 30].
Prognosis of PA reached a high 94.5 % 10-year survival, which significantly increased from 79 to 94 % since 1990 in SEE, whereas in SEER remained stable over 95 % thereafter. The cerebellar PA have a diachronic excellent prognosis and survival gains pertain exclusively to outcome improvements of non-cerebellar tumors. In fact, it is now well-established that gross-total resection of PA is a major predictor of outcome [31], with the greater amount of resection leading to higher possibility of cure [32]. The diagnostic advancements and the improved access to healthcare delivery, leading to earlier diagnosis and, thus, surgery with higher probabilities of total resection, along with management improvements through development of pediatric neurosurgery and establishment of specified childhood CNS tumors centers have contributed to survival gains. The considerable improvement in SEE is also in accordance with the overall rather declining trends of malignant CNS tumors mortality and improved survival over time, which was recently reported for the same area [17]. Significant outcome disparities between the less affluent SEE countries and the US were found; similar disparities, impacting on prognosis of childhood CNS tumors between European regions, as well as between UK and the US have been described [10, 13, 33]. Apart from the availability of specified pediatric neuro-oncological centers in the US, differences in treatment-related factors including type of adjuvant therapy, aggressiveness of relapse treatment and supportive care could partly explain the discrepancy. Completeness of registration should be also taken into account, however, in assessing the SEER vs. SEE survival discrepancies. In particular, if less aggressive tumors are more likely not to be registered in SEE due to their management in non-oncology departments, then a falsely worse prognosis might emerge.
Intriguingly, rural residence, considered as proxy of healthcare access [17, 34], was associated with a twofold increased risk of death in our study. Previous studies have shown similar worse outcomes for other childhood and adult tumors [35, 36]; rural residence would be expected to either prolong the time needed for diagnosis or impact on the treatment received by the patient. Given the non-malignant nature of PA and the fact that in most cases treatment is limited to surgery, the former seems more possible in this occasion. To further evaluate this notion, we examined the age at diagnosis of residents of rural and urban areas; indeed, urban residence was associated with a lower age at diagnosis in both SEE (7.5 years vs. 8.4 years) and SEER (6.7 years vs. 7.3 years) implying possibly diagnosis at an earlier and possibly more favorable stage. The finding was more pronounced in SEER, as contrasted to SEE; possibly the difference in rural definition between countries, as well as the between-country differences in the healthcare systems could explain this discrepancy.
Data regarding tumor location, derived mainly from single-center case series, as well as our data confirm that childhood PA are most frequently (37 %) located in cerebellum apart from infants in whom supratentorial and optic nerve tumors prevail [37–40]. The excellent prognosis of cerebellar PA (10-year survival exceeding 99 %) compared to any other CNS location [14, 41], has been traditionally attributed to both the feasible gross-total resection, [41] as well as the greater plasticity of cerebellum [42] in childhood leading to fewer neurological deficits [43, 44]. It should be also taken into account, however, that cerebellar PA have been reported to be also characterized by differential genetic origins compared to supratentorial tumors [45].
Regardless of socioeconomic, geographical and tumor-specific characteristics, however, non-modifiable individual factors, notably age and gender, impact also on survival. The poorer outcome of infants with low-grade gliomas is poorly understood; there might be an interaction between age and non-surgical treatment, which is more frequently preferred in this age group given the adversities of performing neurological surgeries [46, 47]. Likewise, radiation, the presumably most effective treatment for unresectable PA, may be substituted in infants and young children by chemotherapy given concerns for its neurocognitive and neuroendocrine toxicities [48]. On the other hand, the differential topographic pattern of infant PA, including lower prevalence of cerebellar-located tumors, could impact on survival; the optic nerve PA preponderance in this age is possibly attributed to neurofibromatosis-related tumors [49, 50], anyway linked to worse prognosis [51], especially in children <1 year [52]. Notably though, the effect of age remained unchanged after adjustment for topography. Recent findings, however, indicate that infant low-grade gliomas might comprise a more aggressive disease, compared to PA in older children, as they are characterized by a different genetic composition, including mutations of components of the MAPK pathway, which has been identified as of paramount importance in pediatric low-grade gliomas [16]; particularly, Ho et al. showed that the BRAF-V600E mutation is more common in infants and is associated with worse prognosis, independently of topography and histology [53].
Statistically significant, but of low magnitude, gender differences in childhood PA outcome were shown for the first time in this study, of similar direction in both SEE registries and SEER. No difference in topography or age at diagnosis by gender that could explain this differential was evident. Previous molecular analyses for prognostic factors have either not evaluated or not reported, possibly due to lack of statistical power, gender differences; therefore, future research is needed to confirm this finding and stipulate on potential differential pathogenetic features by gender.
Our findings should be viewed under limitations mainly related to registration. Despite the lack of DCO cases on account of the non-lethal PA nature, the high proportion of astrocytoma NOS, approaching 30 % of all astrocytomas in SEER and SEE registries, may underestimate incidence and impact on time trends and survival findings. The much higher incidence of PA in SEER, compared to SEE, as well as the variations between SEE registries indicate potential underreporting of this non-malignant tumor in SEE; this could have led to selection bias, as less aggressive tumors would be more likely to slip registration (as they are usually treated at non-oncology departments, which may not be encompassed in the registration networks) and possibly underestimation of the survival rates in SEE. Furthermore, changes in classification of PA could have influenced registration process and consequently the reported findings. Other limitations entail the small, by necessity, number of PA in some participating registries, the variable study periods and the different registration policies, especially in SEE. Lastly, no treatment-related data or data on molecular markers with prognostic value were available in this dataset.
In conclusion, this study, exploiting publicly available registration data, comprises the first comprehensive overview on epidemiologic features, including incidence, time trends and survival, of childhood PA. Despite significant survival gains over time in the SEE countries there are still significant disparities compared to the US for this non-malignant childhood tumor, on account mainly of healthcare delivery patterns. The worse survival among infants indicates the need for innovative treatment modalities tailored for the youngest patients, whereas the identification of female gender as a potential adverse predictor of outcome merits further research. It is anticipated that registration improvements, especially in the less affluent SEE area will allow to unveil whether the lower PA incidence is genuine, as well as to deeply evaluate the increasing incidence. Similarly, expanding registration processes to include molecular and cytogenetic markers will provide further room for an in-depth evaluation of their prognostic significance.
References
Collins VP, Jones DT, Giannini C (2015) Pilocytic astrocytoma: pathology, molecular mechanisms and markers. Acta Neuropathol 129:775–788. doi:10.1007/s00401-015-1410-7
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J (2014) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol 16(Suppl 4):iv1–i63. doi:10.1093/neuonc/nou223
Ohgaki H, Kleihues P (2005) Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol 64:479–489
Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin D, Whelan S (2013) International classification of diseases for oncology, third edition, first revision. World Health Organization, Geneva
Burkhard C, Di Patre PL, Schuler D, Schuler G, Yasargil MG, Yonekawa Y, Lutolf UM, Kleihues P, Ohgaki H (2003) A population-based study of the incidence and survival rates in patients with pilocytic astrocytoma. J Neurosurg 98:1170–1174. doi:10.3171/jns.2003.98.6.1170
Arora RS, Alston RD, Eden TO, Estlin EJ, Moran A, Birch JM (2009) Age-incidence patterns of primary CNS tumors in children, adolescents, and adults in England. Neuro Oncol 11:403–413. doi:10.1215/15228517-2008-097
Jukich PJ, McCarthy BJ, Surawicz TS, Freels S, Davis FG (2001) Trends in incidence of primary brain tumors in the United States, 1985–1994. Neuro Oncol 3:141–151
McKean-Cowdin R, Razavi P, Barrington-Trimis J, Baldwin RT, Asgharzadeh S, Cockburn M, Tihan T, Preston-Martin S (2013) Trends in childhood brain tumor incidence, 1973–2009. J Neurooncol 115:153–160. doi:10.1007/s11060-013-1212-5
Smith MA, Freidlin B, Ries LA, Simon R (1998) Trends in reported incidence of primary malignant brain tumors in children in the United States. J Natl Cancer Inst 90:1269–1277
Peris-Bonet R, Martinez-Garcia C, Lacour B, Petrovich S, Giner-Ripoll B, Navajas A, Steliarova-Foucher E (2006) Childhood central nervous system tumours–incidence and survival in Europe (1978–1997): report from Automated Childhood Cancer Information System project. Eur J Cancer 42:2064–2080. doi:10.1016/j.ejca.2006.05.009
Papathoma P, Thomopoulos TP, Karalexi MA, Ryzhov A, Zborovskaya A, Dimitrova N, Zivkovic S, Eser S, Antunes L, Sekerija M, Zagar T, Bastos J, Demetriou A, Cozma R, Coza D, Bouka E, Dessypris N, Kantzanou M, Kanavidis P, Dana H, Hatzipantelis E, Moschovi M, Polychronopoulou S, Pourtsidis A, Stiakaki E, Papakonstantinou E, Oikonomou K, Sgouros S, Vakis A, Zountsas B, Bourgioti C, Kelekis N, Prassopoulos P, Choreftaki T, Papadopoulos S, Stefanaki K, Strantzia K, Cardis E, Steliarova-Foucher E, Petridou ET (2015) Childhood central nervous system tumours: incidence and time trends in 13 Southern and Eastern European cancer registries. Eur J Cancer 51:1444–1455. doi:10.1016/j.ejca.2015.04.014
Nejat F, El Khashab M, Rutka JT (2008) Initial management of childhood brain tumors: neurosurgical considerations. J Child Neurol 23:1136–1148. doi:10.1177/0883073808321768
Mathew RK, O’Kane R, Parslow R, Stiller C, Kenny T, Picton S, Chumas PD (2014) Comparison of survival between the UK and US after surgery for most common pediatric CNS tumors. Neuro Oncol 16:1137–1145. doi:10.1093/neuonc/nou056
Colin C, Padovani L, Chappe C, Mercurio S, Scavarda D, Loundou A, Frassineti F, Andre N, Bouvier C, Korshunov A, Lena G, Figarella-Branger D (2013) Outcome analysis of childhood pilocytic astrocytomas: a retrospective study of 148 cases at a single institution. Neuropathol Appl Neurobiol 39:693–705. doi:10.1111/nan.12013
Znaor A, van den Hurk C, Primic-Zakelj M, Agius D, Coza D, Demetriou A, Dimitrova N, Eser S, Karakilinc H, Zivkovic S, Bray F, Coebergh JW (2013) Cancer incidence and mortality patterns in South Eastern Europe in the last decade: gaps persist compared with the rest of Europe. Eur J Cancer 49:1683–1691. doi:10.1016/j.ejca.2012.11.030
Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, Orisme W, Punchihewa C, Parker M, Qaddoumi I, Boop FA, Lu C, Kandoth C, Ding L, Lee R, Huether R, Chen X, Hedlund E, Nagahawatte P, Rusch M, Boggs K, Cheng J, Becksfort J, Ma J, Song G, Li Y, Wei L, Wang J, Shurtleff S, Easton J, Zhao D, Fulton RS, Fulton LL, Dooling DJ, Vadodaria B, Mulder HL, Tang C, Ochoa K, Mullighan CG, Gajjar A, Kriwacki R, Sheer D, Gilbertson RJ, Mardis ER, Wilson RK, Downing JR, Baker SJ, Ellison DW, St. Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project (2013) Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet 45:602–612 doi:10.1038/ng.2611
Karalexi MA, Papathoma P, Thomopoulos TP, Ryzhov A, Zborovskaya A, Dimitrova N, Zivkovic S, Eser S, Antunes L, Sekerija M, Zagar T, Bastos J, Demetriou A, Agius D, Cozma R, Coza D, Bouka E, Dessypris N, Belechri M, Dana H, Hatzipantelis E, Papakonstantinou E, Polychronopoulou S, Pourtsidis A, Stiakaki E, Chatziioannou A, Manolitsi K, Orphanidis G, Papadopoulos S, Papathanasiou M, Patsouris E, Sgouros S, Zountsas B, Moschovi M, Steliarova-Foucher E, Petridou ET (2015) Childhood central nervous system tumour mortality and survival in Southern and Eastern Europe (1983–2014): gaps persist across 14 cancer registries. Eur J Cancer 51:2665–2677. doi:10.1016/j.ejca.2015.08.018
Surveillance, Epidemiology, End Results (SEER) Program (http://www.seer.cancer.gov) Research data (1973–2012), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission
Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin D et al (2000) International classification of diseases for oncology, 3rd edn. World Health Organization, Geneva
Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P (2005) International classification of childhood cancer, third edition. Cancer 103:1457–1467. doi:10.1002/cncr.20910
Bramer GR (1988) International statistical classification of diseases and related health problems. Tenth revision. World Health Stat Q 41:32–36
McDade TW, Adair LS (2001) Defining the “urban” in urbanization and health: a factor analysis approach. Soc Sci Med 53:55–70
Zanetti R, Sera F, Sacchetto L, Coebergh JW, Rosso S (2015) Power analysis to detect time trends on population-based cancer registries data: when size really matters. Eur J Cancer 51:1082–1090. doi:10.1016/j.ejca.2013.10.008
Steliarova-Foucher E, Stiller C, Colombet M, Kaatsch P, Zanetti R, Peris-Bonet R (2015) Registration of childhood cancer: moving towards pan-European coverage? Eur J Cancer 51:1064–1079. doi:10.1016/j.ejca.2015.03.009
Kaderali Z, Lamberti-Pasculli M, Rutka JT (2009) The changing epidemiology of paediatric brain tumours: a review from the Hospital for Sick Children. Childs Nerv Syst 25:787–793. doi:10.1007/s00381-008-0771-9
McNally RJ, Kelsey AM, Cairns DP, Taylor GM, Eden OB, Birch JM (2001) Temporal increases in the incidence of childhood solid tumors seen in Northwest England (1954–1998) are likely to be real. Cancer 92:1967–1976
Reutfors J, Kramarova E, Weiderpass E, Monge P, Wesseling C, Ahlbom A (2002) Central nervous system tumours in children in Costa Rica, 1981–96. Paediatr Perinat Epidemiol 16:219–225
Stiller CA, Nectoux J (1994) International incidence of childhood brain and spinal tumours. Int J Epidemiol 23:458–464
Hoffman S, Propp JM, McCarthy BJ (2006) Temporal trends in incidence of primary brain tumors in the United States, 1985–1999. Neuro Oncol 8:27–37. doi:10.1215/S1522851705000323
Patel S, Bhatnagar A, Wear C, Osiro S, Gabriel A, Kimball D, John A, Fields PJ, Tubbs RS, Loukas M (2014) Are pediatric brain tumors on the rise in the USA? Significant incidence and survival findings from the SEER database analysis. Childs Nerv Syst 30:147–154. doi:10.1007/s00381-013-2307-1
Dodgshun AJ, Maixner WJ, Hansford JR, Sullivan MJ (2016) Low rates of recurrence and slow progression of pediatric pilocytic astrocytoma after gross-total resection: justification for reducing surveillance imaging. J Neurosurg Pediatr. doi:10.3171/2015.9.PEDS15449
Buder T, Deutsch A, Klink B, Voss-Bohme A (2015) Model-based evaluation of spontaneous tumor regression in pilocytic astrocytoma. PLoS Comput Biol 11:e1004662. doi:10.1371/journal.pcbi.1004662
Gatta G, Botta L, Rossi S, Aareleid T, Bielska-Lasota M, Clavel J, Dimitrova N, Jakab Z, Kaatsch P, Lacour B, Mallone S, Marcos-Gragera R, Minicozzi P, Sanchez-Perez MJ, Sant M, Santaquilani M, Stiller C, Tavilla A, Trama A, Visser O, Peris-Bonet R, EUROCARE Working Group (2014) Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5–a population-based study. Lancet Oncol 15:35–47. doi:10.1016/S1470-2045(13)70548-5
Sergentanis T, Dessypris N, Kanavidis P, Skalkidis I, Baka M, Polychronopoulou S, Athanassiadou F, Stiakaki E, Frangandrea I, Moschovi M, Petridou ET (2013) Socioeconomic status, area remoteness, and survival from childhood leukemia: results from the Nationwide Registry for Childhood Hematological Malignancies in Greece. Eur J Cancer Prev 22:473–479. doi:10.1097/CEJ.0b013e32835c7f69
Petridou ET, Sergentanis TN, Perlepe C, Papathoma P, Tsilimidos G, Kontogeorgi E, Kourti M, Baka M, Moschovi M, Polychronopoulou S, Sidi V, Hatzipantelis E, Stiakaki E, Iliadou AN, La Vecchia C, Skalkidou A, Adami HO (2015) Socioeconomic disparities in survival from childhood leukemia in the United States and globally: a meta-analysis. Ann Oncol 26:589–597. doi:10.1093/annonc/mdu572
Meilleur A, Subramanian SV, Plascak JJ, Fisher JL, Paskett ED, Lamont EB (2013) Rural residence and cancer outcomes in the United States: issues and challenges. Cancer Epidemiol Biomarkers Prev 22:1657–1667. doi:10.1158/1055-9965.EPI-13-0404
Di Rocco C, Ceddia A, Iannelli A (1993) Intracranial tumours in the first year of life. A report on 51 cases. Acta Neurochir (Wien) 123:14–24
Rickert CH, Probst-Cousin S, Gullotta F (1997) Primary intracranial neoplasms of infancy and early childhood. Childs Nerv Syst 13:507–513. doi:10.1007/s003810050127
Sala F, Colarusso E, Mazza C, Talacchi A, Bricolo A (1999) Brain tumors in children under 3 years of age. Recent experience (1987–1997) in 39 patients. Pediatr Neurosurg 31:16–26
Spennato P, Nicosia G, Quaglietta L, Donofrio V, Mirone G, Di Martino G, Guadagno E, del Basso de Caro ML, Cascone D, Cinalli G (2015) Posterior fossa tumors in infants and neonates. Childs Nerv Syst 31:1751–1772. doi:10.1007/s00381-015-2783-6
Fernandez C, Figarella-Branger D, Girard N, Bouvier-Labit C, Gouvernet J, Paz Paredes A, Lena G (2003) Pilocytic astrocytomas in children: prognostic factors–a retrospective study of 80 cases. Neurosurgery 53:544–553 (discussion 554–545)
Cantelmi D, Schweizer TA, Cusimano MD (2008) Role of the cerebellum in the neurocognitive sequelae of treatment of tumours of the posterior fossa: an update. Lancet Oncol 9:569–576. doi:10.1016/S1470-2045(08)70148-7
Ogiwara H, Bowman RM, Tomita T (2012) Long-term follow-up of pediatric benign cerebellar astrocytomas. Neurosurgery 70:40–47. doi:10.1227/NEU.0b013e31822ff0ed (discussion 47–48)
Due-Tonnessen BJ, Helseth E, Scheie D, Skullerud K, Aamodt G, Lundar T (2002) Long-term outcome after resection of benign cerebellar astrocytomas in children and young adults (0–19 years): report of 110 consecutive cases. Pediatr Neurosurg 37:71–80
Sharma MK, Mansur DB, Reifenberger G, Perry A, Leonard JR, Aldape KD, Albin MG, Emnett RJ, Loeser S, Watson MA, Nagarajan R, Gutmann DH (2007) Distinct genetic signatures among pilocytic astrocytomas relate to their brain region origin. Cancer Res 67:890–900. doi:10.1158/0008-5472.CAN-06-0973
Qaddoumi I, Sultan I, Gajjar A (2009) Outcome and prognostic features in pediatric gliomas: a review of 6212 cases from the surveillance, epidemiology, and end results database. Cancer 115:5761–5770. doi:10.1002/cncr.24663
Stokland T, Liu JF, Ironside JW, Ellison DW, Taylor R, Robinson KJ, Picton SV, Walker DA (2010) A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: a population-based cohort study (CCLG CNS9702). Neuro Oncol 12:1257–1268. doi:10.1093/neuonc/noq092
Mansur DB, Rubin JB, Kidd EA, King AA, Hollander AS, Smyth MD, Limbrick DD, Park TS, Leonard JR (2011) Radiation therapy for pilocytic astrocytomas of childhood. Int J Radiat Oncol Biol Phys 79:829–834. doi:10.1016/j.ijrobp.2009.11.015
Czyzyk E, Jozwiak S, Roszkowski M, Schwartz RA (2003) Optic pathway gliomas in children with and without neurofibromatosis 1. J Child Neurol 18:471–478
Listernick R, Louis DN, Packer RJ, Gutmann DH (1997) Optic pathway gliomas in children with neurofibromatosis 1: consensus statement from the NF1 Optic Pathway Glioma Task Force. Ann Neurol 41:143–149. doi:10.1002/ana.410410204
Rasmussen SA, Yang Q, Friedman JM (2001) Mortality in neurofibromatosis 1: an analysis using U.S. death certificates. Am J Hum Genet 68:1110–1118. doi:10.1086/320121
Opocher E, Kremer LC, Da Dalt L, van de Wetering MD, Viscardi E, Caron HN, Perilongo G (2006) Prognostic factors for progression of childhood optic pathway glioma: a systematic review. Eur J Cancer 42:1807–1816. doi:10.1016/j.ejca.2006.02.022
Ho CY, Mobley BC, Gordish-Dressman H, VandenBussche CJ, Mason GE, Bornhorst M, Esbenshade AJ, Tehrani M, Orr BA, LaFrance DR, Devaney JM, Meltzer BW, Hofherr SE, Burger PC, Packer RJ, Rodriguez FJ (2015) A clinicopathologic study of diencephalic pediatric low-grade gliomas with BRAF V600 mutation. Acta Neuropathol 130:575–585. doi:10.1007/s00401-015-1467-3
Acknowledgments
We would like to acknowledge the individual Southern Eastern European cancer registries personnel for their dedication in running the registry process; the kind responsiveness and assistance of SEER officials in providing access to USA data; and Nick Dessypris from NARECHEM for his valuable contribution in data quality control and statistical analysis along with Dr Achilles Chatziioannou, Katerina Manolitsi, Mathilda Papathanasiou, Antonios Vakis, Nikolaos Kelekis and Panos Prassopoulos, who have assisted in the compilation and localization of the NARECHEM-BT data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Georgakis, M.K., Karalexi, M.A., Kalogirou, E.I. et al. Incidence, time trends and survival patterns of childhood pilocytic astrocytomas in Southern-Eastern Europe and SEER, US. J Neurooncol 131, 163–175 (2017). https://doi.org/10.1007/s11060-016-2284-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2284-9