Abstract
Background
Angiotensin signaling is suggested to be involved in tumorigenesis, tumor proliferation, and metastases. In colorectal cancer (CRC), it was demonstrated that angiotensin I-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) may reduce the risk of CRC; however, their impact on tumor recurrence remains unknown. Therefore, in this study, we evaluated the impact of ACEIs/ARBs on tumor recurrence in CRC patients.
Patients and methods
We retrospectively investigated the clinicopathological data of 461 stage I–III CRC patients. We divided the patients into those who took an ACEI and/or ARB (the ACEI/ARB+ group) and those who did not (the ACEI/ARB− group), and we compared the two groups’ recurrence-free survival (RFS) using a Kaplan-Meier curve analysis and log rank test. We also examined the impact of AGTR1 expression on tumor recurrence, using two public CRC datasets.
Results
The Kaplan-Meier curves showed a trend toward improved RFS in the ACEI/ARB+ group versus the ACEI/ARB− group (p = 0.063). Subgroup analyses demonstrated that the RFS was significantly better in the ACEI/ARB+ group versus the ACEI/ARB− group in the patients with left-sided CRC (p = 0.030) and those with stage I CRC (p = 0.009). Consistent with these findings, the AGTR1 expression was higher in the left-sided versus right-sided colon (p = 0.048). High AGTR1 expression levels were associated with poor RFS in the GSE39582 dataset’s stage I–III CRC patients (p < 0.001), and this finding was also validated in the GSE17536 dataset (p = 0.023).
Conclusion
ACEI/ARB treatment may reduce tumor recurrence in left-sided CRC and early-stage CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC), one of the most frequently diagnosed malignancies, is a leading cause of cancer-related deaths worldwide [1]. The overall survival of individuals with CRC has improved dramatically due to the appearance of molecular targeted therapies that suppress specific pathways involved in CRC progression [2]. One such therapy is the use of a vascular endothelial growth factor (VEGF) inhibitor, which suppresses the neovascularization that is crucial for tumor progression or metastases [3]. However, molecular targeted therapy drugs have been insufficient to reduce the risk of tumor recurrence in adjuvant settings, and the induction of conventional chemotherapy is still necessary to decrease tumor recurrence [4]. Novel strategies in addition to surgery and the current chemotherapies/molecular targeted therapies must be developed.
Cumulative evidence has suggested that the renin-angiotensin system (which is associated with hypertension) is also involved in tumor progression, mainly through angiogenesis [5]. Drugs that suppress the renin-angiotensin system may thus have anti-tumor effects. The widely used antihypertensive agents, angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), have been demonstrated to reduce tumor proliferation and metastases in many basic research studies. Cohort studies have also shown that ACEIs and ARBs may reduce the risk of tumor recurrence and improve the prognosis in many types of cancers, including pancreatic, gastric, renal cell, and lung cancers [6,7,8,9].
Angiotensin II receptor type 1 (AT1) is the main target of angiotensin, and AGTR1 is the coding gene of AT1. AGTR1 was shown to be up-regulated and correlated with advanced tumor stage in CRC [10, 11]. Moreover, blockade of AGTR1 by the administration of an ACEI or ARB suppressed tumor growth and the incidence of liver metastases [12,13,14]. Large epidemiological studies revealed that patients who were taking ARBs had decreased incidences of advanced polyp and CRC [15,16,17]. This result may be supported by the results of other basic research suggesting that there is cross-talk between angiotensin signaling and Wnt signaling, which is one of the main pathways of tumorigenesis and tumor progression in CRC [18]. These findings support the ideas that [1] angiotensin signaling is one of the important pathways for tumorigenesis and tumor progression in CRC, and [2] its blockade may reduce the risk of tumor recurrence. We thus conducted the present study to investigate the effects of ACEIs and ARBs on tumor recurrence in a large number of CRC patients. Intriguingly, our results demonstrated, for the first time, that these agents may reduce the risk of tumor recurrence, especially in early-stage and left-sided CRCs.
To test the findings we obtained in the clinical cohort, we also performed an in silico evaluation of the association between tumor recurrence and AGTR1 expression, using two independent public datasets of large numbers of CRC patients. Consistent with our findings in the clinical cohort, we observed that the AGTR1 expression was highly activated in left-sided colon cancer and significantly associated with tumor recurrence in CRCs. We conclude that angiotensin signaling is important in CRC progression, and its blockade by the use of an ACEI and/or ARB may reduce the risk of tumor recurrence in CRC patients.
Patients and methods
Evaluation of the association between ACEI/ARB intake and tumor recurrence in the clinical patient cohort
We retrospectively evaluated the cases of the patients with stage I–III colorectal adenocarcinoma who had undergone curative resection in the period from 2009 to 2014 at Teikyo University Hospital. Patients who underwent neoadjuvant therapies (radiation, chemotherapy, and chemoradiotherapy) and those who had multiple advanced CRCs were excluded. The patients’ clinical information was examined retrospectively, and their use of an ACEI and/or ARB, other antihypertensive drugs (beta-blockers and calcium blockers), and aspirin was evaluated at the time of surgery.
We divided the enrolled patients into two groups: those who took an ACEI and/or ARB (the ACEI/ARB+ group) and those who did not (the ACEI/ARB− group), and we compared the clinicopathological features and survival between the two groups. We defined the location from the cecum to the transverse colon as the right side, and the colorectum from the descending colon to the rectum as the left side. The 5-year recurrence-free survival (RFS) was defined as the period between the date of surgery and the date of tumor recurrence within 5 years after surgery.
Typically, a patient underwent the removal of the affected colorectum and regional lymph nodes up to the root of the feeding artery. Post-operative surveillance including the determination of the carcinoembryonic antigen (CEA) level was performed at 3-month intervals, and a computed tomography (CT) examination was conducted at 6-month intervals. Five fluorouracil-based adjuvant chemotherapies were administered to patients with stage II or III CRC and high-risk factors (i.e., the presence of serosal invasion, lymphovascular invasion, poorly differentiated histology, and the presence of obstruction or perforation at the time of surgery). Written informed consent was obtained from all of the patients involved. The study was approved by the ethical committee of Teikyo University.
Data acquisition and evaluation of AGTR1 expression in public CRC datasets
We evaluated the association between AGTR1 expression and tumor recurrence using two large public datasets of colon cancer cases, the GSE39582 dataset (stages I–III, n = 502 and stage IV, n = 60), and the GSE17536 (stages I–III, n = 176) in the Gene Expression Omnibus (data acquisition date: 2/1/2019) [19, 20]. Both datasets included microarray data (the Affymetrix Human Genome U133 Plus 2.0 Array) of the transcriptome from tumor tissue samples of colon cancer. Only the expression data of tumor tissues from stage I–IV colon cancer were included.
Both datasets had two probes for AGTR1, i.e., 205357_s_at and 208016_s_at, and the higher expression was regarded as the AGTR1 expression of each sample. In the GSE39582 dataset, recurrence-free survival (RFS) was available and in the GSE17536 dataset disease-free survival (DFS) was accessible. We included the stage I–III colon cancers when evaluating the RFS or DFS. We divided each cohort into low and high groups based on the expression levels of AGTR1, and we compared the RFS and DFS of the low and high groups using the log-rank test. The cut-off values for RFS and DFS were decided with the use of Youden’s index in receiver operating characteristic (ROC) curves. We also compared the AGTR1 expression between non-metastatic (stage I–III) and metastatic (stage IV) CRC cases, and between proximal colon and distal colon cases. We used the two public datasets for these analyses; ethical approval was not necessary.
Statistical analyses
As noted above, we divided the patients into those treated versus not treated with an ACEI/ARB based on the patients’ information at the time of surgery, and we compared several clinicopathological characteristics between the two groups. The χ2 test was used for categorical data, and the Mann-Whitney U test was applied for continuous variables. We compared the groups’ RFS and DFS by determining the Kaplan-Meier curves, and differences were evaluated with the log-rank test. Univariate and multivariate analyses using a Cox-proportional hazard model were performed to investigate the factors affecting RFS in the clinical patient cohort, and a multivariate analysis was performed using factors with a p value < 0.05 in the univariate analysis. In all of the statistics, differences with a p value < 0.05 were considered significant. All statistical calculations were performed using JMP Pro 13 statistical software (SAS Institute Japan, Tokyo).
Results
The clinical cohort: the patients with left-sided CRC in the ACEI/ARB+ group achieved significantly better RFS
Of the 461 eligible patients, at the time of surgery, a total of 144 (31%) patients were taking an ACEI and/or ARB and 54 patients (12%) were taking aspirin. Ninety-four patients (20%) had recurrences during the median-follow up period of 57 months. The clinicopathological features of the ACEI/ARB+ group and ACEI/ARB− group are summarized in Table 1, and the details of the patients taking anti-hypertensive drugs or aspirin are shown in Table 2.
The patients who were taking an ACEI/ARB were significantly more likely to be older (> 75 years) and to have poorly differentiated CRC (p = 0.003 and 0.021, respectively). The other clinicopathological features were not significantly different between the patient who were using an ACEI/ARB and those who were not (Table 3).
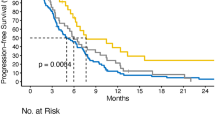
The Kaplan-Meier curves showed a trend toward improved RFS in the ACEI/ARB+ patients compared to the ACEI/ARB− patients (5-year RFS; 81% vs 74%, p = 0.063, Fig. 1a). Because recent studies have suggested that the etiology and biology differ between right-sided and left-sided colorectums, we evaluated the impact of ACEI/ARB intake in subgroups of right-sided colon and left-sided colon cases [21]. Intriguingly, the RFS was significantly better among the patients with left-sided CRC in the ACEI/ARB+ group compared to those in the ACEI/ARB− group (5-year RFS; 84% vs 73%, p = 0.030, Fig. 1b), although this difference was not observed in the patients with right-sided CRC (5-year RFS; 77% vs 77%, p = 0.876, Fig. 1c).
Kaplan-Meier curves comparing recurrence-free survival (RFS) between the ACEI/ARB+ and ACEI/ARB− groups in CRC. a In the stage I–III CRC cases, the ACEI/ARB+ patients showed a trend toward improved RFS compared to the ACEI/ARB− patients (p = 0.063). b In the left-sided CRC cases, the ACEI/ARB+ patients showed significantly improved RFS compared to the ACEI/ARB− patients (p = 0.030). c In the right-sided CRC cases, the RFS was not significantly different between the ACEI/ARB+ and ACEI/ARB− patients (p = 0.876). d In the stage I CRC cases, the ACEI/ARB+ patients showed significantly improved RFS compared to the ACEI/ARB− patients (p = 0.009). e In the stage II CRC cases, the RFS was not significantly different between the ACEI/ARB+ and ACEI/ARB− patients (p = 0.442). f In stage III CRC, the RFS was not significantly different between the ACEI/ARB+ and ACEI/ARB− patients (p = 0.636)
The clinical cohort: the stage I patients in the ACEI/ARB intake group had significantly better RFS
We next evaluated the effect of ACEI/ARB intake at each CRC stage. The Kaplan-Meier curves revealed significantly better RFS in the stage I patients in the ACEI/ARB+ group (n = 43) compared to the stage I patients in the ACEI/ARB− group (n = 71) (5-year RFS; 100% vs 82%, p = 0.009, Fig. 1d). No significant difference was observed in the stage II (5-year RFS; 84% vs 80%, p = 0.442, Fig. 1e) or stage III patients (5-year RFS; 64% vs 63%, p = 0.636, Fig. 1f).
Univariate and multivariate analyses for RFS in stage I and left-sided CRC
We used a Cox proportional hazard model to examine the stage I CRC cases and the left-sided CRC cases to investigate the factors affecting RFS. In the stage I CRC patients (n = 114), the univariate analysis showed that only ACEI/ARB intake was significantly associated with decreased RFS: hazard ratio (HR), not available (NA); 95% confidence interval (CI), 0.31; p < 0.001 (Table 4).
In the left-sided CRC patients (n = 307), the following factors were significantly associated with RFS: tumor depth (T3/4) (HR 1.95; 95%CI 1.11–3.67, p = 0.020), presence of lymph node metastases (HR 2.48; 95%CI 1.51–4.12, p < 0.001), serum CEA (> 5 ng/ml) (HR 1.79; 95%CI 1.07–2.95, p = 0.027), and ACEI/ARB intake (HR 0.51; 95%CI 0.26–0.92, p = 0.023). The results of the multivariate analysis revealed that the presence of lymph node metastases (HR 2.39; 95%CI 1.44–4.01, p < 0.001) and ACEI/ARB intake (HR 0.54; 95%CI 0.28–0.99, p = 0.045) were independently associated with RFS in left-sided CRC.
The public datasets: AGTR1 expression was significantly up-regulated in metastatic colon cancer and distal colon
We first compared the levels of ATGR1 expression between non-metastatic and metastatic colon cancer in the GSE39582 dataset, and we observed that the expression levels of ATGR1 were highly up-regulated in metastatic CRC compared to non-metastatic colon cancer (p = 0.017, Fig. 2a). This result supports the notion that ATGR1 expression is associated with metastases of colon cancer. Then, because ACEI/ARB intake was associated with decreased tumor recurrence in the left-sided colorectum, we also evaluated the difference in the expression levels of ATGR1 between the proximal colon cases and the distal colon cases in the public datasets. Intriguingly, the levels of ATGR1 expression were significantly up-regulated in the distal colon compared to the proximal colon (p = 0.048, Fig. 2b), suggesting that angiotensin signaling was more activated in left-sided colon than in right-sided colon.
The relationship between AGTR1 expression and CRC in the two public CRC datasets. a The expression levels of AGTR1 were significantly up-regulated in the stage IV cases compared to the stage I–III cases in the GSE39582 dataset (p = 0.017). b The expression levels of AGTR1 were significantly up-regulated in distal colon compared to proximal colon in the GSE39582 dataset (p = 0.048). c High AGTR1 expression was associated with decreased RFS in the GSE39582 dataset (p < 0.001). d High AGTR1 expression was associated with decreased DFS in the GSE17536 dataset (p = 0.023)
The public datasets: AGTR1 expression was associated with CRC recurrence
The Kaplan-Meier curves showed that high ATGR1 expression was significantly associated with poor RFS (p < 0.001, Fig. 2c) among the stage I–III colon cancer patients in the GSE39582 dataset. We tested this result in the other CRC dataset (GSE17536) and observed that it was consistent with the GSE39582 dataset finding; i.e., high levels of ATGR1 expression were significantly correlated with decreased DFS in stage I–III colon cancers (p = 0.023, Fig. 2d).
Discussions
Surgical removal is the main treatment for non-metastatic CRC, but even after curative resection, we sometimes encounter tumor recurrence, particularly in advanced-stage CRC. For advanced stage CRC, adjuvant chemotherapy is recommended to reduce the likelihood of tumor recurrence [4]. However, patients often suffer from side effects of such chemotherapy, and the use of an alternative therapy may be necessary.
The involvement of the renin-angiotensin system has been proposed in the progression of various types of cancers including CRC, and it has been shown that AGTR1 may be a promising target in the treatment of CRC [6,7,8,9, 22, 23]. Intriguingly, the results of our present analyses revealed that AGTR1 expression was significantly associated with tumor recurrence in two large public CRC datasets.
ACEIs and ARBs are commonly used anti-hypertensive drugs. ACEIs suppress angiotensin II production, and ARBs block AT1. These drugs thus have the potential to suppress tumor progression in CRC. In both in vivo and in vitro studies, angiotensin-signaling regulated tumor proliferation or metastases by controlling angiogenesis [12, 13]. Several studies have demonstrated an interaction between angiotensin signaling and VEGF signaling, and the concentration of angiotensinogen in the blood can be a marker for predicting the patient response to anti-VEGF therapy [5, 10, 24]. In addition, large epidemiological studies have shown a preventative effect of ACEI/ARB against the incidence of CRC [6, 15, 16].
Regarding the association between ACEI/ARB and survival, a meta-analysis revealed that among patients with several types of cancers (e.g., renal cell carcinomas, pancreatic cancer, and gastric cancer), the ACEI/ARB users achieved significantly better overall survival, whereas in CRC, only a trend toward better overall survival was shown [25]. However, because patients who take ACEIs/ARBs usually have more comorbidities compared to those who do not, the overall survival might be not the appropriate outcome for investigation. We therefore examined the effect of ACEI/ARB on tumor recurrence, and to our knowledge, this is the first study showing a positive impact of ACEIs/ARBs on reducing tumor recurrence in CRC patients.
Our analyses revealed two important findings. First, the use of ACEIs/ARBs made a significant contribution to decreasing tumor recurrence in the patients with early CRCs but not in those with advanced CRCs. This result suggests that ACEIs/ARBs may have some effect against tumor recurrence, but their preventative effect is not so strong that it reduces tumor recurrence in advanced CRCs. Conventional adjuvant chemotherapy may thus be necessary to reduce tumor recurrence in patients with advanced CRC. Our finding is supported by the result of a study in which a more marked reduction in cancer-specific mortality was observed in early-stage CRC compared to advanced-stage CRC with ACEIs [25].
Our second important finding is that the ACEI/ARB use had a significant impact in patients with left-sided CRCs. Right-sided and left-sided CRCs are now known to be embryologically and genetically different, and the effects of chemotherapies differ between them [21]. Consistent with this, we observed that the expression levels of AGTR1 were significantly up-regulated in the left-sided CRCs compared to the right-sided CRCs. It can thus be speculated that angiotensin signaling may be more activated in left-sided CRCs, and that ACEIs/ARBs’ preventative effect against tumor recurrence had much more impact in left-sided CRCs compared to right-sided CRCs. This speculation is supported by the results of studies that used an in-silico approach to investigate potential therapeutic drugs; ARBs were revealed as a potential candidate treatment for KRAS wild-type CRC (which is more dominant in left-sided CRCs) [26, 27]. However, we did not have access to the information about RAS mutation in the present cohort, and this finding remains to be confirmed in future studies.
In addition, patients who take ACEIs and/or ARBs are often prescribed other types of anti-hypertensive drugs or anti-coagulative drugs such as aspirin, because hypertension sometimes causes cardiovascular disease. The results of several studies indicated an anti-tumorigenic or anti-tumor proliferation effect of anti-hypertensive drugs (including beta-blockers and aspirin) [6, 28,29,30]; we therefore also investigated the impacts of these drugs on tumor recurrence. Our results showed no effect of calcium channel blocker, beta-blocker, or aspirin on the recurrence-free survival rate in CRC (data not shown).
We acknowledge several limitations of this study. It was a retrospective analysis, and we could not evaluate the effect of ACEIs and ARBs separately. We also could not determine the dose-response relationships because of the limited number of patients. Large-scale prospective studies are needed to confirm our findings.
In conclusion, the use of ACEIs/ARBs may have a positive impact on the reduction of the likelihood of tumor recurrence in CRC, particularly among left-sided CRC and early-stage CRC cases.
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68(1):7–30
Harris M (2004) Monoclonal antibodies as therapeutic agents for cancer. Lancet Oncol 5(5):292–302
Hicklin DJ, Ellis LM (2005) Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23(5):1011–1027
Brenner H, Kloor M, Pox CP (2014) Colorectal cancer. Lancet 383(9927):1490–1502
Childers WK (2015) Interactions of the renin-angiotensin system in colorectal cancer and metastasis. Int J Color Dis 30(6):749–752
Holmes S, Griffith EJ, Musto G, Minuk GY (2013) Antihypertensive medications and survival in patients with cancer: a population-based retrospective cohort study. Cancer Epidemiol 37(6):881–885
Nakai Y, Isayama H, Ijichi H, Sasaki T, Sasahira N, Hirano K, Kogure H, Kawakubo K, Yagioka H, Yashima Y, Mizuno S, Yamamoto K, Arizumi T, Togawa O, Matsubara S, Tsujino T, Tateishi K, Tada M, Omata M, Koike K (2010) Inhibition of renin-angiotensin system affects prognosis of advanced pancreatic cancer receiving gemcitabine. Br J Cancer 103(11):1644–1648
Sun H, Li T, Zhuang R, Cai W, Zheng Y (2017) Do renin-angiotensin system inhibitors influence the recurrence, metastasis, and survival in cancer patients?: evidence from a meta-analysis including 55 studies. Medicine (Baltimore) 96(13):e6394
Dolley-Hitze T, Jouan F, Martin B, Mottier S, Edeline J, Moranne O, le Pogamp P, Belaud-Rotureau MA, Patard JJ, Rioux-Leclercq N, Vigneau C (2010) Angiotensin-2 receptors (AT1-R and AT2-R), new prognostic factors for renal clear-cell carcinoma? Br J Cancer 103(11):1698–1705
Ager EI, Wen SW, Chan J, Chong WW, Neo JH, Christophi C (2011) Altered efficacy of AT1R-targeted treatment after spontaneous cancer cell-AT1R upregulation. BMC Cancer 11:274
Zhou L, Luo Y, Sato S, Tanabe E, Kitayoshi M, Fujiwara R, Sasaki T, Fujii K, Ohmori H, Kuniyasu H (2014) Role of two types of angiotensin II receptors in colorectal carcinoma progression. Pathobiology. 81(4):169–175
Neo JH, Malcontenti-Wilson C, Muralidharan V, Christophi C (2007) Effect of ACE inhibitors and angiotensin II receptor antagonists in a mouse model of colorectal cancer liver metastases. J Gastroenterol Hepatol 22(4):577–584
Neo JH, Ager EI, Angus PW, Zhu J, Herath CB, Christophi C (2010) Changes in the renin angiotensin system during the development of colorectal cancer liver metastases. BMC Cancer 10:134
Shimizu Y, Amano H, Ito Y, Betto T, Yamane S, Inoue T, Nishizawa N, Matsui Y, Kamata M, Nakamura M, Kitasato H, Koizumi W, Majima M (2017) Angiotensin II subtype 1a receptor signaling in resident hepatic macrophages induces liver metastasis formation. Cancer Sci 108(9):1757–1768
Azoulay L, Assimes TL, Yin H, Bartels DB, Schiffrin EL, Suissa S (2012) Long-term use of angiotensin receptor blockers and the risk of cancer. PLoS One 7(12):e50893
Makar GA, Holmes JH, Yang YX (2014) Angiotensin-converting enzyme inhibitor therapy and colorectal cancer risk. J Natl Cancer Inst 106(2):djt374
Mansouri D, McMillan DC, Roxburgh CS, Crighton EM, Horgan PG (2013) The impact of aspirin, statins and ACE-inhibitors on the presentation of colorectal neoplasia in a colorectal cancer screening programme. Br J Cancer 109(1):249–256
Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J, Hou FF, Kahn M, Liu Y (2015) Multiple genes of the renin-angiotensin system are novel targets of Wnt/beta-catenin signaling. J Am Soc Nephrol 26(1):107–120
Marisa L, de Reynies A, Duval A et al (2013) Gene expression classification of colon cancer into molecular subtypes: characterization, validation, and prognostic value. PLoS Med 10(5):e1001453
Smith JJ, Deane NG, Wu F, Merchant NB, Zhang B, Jiang A, Lu P, Johnson JC, Schmidt C, Bailey CE, Eschrich S, Kis C, Levy S, Washington MK, Heslin MJ, Coffey RJ, Yeatman TJ, Shyr Y, Beauchamp RD (2010) Experimentally derived metastasis gene expression profile predicts recurrence and death in patients with colon cancer. Gastroenterology. 138(3):958–968
Petrelli F, Tomasello G, Borgonovo K et al (2016) Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol
Rocken C, Rohl FW, Diebler E, Lendeckel U, Pross M, Carl-McGrath S, Ebert MPA (2007) The angiotensin II/angiotensin II receptor system correlates with nodal spread in intestinal type gastric cancer. Cancer Epidemiol Biomarkers Prev 16(6):1206–1212
Fujita M, Hayashi I, Yamashina S, Itoman M, Majima M (2002) Blockade of angiotensin AT1a receptor signaling reduces tumor growth, angiogenesis, and metastasis. Biochem Biophys Res Commun 294(2):441–447
Osumi H, Matsusaka S, Wakatsuki T, Suenaga M, Shinozaki E, Mizunuma N (2015) Angiotensin II type-1 receptor blockers enhance the effects of bevacizumab-based chemotherapy in metastatic colorectal cancer patients. Mol Clin Oncol 3(6):1295–1300
Cardwell CR, Mc Menamin UC, Hicks BM, Hughes C, Cantwell MM, Murray LJ (2014) Drugs affecting the renin-angiotensin system and survival from cancer: a population based study of breast, colorectal and prostate cancer patient cohorts. BMC Med 12:28
Bleeker WA, Hayes VM, Karrenbeld A, Hofstra RMW, Hermans J, Buys CHCM, Plukker JTM (2000) Impact of KRAS and TP53 mutations on survival in patients with left- and right-sided Dukes’ C colon cancer. Am J Gastroenterol 95(10):2953–2957
Wen Q, Dunne PD, O'Reilly PG et al (2017) KRAS mutant colorectal cancer gene signatures identified angiotensin II receptor blockers as potential therapies. Oncotarget. 8(2):3206–3225
Ahl R, Matthiessen P, Fang X et al (2018) Beta-blockade in rectal Cancer surgery: a simple measure of improving outcomes. Ann Surg
Li P, Wu H, Zhang H, Shi Y, Xu J, Ye Y, Xia D, Yang J, Cai J, Wu Y (2015) Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: a meta-analysis. Gut. 64(9):1419–1425
Restivo A, Cocco IM, Casula G et al (2015) Aspirin as a neoadjuvant agent during preoperative chemoradiation for rectal cancer. Br J Cancer 113(8):1133–1139
Author information
Authors and Affiliations
Contributions
Study concept and design, statistical analysis, and manuscript preparation were done by T.O. Data acquisition was done by T.O, Y.O, K.O, T.Y, Y.F, R.S, T.H, T.T, K.N, and H.I. Manuscript editing and review were done by Y.H and S.I.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ozawa, T., Hashiguchi, Y., Yagi, T. et al. Angiotensin I-converting enzyme inhibitors/angiotensin II receptor blockers may reduce tumor recurrence in left-sided and early colorectal cancers. Int J Colorectal Dis 34, 1731–1739 (2019). https://doi.org/10.1007/s00384-019-03379-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-019-03379-y