Abstract
Purpose
We aim to investigate the association between angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) therapy and colorectal cancer (CRC) by conducting a systematic review with meta-analysis.
Methods
Literature was searched on PubMed, Scopus, and the Cochrane library to identify relevant studies evaluating ACEIs/ARBs therapy and risk of CRC incidence or survival of CRC patients. Pooled risk ratio (RR) with 95 % confidence intervals was calculated for the association between ACEIs/ARBs and CRC risk and mortality.
Results
Eleven observational studies were included in the systematic review. A meta-analysis of six studies totaling 113,048 individuals indicated a 6 % decreased risk of CRC in ACEIs/ARBs users compared to non-users (95 % CI 0.89–0.98). In the four case–control studies, individuals using ACEIs/ARBs were associated with a 6 % decreased risk of CRC (95 % CI 0.90–0.99). The meta-analysis of three studies investigating the relationship between ACEIs/ARBs and survival of CRC did not show a significantly decreased mortality in ACEIs/ARBs users (RR 0.81, 95 % CI 0.60–1.09). Seven studies evaluated the dose–response relationship between ACEIs/ARBs therapy and CRC, and two of them showed that the association was related to longer duration and higher dose.
Conclusions
CEIs/ARBs therapy might be associated with a reduce risk of CRC development, but whether use of these medications improves the outcomes of CRC remains unknown. Large-scale and more robust studies are needed to further explore this association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As one of the most common types of carcinomas, colorectal cancer (CRC) is a leading cause of cancer-related morbidity and mortality worldwide [1]. In western countries, the estimated cumulative lifetime risk of developing CRC is approximately 5 % in the general population [2]. The progress of CRC screening technologies has increased the diagnostic rate of early-stage CRC and colorectal adenoma (CRA), a precursor lesion of CRC [3, 4]. Although screening might contribute to the prevention of CRC and the reduction in cancer-specific mortality [5, 6], it is still urgent to explore the field of chemoprevention and adjuvant therapies against CRC.
There is some evidence that angiotensin-converting enzyme inhibitor (ACEI) might reduce an individual’s risk of cancer [7]. Angiotensin receptor blocker (ARB), another class of renin-angiotensin system (RAS) inhibitors, has also been suggested to be associated with a lower incidence of cancer occurrence [8]. In addition, a systematic review conducted in 2011 has indicated that ACEIs/ARBs use may be related to improved outcomes in cancer patients [9]. Nevertheless, a few studies failed to find any association between ACEIs/ARBs and cancer [10, 11].
In particular for CRC, previous studies have reported inconsistent findings concerning the association of ACEIs/ARBs and risk of CRC or the prognosis of CRC patients [12]. Therefore, we conducted a systematic review with meta-analysis to investigate the association between ACEIs/ARBs therapy and CRC, which still remains controversial by now.
Methods
Literature search and search strategy
This study was carried out based on the guidelines of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [13]. The PubMed database, Scopus, and the Cochrane library were systematically searched from inception to November 2014, with the aim of finding original epidemiological and clinical studies regarding to the association between use of ACEIs/ARBs and CRC.
The search strategy adapted for PubMed was as follows: ((angiotensin-converting enzyme inhibitors[Mesh] OR angiotensin-converting enzyme inhibitor* OR angiotensin-converting enzyme inhibitor* OR ACE inhibitor* OR ACEI* OR captopril OR ramipril OR cilazapril OR enalapril OR fosinopril OR perindopril OR imidapril OR lisinopril OR moexipril OR quinapril OR trandolapril) OR (angiotensin receptor antagonists[Mesh] OR angiotensin receptor blocker* OR angiotensin receptor antagonist* OR angiotensin receptor blockade OR angiotensin-receptor blocker* OR angiotensin-receptor antagonist* OR angiotensin-receptor blockade OR ARB OR ARBs OR irbesartan OR eprosartan OR losartan OR telmisartan OR valsartan OR olmesartan OR candesartan) OR (renin angiotensin system inhibitor* OR renin-angiotensin system inhibitor* OR RAS inhibitor*)) AND (Colorectal neoplasms[Mesh] OR ((colon OR rectum OR rectal OR colorectal OR colorectum OR bowel) AND (cancer OR carcinoma OR adenoma OR tumor OR tumour OR neoplasm* OR malignan* OR polyp OR lesion))). No language restriction was imposed.
Selection criteria and quality assessment
Studies were eligible for the systematic review if they met the following criteria: (1) original observational studies, including case–control studies, cohort studies, and cross-sectional studies; (2) assessing the association between exposure to ACEIs/ARBs and the incidence or prognosis of CRC; (3) reporting outcomes of interest, i.e., odds ratio (OR), relative risk (RR), hazard ratio (HR), or standardized incidence ratio (SIR) (the ratio of the observed to the expected number of cases) with 95 % confidence intervals (CI). Study selection was processed by two authors (Y. N. Dai and J. H. Wang) independently, and disagreements were resolved by discussion.
The Newcastle–Ottawa scale (NOS) was used to assess the quality of the included studies by two independent authors [14]. Studies were considered as high quality if the score was 7–9 points.
Data extraction
Data were extracted by two authors (Y. N. Dai and J. Z. Zhu) working independently. Differences in data extraction were discussed and resolved. The main outcome measures were CRC risk, as well as survival and prognosis of CRC. The risk of CRA incidence was also an outcome of interest for a secondary analysis. The following information of the included studies was abstracted: author, year of publication, study location, study type, patient population, number of subjects, detailed comparison, OR, RR, HR, or SIR with 95 % CI, and variables adjusted in the analysis. Moreover, the outcomes were abstracted additionally according to cumulative duration of ACEIs/ARBs therapy and dosage of the relevant drugs to investigate the dose–response relationship.
Statistical analysis
A meta-analysis was conducted to investigate the association between ACEIs/ARBs and risk of CRC incidence. Adjusted RRs were combined to estimate the overall effect if possible. If adjusted RRs were not available, the RR was calculated from the raw data provided. In cases when neither an adjusted RR nor raw data were available in the individual study, we calculated crude RRs with 95 % CIs by creating a 2 × 2 table of CRC cases and controls by ACEIs/ARBs using status. OR and HR were considered to be approximate to the RR because the cancer risk is small in both groups. Data on SIRs were not pooled with RRs. We used the inverse variance method with a fixed- or random-effects model to calculate the summarized RR of CRC risk with 95 % CI.
Statistical heterogeneity was evaluated by the Chi-squared test, and the I-squared statistic. Heterogeneity was considered significant by the Chi-squared test with p < 0.10 or by I-squared >50 % [15, 16]. Moreover, sensitivity analysis was performed, where the outcome of CRC risk was explored using a fixed-effects model, and the meta-analysis was restricted to subgroups based on study types, studies with adjustments of confounders, and studies comparing ACEI users with non-users. Publication bias was evaluated by Egger’s test [17, 18]. The meta-analysis was conducted using Review Manager Software, version 5.2, and Egger’s test was carried out with STATA software, version 12.0.
Results
Study selection and characteristics
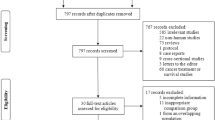
Figure 1 illustrates the study selection process. A total of 916 articles were identified through database search according to the previously established medical terms. After each publication was screened through titles and abstracts, 898 of these were excluded. Following detailed evaluation among the remaining eighteen records, a total of eleven articles were finally included in this systematic review.
The total number of participants enrolled amounted to 135,605 in the incidence studies and 13,031 in the mortality studies. The characteristics of the included studies are exhibited in Table 1. Five of the studies were population-based cohort studies [10, 19–22]; five of these were case–control studies [23–27]; and one was cross-sectional study [28]. Six of the studies were based in Europe [10, 22, 24, 26–28]; five were performed in North America [19–21, 23, 25]. Overall, the quality of the included studies was good: The median (range) NOS score was 7 (5–8).
The summary of the results of each included study is showed in Table 2. There were inconsistencies among the adjustments of confounding factors in each study. Eight studies reported outcomes with a variety of adjustments [10, 19–25, 27], e.g., age, gender, BMI, hypertension, and other established risk factors for CRC, as well as medication use, whereas three studies did not adjust for potential confounders [10, 26, 28].
Risk of CRC incidence
The association between ACEIs/ARBs use and risk of CRC incidence was examined in seven studies, four of which were case–control studies within a cohort [23–26], one was a cross-sectional study using a prospectively maintained database [28], and two were cohort studies [10, 22]. Among these studies, one [10] reported outcomes in SIRs and was consequently excluded from the meta-analysis.
The meta-analysis of the remaining six studies [22–26, 28] involving 113,048 patients showed that the risk of CRC significantly decreased in ACEIs/ARBs users compared to non-users. In general, the pooled RR for CRC was 0.94 (95 % CI 0.89–0.98, p = 0.006). There was no statistically heterogeneity among studies (I 2 = 0 %, p = 0.89; Fig. 2a).
Forest plot of comparison. Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers users versus non-users; outcome: risk of colorectal cancer incidence. A fixed-effects model was adopted. a Total risk; b subgroup analysis in case–control studies; c subgroup analysis in studies with adjustment of confounders
In addition, sensitivity analyses were conducted (Table 3). When a random-effects model was adopted, the results did not change (RR 0.94, 95 % CI 0.89–0.98, p = 0.006). In subgroup analysis according to study type, individuals using ACEIs/ARBs were associated with a 6 % decreased risk of CRC among the case–control studies (95 % CI 0.90–0.99, p = 0.010). No significant heterogeneity was observed (I 2 = 0 %, p = 0.910; Fig. 2b). Because there was only one cohort study [22] and one cross-sectional study [28], no subgroup analysis was conducted among these groups of patients. Furthermore, when two trials [26, 28] without adjusting for confounding factors were removed, the pooled RR was 0.93 (95 % CI 0.88–0.98, p = 0.010) (I 2 = 0 %, p = 0.980; Fig. 2c). Another subgroup analysis indicated a 13 % decreased risk of CRC in ACEI users compared with non-users (95 % CI 0.81–0.93, p < 0.0001; Fig. 3). Comparison between ARB users and non-users was not allowed due to insufficient data. Nevertheless, the findings did not remain robust after excluding the study by Azoulay et al. [24] (RR 0.95, 95 % CI 0.88–1.03, p = 0.210; Table 3). Furthermore, there was no indication of publication bias with Egger’s test (p = 0.801).
Risk of CRA incidence
A retrospective cohort study [19] involving 1,760 continuous lisinopril (a kind of ACEIs) users and 2,900 non-users, who all had a history of adenomatous polyps (AP), and were on a follow-up colonoscopy, was performed in the USA. It indicated a 41 % reduction in the incidence of advanced APs in lisinopril users compared to non-users (95 % CI 0.49–0.69). After adjusting for known polyp risk factors, as well as NSAID and statin treatment, the association was significant.
Survival and prognosis of CRC
The relationship between ACEIs/ARBs use with survival and prognosis of established CRCs was evaluated in three studies. A retrospective cohort study by Engineer et al. [20] demonstrated that patients with stage III and IV CRC exposed to a combination of ACEIs/ARBs and beta blockers had a decreased mortality compared to unexposed patients (HR 0.50, 95 % CI 0.29–0.85; Cox regression, p = 0.010). Moreover, it also showed a decline of hospitalizations and cancer progression in the exposed group (HR 0.59, 95 % CI 0.36–0.99, p = 0.047). A nested case–control study by Cardwell et al. [27] found a reduction in cancer-specific mortality in CRC patients using ACEI compared to non-users (adjusted OR 0.78, 95 % CI 0.66, 0.92), but the association was not significant among ARB users (adjusted OR 0.82, 95 % CI 0.64, 1.07). However, Holmes et al. [21] did not find any difference of mortality in ACEIs/ARBs users compared to non-users in CRC individuals (HR 1.03, 95 % CI 0.93–1.15, p = 0.560) (Tables 1, 2). In the meta-analysis, the combined RR was 0.81 for CRC mortality (95 % CI 0.60–1.09, p = 0.160; Fig. 4). There was significant heterogeneity among the studies (I 2 = 85 %, p = 0.002), as a result of the different study designs and heterogeneity in the study population.
Dose–response relationship
Seven studies investigated the dose–response relationship between ACEIs/ARBs therapy and CRC, the results of which are listed in Table 4. Most studies did not observe any apparent dose–response relationships based on categories of cumulative duration and dosage, except for the two studies by Makar et al. [26] and Kedika et al. [19].
Discussion
The present systematic review with meta-analysis has indicated that ACEIs/ARBs might be associated with a reduced risk of CRC, as well as its precancerous lesion, CRA. Furthermore, there was some evidence to suggest that ACEIs/ARBs treatment might improve the outcomes in patients suffering from CRC, but the evidence was not robust. The dose–response relationship was uncertain, while some evidence has illustrated that longer duration and higher dose of ACEIs/ARBs therapy are associated with lower risk of CRC or advanced AP.
There is a body of evidence that many of the agents used in the cardiovascular system, such as statins [29] and aspirins [30], play a protective role in CRC. In addition, combination therapy with ACEIs or ARBs and cyclooxygenase-2 inhibitors has been indicated to have an anticancer effect through down-regulation of insulin-like growth factor I receptors in colon cancer cells [31]. ACEIs and ARBs are antihypertensive medications which act specifically on the RAS. Accumulating data has suggested that RAS is involved in certain steps of carcinogenesis and consequently regulates cell proliferation and tumor growth [32]. It is demonstrated that RAS inhibitors might exert an inhibitory effect on tumor angiogenesis by reducing the expression of vascular endothelial growth factor [33], induce cancer cell apoptosis, and disrupt the microenvironment of tumor [34].
In particular, a previous in vivo study has showed that ACEIs or ARBs reduce the number of colonic pre-neoplastic lesions in metabolically disordered mice [35]. As an obesity-related metabolic abnormality, CRC is prevented by RAS inhibitors through attenuating chronic inflammation in the colonic mucosa [36]. Furthermore, there is convincing evidence that ACEIs or ARBs suppress CRC liver metastases [37–39] and improve survival in established CRC patients.
Moreover, the ACE insertion/deletion (I/D) gene polymorphism is related to the positive association between ACEIs/ARBs and CRC. The cohort study by van der Knaap et al. [22] has demonstrated that individuals with the DD genotype, which is associated with high levels of ACE, are protected against cancer by RAS inhibitors.
It is commonly accepted that most CRCs develop from CRAs via an adenoma–carcinoma sequence [40]. The cohort study by Kedika et al. [19] has indicated that the long-term use of lisinopril is related to decreased incidence of advanced APs. Therefore, the authors speculated that ACEIs might lower the risk of CRC by reducing the development of advanced AP.
However, there had been some potential confounders that should be taken into consideration before we drew a conclusion. Firstly, users of ACEIs/ARBs tend to suffer from obesity, hypertension, diabetes mellitus, and other conditions of the metabolic syndrome. Meanwhile, they may be more likely to be smokers [24]. These factors were associated with higher CRC risk. On the contrary, users of ACEIs/ARBs are more probable to be prescribed statins and aspirin [24], which have been recognized as pharmaceutical approaches for CRC prevention [36]. Fortunately, most included studies have adjusted these confounders except two [26, 28], and the result remained significant when excluding these two studies. Among studies evaluating the survival and prognosis of CRC, all three articles have adjusted for cancer stage.
The current study has some limitations. First of all, current evidence on this topic is still limited; further research is likely to have an important impact on the estimate of effect. Secondly, other certain confounders of CRC, such as family history, occupational exposure, and race, were not adjusted. Thirdly, all the included articles were observational studies, and no randomized controlled trials were identified, which prevented us from determining the causality of the association. Fourthly, many of the included studies did not ascertain medication compliance of the patients. Meanwhile, individuals treated with ACEIs/ARBs were under increased medical surveillance, leading to follow-up bias. Furthermore, in the meta-analysis of mortality studies, statistical heterogeneity existed. Last but not least, in the study by Marker et al. [26] that analyzed the dose–response relationship, there might be misclassification of exposure duration, given the lack of information about medication use before enrollment.
To our knowledge, it is the first systematic review with meta-analysis to investigate the association between ACEIs/ARBs therapy and CRC. The included studies were of high quality, and many of them were based on a large prescription database. Therefore, a large study population was involved, relatively complete follow-ups were assured, and reliable data on CRC incidence or mortality were provided. The meta-analysis of incidence studies enrolled a total of 113,048 participants, with no heterogeneity observed among the individual studies, which ascertained considerable statistical power. In addition, sensitivity and subgroup analyses were performed to observe the instability of the meta-analysis.
In conclusion, there was evidence that ACEIs/ARBs therapy might be associated with a reduced risk of CRC, but whether use of these agents improves the outcomes of CRC remained unknown. The dose–response relationship was vague, whereas some evidence has suggested that the association between ACEIs/ARBs and CRC development might be related to longer duration and higher dose. However, the results from this study did not allow for a definitive conclusion. Further large-scale studies are required to confirm this relationship, and to explore the optimal dose and duration of ACEIs/ARBs for preventive or adjuvant therapy in CRC.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E et al (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Andrieu N, Launoy G, Guillois R, Ory-Paoletti C, Gignoux M (2003) Familial relative risk of colorectal cancer: a population-based study. Eur J Cancer 39:1904–1911
Klabunde CN, Lanier D, Nadel MR, McLeod C, Yuan G et al (2009) Colorectal cancer screening by primary care physicians: recommendations and practices, 2006-2007. Am J Prev Med 37:8–16
Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM et al (1993) Reducing mortality from colorectal cancer by screening for fecal occult blood. Minn Colon Cancer Control Study N Engl J Med 328:1365–1371
Vogelaar I, van Ballegooijen M, Schrag D, Boer R, Winawer SJ et al (2006) How much can current interventions reduce colorectal cancer mortality in the US? Mortality projections for scenarios of risk-factor modification, screening, and treatment. Cancer 107:1624–1633
Force USPST (2008) Screening for colorectal cancer: US preventive services task force recommendation statement. Ann Intern Med 149:627–637
Lever AF, Hole DJ, Gillis CR, McCallum IR, McInnes GT et al (1998) Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet 352:179–184
Huang CC, Chan WL, Chen YC, Chen TJ, Lin SJ et al (2011) Angiotensin II receptor blockers and risk of cancer in patients with systemic hypertension. Am J Cardiol 107:1028–1033
Mc Menamin UC, Murray LJ, Cantwell MM, Hughes CM (2012) Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in cancer progression and survival: a systematic review. Cancer Causes Control 23:221–230
Friis S, Sorensen HT, Mellemkjaer L, McLaughlin JK, Nielsen GL et al (2001) Angiotensin-converting enzyme inhibitors and the risk of cancer: a population-based cohort study in Denmark. Cancer 92:2462–2470
Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC (2010) Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol 11:627–636
Song M, Giovannucci EL (2014) Antihypertension and colorectal cancer prevention: getting two birds with one stone? J Natl Cancer Inst 106:djt438
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(264–269):W264
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Thornton A, Lee P (2000) Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol 53:207–216
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Kedika R, Patel M, Pena Sahdala HN, Mahgoub A, Cipher D et al (2011) Long-term use of angiotensin converting enzyme inhibitors is associated with decreased incidence of advanced adenomatous colon polyps. J Clin Gastroenterol 45:e12–e16
Engineer DR, Burney BO, Hayes TG, Garcia JM (2013) Exposure to ACEI/ARB and beta-blockers is associated with improved survival and decreased tumor progression and hospitalizations in patients with advanced colon cancer. Transl Oncol 6:539–545
Holmes S, Griffith EJ, Musto G, Minuk GY (2013) Antihypertensive medications and survival in patients with cancer: a population-based retrospective cohort study. Cancer Epidemiol 37:881–885
van der Knaap R, Siemes C, Coebergh JW, van Duijn CM, Hofman A et al (2008) Renin-angiotensin system inhibitors, angiotensin I-converting enzyme gene insertion/deletion polymorphism, and cancer: the Rotterdam Study. Cancer 112:748–757
Assimes TL, Elstein E, Langleben A, Suissa S (2008) Long-term use of antihypertensive drugs and risk of cancer. Pharmacoepidemiol Drug Saf 17:1039–1049
Azoulay L, Assimes TL, Yin H, Bartels DB, Schiffrin EL et al (2012) Long-term use of angiotensin receptor blockers and the risk of cancer. PLoS One 7:e50893
Boudreau DM, Koehler E, Rulyak SJ, Haneuse S, Harrison R et al (2008) Cardiovascular medication use and risk for colorectal cancer. Cancer Epidemiol Biomark Prev 17:3076–3080
Makar GA, Holmes JH, Yang YX (2014) Angiotensin-converting enzyme inhibitor therapy and colorectal cancer risk. J Natl Cancer Inst 106:djt374
Cardwell CR, Mc Menamin UC, Hicks BM, Hughes C, Cantwell MM et al (2014) Drugs affecting the renin-angiotensin system and survival from cancer: a population based study of breast, colorectal and prostate cancer patient cohorts. BMC Med 12:28
Mansouri D, McMillan DC, Roxburgh CS, Crighton EM, Horgan PG (2013) The impact of aspirin, statins and ACE-inhibitors on the presentation of colorectal neoplasia in a colorectal cancer screening programme. Br J Cancer 109:249–256
Bardou M, Barkun A, Martel M (2010) Effect of statin therapy on colorectal cancer. Gut 59:1572–1585
Dube C, Rostom A, Lewin G, Tsertsvadze A, Barrowman N et al (2007) The use of aspirin for primary prevention of colorectal cancer: a systematic review prepared for the US preventive services task force. Ann Intern Med 146:365–375
Yasumaru M, Tsuji S, Tsujii M, Irie T, Komori M et al (2003) Inhibition of angiotensin II activity enhanced the antitumor effect of cyclooxygenase-2 inhibitors via insulin-like growth factor I receptor pathway. Cancer Res 63:6726–6734
Deshayes F, Nahmias C (2005) Angiotensin receptors: a new role in cancer? Trends Endocrinol Metab 16:293–299
Greene AS, Amaral SL (2002) Microvascular angiogenesis and the renin-angiotensin system. Curr Hypertens Rep 4:56–62
George AJ, Thomas WG, Hannan RD (2010) The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer 10:745–759
Kubota M, Shimizu M, Sakai H, Yasuda Y, Ohno T et al (2011) Renin-angiotensin system inhibitors suppress azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Biochem Biophys Res Commun 410:108–113
Shirakami Y, Shimizu M, Kubota M, Araki H, Tanaka T et al (2014) Chemoprevention of colorectal cancer by targeting obesity-related metabolic abnormalities. World J Gastroenterol 20:8939–8946
Neo JH, Ager EI, Angus PW, Zhu J, Herath CB et al (2010) Changes in the renin angiotensin system during the development of colorectal cancer liver metastases. BMC Cancer 10:134
Wen SW, Ager EI, Neo J, Christophi C (2013) The renin angiotensin system regulates Kupffer cells in colorectal liver metastases. Cancer Biol Ther 14:720–727
Luo Y, Ohmori H, Shimomoto T, Fujii K, Sasahira T et al (2011) Anti-angiotensin and hypoglycemic treatments suppress liver metastasis of colon cancer cells. Pathobiology 78:285–290
Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61:759–767
Conflict of interest
All authors have declared that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, YN., Wang, JH., Zhu, JZ. et al. Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers therapy and colorectal cancer: a systematic review and meta-analysis. Cancer Causes Control 26, 1245–1255 (2015). https://doi.org/10.1007/s10552-015-0617-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-015-0617-1