Abstract
Purpose
Endometrial cancer (EC) exhibits striking racial disparities with higher mortality in Blacks compared to Whites. The mortality-to-incidence ratio (MIR) provides a population-based measure of survival which accounts for incidence. The objective of this study was to map EC MIRs by race for eight health regions within South Carolina (SC) and chart EC incidence by race and grade across the four cancer stages.
Methods
Cancer incidence and mortality data were obtained from the SC Community Access Network (SCAN), the online data query system provided by the SC Department of Health and Environmental Control (DHEC). The underlying data for SCAN were generated from the SC Central Cancer Registry and SC DHEC Vital Records and used to construct MIRs. ArcGIS 10.1 was used to map EC MIRs by race for eight health regions within SC. Four categories of MIR were derived using the national MIR for EC among Whites as the reference category.
Results
Blacks had higher levels of poorly differentiated tumors across all stages and higher incidence and mortality rates. In all eight health regions, Blacks were in the highest MIR category. By contrast, the MIRs for Whites were more evenly represented over the four categories.
Conclusions
The MIR proved useful for identifying disparities in EC incidence and mortality among Black and White women in SC. Cancer surveillance programs may use the MIR to monitor disparities across racial/ethnic groups and geographic regions going forward. MIRs have the potential to serve as an indicator of the long-term success of cancer surveillance programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometrial cancer (EC) is the most common gynecological cancer and the fourth most common cancer among women in the USA, with an estimated 54,870 cases expected in 2015 [1]. Nationally, there is a 30 % lower incidence among Blacks, relative to their White counterparts [1–3]. However, Black women who are diagnosed with EC are 2.5 times more likely to die (mortality rate 80 % higher) than their White counterparts [1–3]. These disparities may be partly due to advanced stage at diagnosis, increased comorbidities, inequalities in treatment, higher likelihood of poorly differentiated tumors, racial disparities in expression of the HER-2 oncogene, and mutations in the TP53 tumor suppressor genes among Blacks [2, 4–11].
The mortality-to-incidence ratio (MIR) represents a unique way to quantify racial cancer disparities [12, 13]. The MIR is an important indicator that offers additional information beyond what incidence, and mortality rates alone can offer [12]. Few studies have used the MIR to compare cancer rates [12, 14–16]. Wagner et al. [13] described racial cancer disparities and their potential geographic determinants by calculating, comparing and mapping MIRs throughout the state of Georgia (GA, USA) and found that Blacks in GA had more fatal cancers than Whites for all cancer sites evaluated. Sunkara et al. [17] demonstrated a strong linear relationship between the colorectal cancer MIR and health system ranking across countries evaluated.
Some previous studies on racial disparities in EC between Blacks and Whites focused on differences by histologic grade, and they found that part of the observed disparity between Blacks and Whites in EC may be explained by the differences in tumor grade and stage of EC [18–20]. Sabatino et al. [19] found that Blacks had the lowest rate of low-grade and early-stage (i.e., localized) epithelial EC and the highest rates of high-grade and late-stage (i.e., regional/distant) EC. Similarly, Oliver et al. [20] reported that Blacks were more likely to present with non-localized EC (31.8 vs. 24.5 % p = 0.02) and to have poorly differentiated tumors (20.5 vs. 15.0 %, p < 0.01) than Whites, while Alagkiozidis et al. [21] found high incidence of poorly differentiated tumors and late-stage/advanced-stage tumors among Blacks with EC. Higher grade also is strongly associated with histologic type of EC, and this is more common among Black women [22]. The objective of this study, therefore, was to (1) describe the EC disparities in South Carolina (SC) among Blacks and Whites using several indicators of incidence and mortality, including the MIRs by race for eight health regions within SC and (2) chart EC incidence by race and grade across the four cancer stages.
Materials and methods
Data for this study were obtained from SC Central Cancer Registry (SCCCR) which is located in the SC Department of Health and Environmental Control (DHEC) Office of Public Health Statistics and Information Services. Aggregate data for the age-adjusted mortality and incidence rates were obtained from the SC DHEC. Data on corpus uterus cancer were used as a proxy for endometrial cancer. In computing the incidence and mortality rates for EC cancer, the third edition International Classification of Disease (ICD) codes were used: isthmus uteri (C54.0), endometrium (C54.1), myometrium (C54.2), fundus uteri (C54.3), and overlapping lesion of corpus uteri (C54.8). All possible histopathologic types for EC were included in the calculation of rates with the exception of malignant mesothelioma, Kaposi sarcoma, and lymphomas (Surveillance, Epidemiology and End Results Program codes 9050-9055, 9140, 9590-9992).
All incident cancer cases are required by law to be reported to SCCCR, a resource established with funding from an award from the National Program of Cancer Registries (NPCR) since 1994. Enabling legislation from the SC General Assembly was enacted in 1996. Data are collected by SCCCR on all cancers, both in situ and invasive, from hospitals, pathology laboratories, freestanding treatment centers, and physician offices. The only exceptions are in situ forms of cervical cancer and invasive form of basal and squamous cell skin cancers of non-genital sites. The US standard population in 2000 was provided by the National Cancer Institute (NCI) for age adjustment.
The SCCCR from which we derived the data for this analysis has a history of receiving the highest/gold rating for data completeness (>94 %), timeliness, and data quality from the North American Association of Central Cancer Registries and NPCR. SCCCR is a member of the CDC National Interstate Data Exchange System (N-IDEAS) such that any member state may share resident incident cases with others to ensure completeness of incident cancer data. Additionally, there is geo-coding of all cancer cases and all cancer deaths in the state of SC. Histologic grade were not available on all pathology reports from the source medical records. If that information is not available, grade is coded to unknown. However, the analyses for this paper use aggregate (i.e., mortality and incidence rates), rather than individual-level data on EC cases.
Mortality data obtained from SC DHEC Vital Registry are based on information from death certificates of residents in SC. The classification of the underlying cause of death is according to ICD-10, and cause of death was restricted to endometrial cancer. There are interjurisdictional exchange agreements in place between SC and other states to capture out-of-state deaths of SC residents. The SCCCR obtains cancer mortality data annually from the Division of Vital Registry and Biostatistics for linkage purposes.
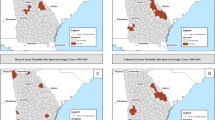
For mapping of EC MIRs, we compared MIRs across the eight DHEC health regions, which SC DHEC defined for the purpose of planning environmental and health programs. This study focused specifically on mapping EC MIRs for these eight DHEC health regions by race (Black vs. White). In situ cancers were excluded given the low probability of cancer-related death. In order to create the MIRs, the age-adjusted incidence and mortality rates were first calculated using incidence data from 1998 to 2009 in the SCCCR and mortality data from 1998 to 2009 in the DHEC Vital Registry. A sensitivity analysis previously described by Sunkara and Hebert [23] examined the effect of moving across different “denominator years” to vary with the alignment of the average incidence-to-mortality time interval. The sensitivity analysis used all combinations of sex and race for cancers involving all anatomic sites, including EC. It was shown that the lines describing the MIR remained parallel with rates observed generally stable over time across eight different 5-year periods beginning in 1996. This was computed using incidence data from the SCCCR [23].
The MIR analysis and tumor stage by grade chart (Figs. 1, 3) consisted of all EC from 1998 to 2009 (12 years) in the DHEC Registry. The information was extracted using the SC Community Assessment Network (SCAN). MIRs were stratified by race, specifically Blacks versus Whites. There were too few Hispanic EC cases in the state of SC to provide stable estimates. There were a total number of 5,594 incident cases of EC. Blacks and Whites make up 5,515 (98.59 %) of these incident cases, while all other races only make up 79 (1.41 %) of cases. Therefore, we did not include Hispanic EC incidence and mortality data in these analyses.
As a ratio with the mortality rate in the numerator and the incidence rate in the denominator, the MIR takes on values ranging from 0 to 1. Values closer to zero indicate more indolent while those closer to one indicate more aggressive cancers. All new cases of EC reported over the 12-year period from 1998 to 2009 were included in these analyses. Mortality was restricted to those whose cause of death was EC from 1998 to 2009 (12 years). The MIR which has been shown to be highly insensitive to time-discordant incidence and mortality [23] does not take into account follow-up time and is not equivalent to Cox proportional hazards-type survival analysis which is a truly multivariate technique that accounts for follow-up time. Similarly, we cannot account for competing risk in this statistic other than the underlying mechanics of the statistic which only counts deaths where EC is the cause of death; deaths for other causes of death are not included in the numerator. Over the 12-year study period, there were 4,035 new cases among Whites and 1,480 among Blacks.
In order to compare racial differences in EC MIR in eight SC DHEC health regions, we defined four categories of EC MIR. Firstly, we computed the MIR for Whites nationally [24] (i.e., for the USA as a whole from the United States Cancer Statistics at the Center for Disease Control and Prevention) as a reference. The upper limit for Category 1 is the reference; the upper limit of Category 2 is 10 % higher than the reference; the upper limit of Category 3 is 20 % higher than the reference; and Category 4 consists of MIR > 20 % higher than the reference. This method of categorization and analysis was previously used by Hebert et al. [12]. The defined categories of each DHEC health region were mapped by race using ArcGIS version 10.1 (ESRI, Redlands, CA). It should be noted, however, that the independent incidence and mortality chart (not involved in computing MIR) are based on data aggregated over 5 years (2005–2009; Figs. 2, 4).
Age-adjusted endometrial cancer incidence rates (2005–2009) in South Carolina by race, cancer stage and cancer grade. a Endometrial cancer incidence rates in South Carolina by race and cancer stage. b Endometrial cancer incidence rates in South Carolina by race and cancer grade. Rate is per 100,000 population
Results
Across all eight DHEC health regions, MIRs were in the fourth (highest) category for Blacks, while the Whites were more evenly divided over the four categories (Fig. 1).
Blacks were more likely to present with a poorly differentiated tumor while Whites were more likely to have a well-differentiated tumor. Similarly, Blacks also were more likely to have a regional or distant (metastatic) tumor while Whites were more likely to have localized tumors (Fig. 2).
When stratified by tumor stage, Blacks had higher levels of poorly differentiated tumor across all stages. This is particularly prominent in stages III and IV where Blacks have strikingly higher levels of poorly differentiated tumors (Fig. 3).
Age-adjusted endometrial cancer incidence rates (1998–2009) in South Carolina by race and grade. a Age-adjusted endometrial cancer incidence rate in South Carolina by race and grade for Stage II (local) cancer. b Age-adjusted endometrial cancer incidence rate in South Carolina by race and grade for Stage III (regional) cancer. c Age-adjusted endometrial cancer incidence rate in South Carolina by race and grade for Stage IV (distant) cancer. Rate is per 100,000 population
The incidence and mortality rates were consistently higher among Blacks over the 5-year period from 2005 to 2009 (Fig. 4).
Table 1 shows the computed MIRs for Blacks and Whites across the eight DHEC regions. Table 1 also shows that the lowest MIR for both Black and White women was in Region 6 while the highest MIR for the two groups was in Region 7. Although the other regions did not have exactly the same pattern, the pattern appears fairly similar. For the state of SC overall, the MIR for Blacks was 2.3 times higher than that of their White counterparts. The most marked racial difference was seen in Region 6 where the MIR among Black women was 3.1 times higher than that of White women.
The proportions of those with localized, regional, and distant EC were 22.09, 34.73, and 41.81 %, respectively (p value <0.001; Tables 2, 3).
When histopathologic subtypes were examined, higher proportions of Blacks had complex, mixed, and stromal neoplasms (57.71 %) and epithelial neoplasms (53.73 %) while other forms had higher proportions among the Whites.
Discussion
This study found that both incidence and mortality of EC were higher among Blacks than Whites in SC; a pattern which is different from what has been found in the previous literatures where incidence is usually higher among Whites and mortality is higher among Blacks [1, 2, 4]. We confirmed well-documented interracial differences in cancer incidence and mortality which has previously been observed for other cancers, but not EC, in SC [25–32]. This study also showed racial differences in the histologic subtypes of EC which has been shown to be important for monitoring aggressive tumors among Black women [33]. This study also described racial disparities in MIR from EC by calculating, comparing, and mapping MIRs throughout the eight DHEC regions in SC. The potential utility of MIR for cancer surveillance programs in monitoring disparities across racial/ethnic groups and geographic regions going forward is substantial, as shown by our study’s examination of MIRs for EC incidence and mortality by race and region.
The higher incidence of EC found in Blacks compared with Whites is not consistent with findings from previous studies. One reason that has been proposed for lower incidence of EC among Blacks includes failure to correct for hysterectomy prevalence among Blacks which may lead to underestimation of EC risk [4]. Hysterectomies have been shown to be three times higher in women who live in the South, which may result in underestimation of EC risk [4, 34–36]. After hysterectomy was corrected for by Siegel et al. [34], incidence rate of EC doubled among Black women in SC and three other states in the South. Given that we did not adjust for hysterectomies because it was not possible to do so, the increased incidence of EC among Blacks in our sample may still be an underestimate of the disease burden among Blacks because of the higher prevalence of hysterectomy among Blacks in the South [4, 35, 36].
Our finding that Black women had higher levels of poorly differentiated tumor across all stages (particularly prominent in stage III and stage IV EC cases; Fig. 3) probably explains the higher EC mortality among Blacks compared to Whites which is consistent with findings from other studies [1–3]. Many reasons have been hypothesized as being responsible for the higher level of EC mortality among Blacks compared to Whites. One reason is later stage at diagnosis as mentioned above [1]. Low socioeconomic status which is more prevalent among Blacks generally also has been shown to be a risk factor for higher mortality among Black women compared with White women [2, 8]. Higher prevalence of obesity among Black women also may explain some of the increased mortality [2, 11, 36]. Although several studies have found an association between obesity and EC, this association observed by Cote et al. was stronger with estrogen-dependent type I tumors. There is, however, a high prevalence of non-estrogen-dependent type II tumors of the type typically found among morbidly obese Black women [22]. Cote et al. also found that while hypertension was higher among Blacks compared to Whites, survival analysis did not show a difference between Blacks and Whites among EC survivors [22]. Physical inactivity which also is known to be more prevalent among Black women has been shown to be an independent risk factor for endometrial cancer [37–45] although some studies did not find physical activity to be associated with EC [38, 39]. Inequality in treatment was shown as another possible factor responsible for higher mortality in Black women with EC [2, 6, 46]. Some researchers found that compared to White women with EC, a higher percentage of Black women with EC did not receive any cancer-directed treatment [2, 6].
Using the MIR as a surveillance tool stresses the point that SC exhibits more extreme interracial differences in cancer incidence, mortality and MIR than other states or the nation [25–32] and specifically the MIR helped to highlight regions where this disparity is highest. For example, Regions 6 and 4 have the highest MIR disparity where the MIR of Blacks is 3.1 and 3.0 times higher than that of Whites, respectively. Regions 6 and 4 represent the “Pee Dee” region which is known for its lower socioeconomic status, rurality, and being medically underserved [12]. Additionally, in four of the 12 counties in the “Pee Dee” regions (Dillon, Lee, Marlboro, and Williamsburg), percentage of adults that report a BMI of 30 or more was greater than 40 % [47]. Similarly, percentage of adults aged 20 and over reporting no leisure-time physical activity was greater than 30 % in seven counties (Chesterfield, Darlington, Dillon, Lee, Marion, Marlboro, and Sumter) out the 12 counties in the “Pee Dee” region [47]. Additional information on Blacks versus Whites disparity between the prevalence of obesity and physical inactivity levels at County/Regional level might help to explain the racial disparities in EC MIR in the future. Urban centers with major hospital systems such as Charleston (Region 7), Columbia (Region 3), Greenville and Spartanburg (Region 2) may be responsible for the relatively smaller MIR disparities seen in Regions 2 and 7.
The MIR serves as a population-based approximation of fatality (1/survival) given incidence by stabilizing the incidence and mortality differences across cancer sites and racial groups [12, 13]. Various survival studies have shown that Black women have poorer EC survival than White women [2, 22, 48–54]. To our knowledge, however, there is no specific report on racial differences in EC survival in SC. In this respect, our computed MIR thus serves as a proxy for EC survival in SC which shows the same racial patterns as observed in the survival studies carried out in other US states [2, 22, 48–55].
The strength of this study is that it combined data over 12 years to compute the MIR making estimates more stable. Migration of EC patients in and out of SC was also mitigated in this study because of the interjurisdictional exchange agreements between SC and other states to capture out-of-state deaths of SC residents. Also, this is a population-based study of the entire population of EC patients whereby incidence and mortality data represented >94 % of all EC cases. Thus, the study is virtually devoid of selection bias, a common problem associated with such studies. Additionally, the MIR is relatively cheap and easy to compute from existing relatively complete data. It can be used as a surrogate measure for a more expensive and time-consuming survival studies. A drawback, however, is that there is no way to compute censoring and loss to follow up. It also was not possible to adjust for covariates such as treatment, comorbidities, or individual socioeconomic status in the analysis. Another weakness is that the relationship between the numerator (mortality) and the denominator (incidence) may not be direct because persons diagnosed of EC may not die of EC and persons who die after the diagnosis of EC survive for varying length of time which could not be accounted for by the MIR. Data on regionally specific prevalence of obesity and physical activity levels which are major risk factors for EC though available were not specified by race.
In conclusion, we were able to demonstrate racial disparities in EC incidence and mortality in SC with higher levels of both factors in Black women compared with White women. The MIR proved useful for identifying disparities in EC’s incidence and mortality among Black and White women in SC. We also showed regional differences in the computed MIR which may reflect some underlying environmental, demographic, and structural risk factors that warrant future studies. MIRs have the potential as an indicator of the long-term success of cancer surveillance programs. Cancer surveillance programs should use the MIR to monitor disparities across racial/ethnic groups and geographic regions going forward. The mapping of the MIR can aid in identifying specific locations that require timely, focused, additional attention in terms of resource allocation, future epidemiological studies, and interventions to reduce disease burden. Future studies can focus on assessment of environmental exposures at individual levels as it relates to racial disparities.
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. Ca Cancer J Clin 65(1):5–29
Farley J, Risinger JI, Rose GS, Maxwell GL (2007) Racial disparities in blacks with gynecologic cancers. Cancer 110(2):234–243
Long B, Liu FW, Bristow RE (2013) Disparities in uterine cancer epidemiology, treatment, and survival among African Americans in the United States. Gynecol Oncol 130(3):652–659
Sherman ME, Carreon JD, Lacey JV, Devesa SS (2005) Impact of hysterectomy on endometrial carcinoma rates in the United States. J. Natl Cancer Inst 97(22):1700–1702
Sherman ME, Devesa SS (2003) Analysis of racial differences in incidence, survival, and mortality for malignant tumors of the uterine corpus. Cancer 98(1):176–186
Freeman HP (2003) Commentary on the meaning of race in science and society. Cancer Epidemiol Biomark Prev 12(3):232S–236S
Kohler MF, Berchuck A, Davidoff AM, Humphrey PA, Dodge RK, Iglehart JD, Soper JT, Clarke-Pearson DL, Bast RC Jr, Marks JR (1992) Overexpression and mutation of p53 in endometrial carcinoma. Cancer Res 52(6):1622–1627
Madison T, Schottenfeld D, James SA, Schwartz AG, Gruber SB (2004) Endometrial cancer: socioeconomic status and racial/ethnic differences in stage at diagnosis, treatment, and survival. Am J Public Health 94(12):2104–2111
Maxwell GL, Risinger JI, Hayes KA, Alvarez AA, Dodge RK, Barrett JC, Berchuck A (2000) Racial disparity in the frequency of PTEN mutations, but not microsatellite instability, in advanced endometrial cancers. Clin Cancer Res 6(8):2999–3005
Plaxe SC, Saltzstein SL (1997) Impact of ethnicity on the incidence of high-risk endometrial carcinoma. Gynecol Oncol 65(1):8–12
Schouten LJ, Goldbohm RA, van den Brandt PA (2004) Anthropometry, physical activity, and endometrial cancer risk: results from the Netherlands cohort study. J Natl Cancer Inst 96(21):1635–1638
Hebert JR, Daguise VG, Hurley DM, Wilkerson RC, Mosley CM, Adams SA, Puett R, Burch JB, Steck SE, Bolick-Aldrich SW (2009) Mapping cancer mortality-to-incidence ratios to illustrate racial and sex disparities in a high-risk population. Cancer 115(11):2539–2552
Wagner SE, Hurley DM, Hebert JR, McNamara C, Bayakly AR, Vena JE (2012) Cancer mortality-to-incidence ratios in Georgia describing racial cancer disparities and potential geographic determinants. Cancer 118(16):4032–4045
Hsing AW, Tsao L, Devesa SS (2000) International trends and patterns of prostate cancer incidence and mortality. Int J Cancer 85(1):60–67
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24(14):2137–2150
Sugerman PB, Savage NW (2002) Oral cancer in Australia: 1983–1996. Aust Dent J 47(1):45–56
Sunkara V, Hebert JR (2015) The colorectal cancer mortality-to-incidence ratio as an indicator of global cancer screening and care. Cancer 121:1563–1569
Armstrong K, Randall TC, Polsky D, Moye E, Silber JH (2011) Racial differences in surgeons and hospitals for endometrial cancer treatment. Med Care 49(2):207–214
Sabatino SA, Stewart SL, Wilson RJ (2009) Racial and ethnic variations in the incidence of cancers of the uterine corpus, United States, 2001–2003. J Womens Health 18(3):285–294
Oliver KE, Enewold LR, Zhu KM, Conrads TP, Rose GS, Maxwell GL, Farley JH (2011) Racial disparities in histopathologic characteristics of uterine cancer are present in older, not younger blacks in an equal-access environment. Gynecol Oncol 123(1):76–81
Alagkiozidis I, Wilson K, Ruffner N, Weedon J, Serur E, Economos K, Abulafia O, Lee YC, Salame G (2014) External validation of a nomogram for predicting survival of women with uterine cancer in a cohort of African American patients. Int J Gynecol Cancer 24(1):85–90
Cote ML, Ruterbusch JJ, Ahmed Q, Bandyopadhyay S, Alosh B, Abdulfatah E, Seward S, Morris R, Ali-Fehmi R (2014) Endometrial cancer in morbidly obese women: do racial disparities affect surgical or survival outcomes? Gynecol Oncol 133(1):38–42
Sunkara V, Hebert JR (2015) The application of the mortality-to-incidence ratio for the evaluation of cancer care disparities globally. Cancer 122(3):487–488. doi:10.1002/cncr.29746
United States Cancer Statistics (1999–2012 cancer incidence and mortality data) https://nccd.cdc.gov/uscs/. Accessed 122315
Adams SA, Hebert JR, Bolick-Aldrich S, Daguise VG, Mosley CM, Modayil MV, Berger SH, Teas J, Mitas M, Cunningham JE et al (2006) Breast cancer disparities in South Carolina: early detection, special programs, and descriptive epidemiology. J S C Med Assoc 102(7):231–239
Alberg AJ, Horner MJ, Daguise VG, Carpenter MJ, Mosley CM, Vincent B, Silvestri G, Reed CE, Hebert JR (2006) Lung and bronchus cancer disparities in South Carolina: epidemiology and strategies for prevention. J S C Med Assoc 102(7):183–191
Brandt HM, Modayil MV, Hurley D, Pirisi-Creek LA, Johnson MG, Davis J, Mathur SP, Hebert JR (2006) Cervical cancer disparities in South Carolina: an update of early detection, special programs, descriptive epidemiology, and emerging directions. J S C Med Assoc 102(7):223–230
Daguise VG, Burch JB, Horner MJ, Mosley C, Hofseth LJ, Wargovich MJ, Lloyd SC, Hebert JR (2006) Colorectal cancer disparities in South Carolina: descriptive epidemiology, screening, special programs, and future direction. J S C Med Assoc 102(7):212–220
Drake BF, Keane TE, Mosley CM, Adams SA, Elder KT, Modayil MV, Ureda JR, Hebert JR (2006) Prostate cancer disparities in South Carolina: early detection, special programs, and descriptive epidemiology. J S C Med Assoc 102(7):241–249
Hebert JR, Adams SA, Daguise VG, Hurley D, Smith EW, Purdon C, Lawson A, Mitas M, Reed CE (2006) Esophageal cancer disparities in South Carolina: early detection, special programs, and descriptive epidemiology. J S C Med Assoc 102(7):201–209
Hebert JR, Elder K, Ureda JR (2006) Meeting the challenges of cancer prevention and control in South Carolina: focusing on seven cancer sites, engaging partners. J S C Med Assoc 102(7):177–182
Yen KL, Horner MJ, Reed SG, Daguise VG, Bolick-Aldrich SW, Young MR, Day TA, Wood PA, Hebert JR (2006) Head and neck cancer disparities in South Carolina: descriptive epidemiology, early detection, and special programs. J S C Med Assoc 102(7):192–200
Cote ML, Ruterbusch JJ, Olson SH, Lu K, Ali-Fehmi R (2015) The growing burden of endometrial cancer: a major racial disparity affecting black women. Cancer Epidemiol Biomark Prev 24(9):1407–1415
Siegel RL, Devesa SS, Cokkinides V, Ma JM, Jemal A (2013) State-level uterine corpus cancer incidence rates corrected for hysterectomy prevalence, 2004 to 2008. Cancer Epidemiol Biomark Prev 22(1):25–31
Sighoko D (2014) Ethnic and geographic variations in corpus uteri cancer burden: evidence based on data from 29 states and the District of Columbia. CI5 IX, X and SEER data (1998–2010). Cancer Causes Control 25(9):1197–1209
Cossrow N, Falkner B (2004) Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab 89(6):2590–2594
Arem H, Irwin ML, Zhou Y, Lu LG, Risch H, Yu H (2011) Physical activity and endometrial cancer in a population-based case–control study. Cancer Causes Control 22(2):219–226
Colbert LH, Lacey JV, Schairer C, Albert P, Schatzkin A, Albanes D (2003) Physical activity and risk of endometrial cancer in a prospective cohort study (United States). Cancer Causes Control 14(6):559–567
Conroy MB, Sattelmair JR, Cook NR, Manson JE, Buring JE, Lee IM (2009) Physical activity, adiposity, and risk of endometrial cancer. Cancer Causes Control 20(7):1107–1115
Du MM, Kraft P, Eliassen AH, Giovannucci E, Hankinson SE, De Vivo I (2014) Physical activity and risk of endometrial adenocarcinoma in the nurses’ health study. Int J Cancer 134(11):2707–2716
Friberg E, Mantzoros CS, Wolk A (2006) Physical activity and risk of endometrial cancer: a population-based prospective cohort study. Cancer Epidemiol Biomark Prev 15(11):2136–2140
Friedenreich C, Cust A, Lahmann PH, Steindorf K, Boutron-Ruault MC, Clavel-Chapelon F, Mesrine S, Linseisen J, Rohrmann S, Pischon T et al (2007) Physical activity and risk of endometrial cancer: the European prospective investigation into cancer and nutrition. Int J Cancer 121(2):347–355
Gierach GL, Chang SC, Brinton LA, Lacey JV, Hollenbeck AR, Schatzkin A, Leitzmann MF (2009) Physical activity, sedentary behavior, and endometrial cancer risk in the NIH-AARP diet and health study. Int J Cancer 124(9):2139–2147
Tavani A, Bravi F, Dal Maso L, Zucchetto A, Bosetti C, Pelucchi C, Montella M, Franceschi S, La Vecchia C (2009) Physical activity and risk of endometrial cancer: an Italian case–control study. Eur J Cancer Prev 18(4):303–306
Voskuil DW, Monninkhof EM, Elias SG, Vlems FA, van Leeuwen FE (2007) Task force phys activity C: physical activity and endometrial cancer risk, a systematic review of current evidence. Cancer Epidemiol Biomark Prev 16(4):639–648
Trimble EL, Harlan LC, Clegg LMX, Stevens JL (2005) Pre-operative imaging, surgery and adjuvant therapy for women diagnosed with cancer of the corpus uteri in community practice in the United States. Gynecol Oncol 96(3):741–748
County Health Rankings and Roadmaps; Adult obesity and physical inactivity in South Carolina. http://www.countyrankings.org/app/south-carolina/2015/measure/factors/11/map available on 122315
Cook LS, Kmet LM, Magliocco AM, Weiss NS (2006) Endometrial cancer survival among US black and white women by birth cohort. Epidemiology 17(4):469–472
Elshaikh MA, Munkarah AR, Robbins JR, Laser BS, Bhatt N, Cogan C, Siddiqui F (2013) The impact of race on outcomes of patients with early stage uterine endometrioid carcinoma. Gynecol Oncol 128(2):171–174
Kost ER, Hall KL, Hines JF, Farley JH, Nycum LR, Rose GS, Carlson JW, Fischer JR, Kendall BS (2003) Asian-Pacific Islander race independently predicts poor outcome in patients with endometrial cancer. Gynecol Oncol 89(2):218–226
Maxwell GL, Tian C, Risinger J, Brown CL, Rose GS, Thigpen JT, Fleming GF, Gallion HH, Brewster WR (2006) Racial disparity in survival among patients with advanced/recurrent endometrial adenocarcinoma: a gynecologic oncology group study. Cancer 107(9):2197–2205
Olson SH, Atoria CL, Cote ML, Cook LS, Rastogi R, Soslow RA, Brown CL, Elkin EB (2012) The impact of race and comorbidity on survival in endometrial cancer. Cancer Epidemiol Biomark Prev 21(5):753–760
Ruterbusch JJ, Ali-Fehmi R, Olson SH, Sealy-Jefferson S, Rybicki BA, Hensley-Alford S, Elshaikh MA, Gaba AR, Schultz D, Munkarah AR et al (2014) The influence of comorbid conditions on racial disparities in endometrial cancer survival. Am J Obstet Gynecol 211(6):627-e1
Yap OW, Matthews RP (2006) Racial and ethnic disparities in cancers of the uterine corpus. J Natl Med Assoc 98(12):1930–1933
Cook LS, Kmet LM, Magliocco AM, Weiss NS (2006) Endometrial cancer survival among U.S. black and white women by birth cohort. Epidemiology 17(4):469–472
Acknowledgments
We acknowledge the Department of Health and Environmental Services (DHEC) for making their data available for use and particularly, and we appreciate the SCCCR Director Susan Bolick for giving us additional information.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Babatunde, O.A., Adams, S.A., Eberth, J.M. et al. Racial disparities in endometrial cancer mortality-to-incidence ratios among Blacks and Whites in South Carolina. Cancer Causes Control 27, 503–511 (2016). https://doi.org/10.1007/s10552-016-0724-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-016-0724-7