Abstract
Purpose
We sought to assess survival outcomes of patients with de novo metastatic breast cancer (dnMBC) who did not receive treatment irrespective of the reason.
Methods
Adults with dnMBC were selected from the NCDB (2010–2016) and stratified based on receipt of treatment (treated = received at least one treatment and untreated = received no treatments). Overall survival (OS) was estimated using the Kaplan–Meier method, and groups were compared. Cox proportional hazards models were used to identify factors associated with OS.
Results
Of the 53,240 patients with dnMBC, 92.1% received at least one treatment (treated), and 7.9% had no documented treatments, irrespective of the reason (untreated). Untreated patients were more likely to be older (median 68 y vs 61 y, p < 0.001), have higher comorbidity scores (p < 0.001), have triple-negative disease (17.8% vs 12.6%), and a higher disease burden (≥ 2 metastatic sites: 38.2% untreated vs 29.2% treated, p < 0.001). The median unadjusted OS in the untreated subgroup was 2.5 mo versus 36.4 mo in the treated subgroup (p < 0.001). After adjustment, variables associated with a worse OS in the untreated cohort included older age, higher comorbidity scores, higher tumor grade, and triple-negative (vs HR + /HER2-) subtype (all p < 0.05), while the number of metastatic sites was not associated with survival.
Conclusions
Patients with dnMBC who do not receive treatment are more likely to be older, present with comorbid conditions, and have clinically aggressive disease. Similar to those who do receive treatment, survival in an untreated population is associated with select patient and disease characteristics. However, the prognosis for untreated dnMBC is dismal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Among women in the US, breast cancer is the second most common cause of cancer-related mortality [1]. With the advancement of treatments and systemic therapies, survival for patients with early-stage breast cancer has improved with a 5-year relative survival exceeding 90% in most developed countries [1, 2]. For those with metastatic breast cancer (MBC), survival outcomes have also improved, although not to the same degree [3]. Given the improvements in systemic treatments for select patients with MBC, survival outcomes now vary widely, and our recently proposed staging model was able to stratify patients with de novo MBC (dnMBC) into three distinct subgroups [4], thus providing refined prognostic estimates for patients with dnMBC.
In a recent 2020 study, Afifi et al. evaluated the causes of death among women with breast cancer in the US from 2000 to 2015, using the Surveillance, Epidemiology, and End Results Program (SEER) registries [5]. Within 5–10 years following diagnosis, 38.2% of patients had died from breast cancer, even after assuming that most of these women received standard of care. However, data describing the prognosis for those with dnMBC who forego treatment are lacking. In order to better understand the true advantages of treatment, a solid understanding of the natural history of untreated cases is critical. In most reports, patients with breast cancer of any/all stages survive nearly 3–4 years without any type of treatment, and approximately 5–10% of untreated patients live > 10 years [6]. This likely reflects the spectrum of clinical aggressiveness also observed in patients with non-metastatic disease, suggesting that untreated breast cancer varies between a virulent and chronic disease.
Although studies on patients with untreated dnMBC specifically are lacking, several older studies have looked at survival outcomes in patients with any stage of breast cancer that went untreated. In a study by Phillips et al. (including all stages and all ages) published in 1959, the average duration of life from onset of symptoms was 46.2 months [7], which was similar to other previously published works from 1927 to 1946 (estimates 38–40.5 months) [8]. In a review of untreated breast cancer (all stages) by Galmarini et al., it was noted that most studies were published > 50 years ago [6]. In addition, most studies reported that a “lump” had been present for 3–12 months prior to diagnosis, and 25% of untreated women exhibited distant metastases within a year [6].
Given the limited data in this unique population, we sought to assess outcomes in patients with untreated dnMBC, irrespective of the reason that treatment was not received. Our primary aim was to report survival outcomes for those with untreated dnMBC in a contemporary cohort of patients in the National Cancer Database (NCDB), compared to those who receive treatment. Our secondary aims were (1) to determine what, if any, variables are associated with survival outcomes in those with untreated dnMBC and (2) to determine if our previously proposed staging model could also meaningfully stratify patients with untreated dnMBC.
Methods
Patients age ≥ 18 diagnosed with dnMBC were selected from the National Cancer Database (NCDB, 2017 Participant User File). Patients with distant metastatic disease were identified as those with clinical M1 or pathologic M1 disease. Patients with ICD-O3 histology codes not included in the WHO Classification of Tumors [9] contained in the AJCC 8th Edition Breast Cancer Staging Manual [10] were excluded, as were those with unknown or missing survival data. NCDB administratively masks survival data for all patients diagnosed during the last reporting year of the dataset, so all patients diagnosed in 2017 were excluded from this analysis. Because human epidermal growth factor receptor-2 (HER2) status was not reliably coded until 2010, all patients diagnosed prior to 2010 were also excluded. The final patient cohort for this study includes patients diagnosed 2010–2016. Additionally, patients for whom treatment status was unable to be defined were also excluded. Of note, patients with other cancers were not specifically excluded, although it is presumably uncommon for a patient to have multiple metastatic cancers, albeit not impossible. In addition, patients who underwent excisional biopsy of the primary breast tumor for diagnostic purposes only were included in the treatment subgroup, as this type of surgery would be included in the possible surgery types of “Surgical Procedure of Primary Site” in the NCDB [11]. However, if this was the only “treatment,” they were excluded from the subgroup analyses of patients who received at least one type of systemic therapy.
Patients were classified as undergoing “any treatment” if they underwent any systemic therapy (chemotherapy, immunotherapy, or endocrine therapy), lumpectomy or mastectomy, radiation therapy, or any other therapy that could not be classified as systemic, surgical, or radiation. Other patients were classified as “untreated” if they did not undergo any of the aforementioned treatments, except in the case of palliative treatment, as coded in the NCDB. Of note, the NCDB separately encodes “palliative care” and describes it as “…any care provided in an effort to palliate or alleviate symptoms…may include surgery, radiation therapy, systemic therapy (chemotherapy, hormone therapy, or other systemic drugs), and/or other pain management therapy” [11]. Furthermore, patients were assigned as “untreated” based on documentation of treatment in the NCDB, irrespective of the reason and/or patient knowledge. Patients who underwent treatment were further classified as undergoing any systemic treatment or no systemic treatment. Hormone receptor positive was defined as being estrogen receptor (ER) or progesterone receptor (PR) positive. Triple-negative breast cancer was defined as being ER negative, PR negative, and HER2 negative (ER-/PR-/HER2-). Of note, when determining the number of sites of metastatic disease, the NCDB defines 1 “site” as having only one organ system involved, such as having bone-only metastases, although the patient may have multiple bone metastases. Thus, 2 sites of metastatic disease imply that two different organ systems are involved, such as both bone and lungs, but it does not refer to the actual number of metastatic foci within any given organ system. Although some data are available in the NCDB regarding site of radiation, this was not investigated in the current study (only whether any radiation therapy was given with therapeutic intent or palliative intent).
Patient demographic, disease, and treatment characteristics were summarized with N (%) for categorical variables and median (interquartile range, IQR) for continuous variables. Differences between patient groups (treated vs untreated) were tested using Chi-square tests for categorical variables and t-tests for continuous variables.
Overall survival (OS) was defined as the time from diagnosis to death or last follow-up. Median follow-up was estimated using the reverse Kaplan–Meier method, and unadjusted OS was estimated using the Kaplan–Meier method. Survival rates were estimated at 1, 2, 3, 6, and 12 months. Median OS was estimated for all patients and for those surviving at least 1, 2, 3, 6, and 12 months. OS was estimated after stratifying by select covariates including age (< 40, 40–70, and ≥ 70), gender (male and female), tumor phenotype (HER2 + , hormone receptor + /HER2-, and triple-negative breast cancer), number of metastatic organ systems involved (1, 2, 3, and 4+), and metastatic site (bone only, brain only, liver only, lung only, other only, and multiple). Additional analyses were performed using our previously published stratification system for those with dnMBC (proposed stage IVA, IVB, and IVC), which is based on groupings determined by 3-year OS outcomes [4]. In brief, patients with more favorable disease characteristics (i.e., hormone receptor positive, less disease burden, etc.) were often classified as stage IVA or IVB, while those with less favorable disease characteristics (i.e., triple negative, extensive disease burden, etc.) were generally classified as stage IVC [4]. Cox proportional hazards models were used to identify factors associated with OS in the untreated metastatic subgroup after adjustment for known covariates. This model included age, Charlson/Deyo comorbidity score, facility type and location, insurance status, race/ethnicity, and gender, in addition to either metastatic stage or the variables used to define metastatic stage (grade, tumor phenotype, number of metastatic sites, and metastatic site). Additional models were conducted that excluded patients who died or were lost to follow-up within 1 month. All adjusted survival models included a robust sandwich covariance estimator to account for the correlation of patients treated at the same facility.
No adjustments were made for multiple comparisons, and p-value < 0.05 was considered statistically significant. Only patients with complete data were included in each analysis, and effective sample sizes are listed for each table and figure. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute, Cary NC) or R, version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). Given the use of retrospective de-identified national data, the study was deemed exempt by the Institutional Review Board at our institution.
Results
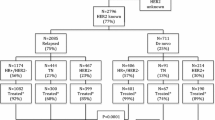
Of the 53,240 patients diagnosed with dnMBC 2010–2016 in the NCDB, 49,040 received some treatment (92.1% treated) and 4200 patients received no treatment (7.9% untreated) (Fig. 1). Of those who received some treatment, 46,476 received at least one systemic therapy (94.8%), while 2245 did not receive any systemic therapy (4.6%). Within the entire cohort, 11,043 patients (20.7%) received palliative care at some point during follow-up, which was more common among those who received treatment for their dnMBC diagnosis than those who were untreated (21.5% vs 12.4%, p < 0.001). The median follow-up for all patients was 53.7 months (95% CI 53.3–54.2), and the median OS was 33.6 months (95% CI 33.1–34.1). Of those who received at least one treatment, 61.7% received chemotherapy, 59.9% received endocrine therapy, 33.8% underwent lumpectomy or mastectomy, and 35.9% received radiation therapy (Supplemental Table 1). Of note, data regarding biopsy of suspected distant metastases in the NCDB are not available. However, 33.1% were listed as having pathologic M1 disease, suggesting that pathology was assessed (likely by biopsy) for those patients; unfortunately, pathologic M stage was missing for 66.4% of patients.
Treated versus untreated dnMBC
Compared to those who received treatment for their dnMBC diagnosis, patients with untreated dnMBC tended to be older (median age 68 years old vs 61 years old, p < 0.001) and have higher comorbidity scores (score ≥ 2: 9.8% vs 4.8%, p < 0.001). Treatment receipt was similar for men and women with breast cancer, while the proportion of non-Hispanic Black patients was higher among the untreated cohort than the treated cohort (19.9% vs 16.8%, overall p for race/ethnicity < 0.001). Patients in the untreated cohort tended to come from areas with lower income levels and lower education levels (both p < 0.001). Furthermore, the proportion of government and uninsured patients was higher among the untreated cohort than the treated cohort (government insurance: 66.3% vs 51.4% and uninsured: 7.3% vs 5%; overall p for insurance type < 0.001). Patients with untreated dnMBC were more likely to have unknown T/N categories (TX: 20.7% vs 10.1% and NX: 19.9% vs 9.7%; both p < 0.001) and missing biomarkers (19.4% vs 7.4%; p < 0.001). Untreated patients were also more likely to have more than 1 site of metastatic disease (38.2% vs 29.2%, p < 0.001) (Table 1).
When comparing survival outcomes for those with untreated versus treated dnMBC, patients with untreated disease had significantly lower OS rates (unadjusted median OS: 2.5 vs 36.4 months, log rank p < 0.001; Fig. 2A). When further stratified by receipt of systemic versus non-systemic treatment, those who received non-systemic treatment had better outcomes than those who were untreated, but still had significantly truncated survival (unadjusted median OS: untreated 2.5 months, treated with non-systemic therapy 7.0 months, and treated with systemic therapy 37.8 months; log rank p < 0.001; Fig. 2B). In order to determine if the observed survival differences were largely due to early deaths by those who did not receive treatment, additional unadjusted survival analyses were conducted based on a defined period of survival (Fig. 3). For example, for those who survived at least 1 month after diagnosis, the unadjusted median OS was 6.9 months for untreated patients versus 38.5 months for those who received systemic therapy (vs 9.8 months for those who received non-systemic therapy). For those who survived at least 3 months, significant differences were again observed (unadjusted median OS: untreated, 18.6 months; treated with non-systemic therapy, 20.9 months; and treated with systemic therapy, 40.8 months).
Unadjusted median overall survival for patients with de novo metastatic breast cancer from the National Cancer Database, diagnosed 2010–2016, stratified by treatment (untreated vs treated w/non-systemic therapy vs treat w/systemic therapy) and by survival time (if patients survived X months, where X = 1 month, 2 months, 3 months, 6 months, or 12 months)
Survival outcomes for patients with untreated dnMBC
When stratified by age group, comparatively younger patients (age < 40 years old) with untreated dnMBC had significantly better survival outcomes compared to the two other cohorts (unadjusted median OS: age < 40, 28.5 months; age 40–70, 2.8 months; and age > 70, 2.2 months; log rank p < 0.001; Supplemental Fig. 1). However, no differences were observed when stratified by patient sex (unadjusted median OS: male, 2.6 months and female, 2.5 months; log rank p = 0.46). As noted for patients who receive treatment for dnMBC, survival outcomes also varied based on tumor phenotype for those with untreated dnMBC (unadjusted median OS: HR + /HER2-, 3.8 months; HER2 + , 2.6 months; and TNBC, 2.1 months; log rank p < 0.001; Fig. 4A). Predictable variations were also observed when stratified by disease burden, as patients with less disease had better survival outcomes (unadjusted median OS: 1 metastatic site, 4.1 months; 2 metastatic sites, 1.8 months; 3 metastatic sites, 1.1 months; and 4 + metastatic sites, 1.2 months; log rank p < 0.001; Fig. 4B). For patients with only 1 site of metastatic disease, those with bone only metastases lived longer than those with lung only, brain only, or liver only metastases (unadjusted median OS: bone only, 5.8 months, lung only, 3.8 months; brain only, 2.0 months; and liver only, 1.2 months; log rank p < 0.001; Fig. 4C). Comparison of patients with untreated dnMBC based on previously proposed staging guidelines for patients with dnMBC [4] demonstrated that those with stage IVA disease (generally defined as those with more limited disease and favorable biology) had the best survival outcomes, compared to those with stage IVB or IVC disease (unadjusted median OS: stage IV, 5.4 months; stage IVB, 2.4 months; and stage IVC, 1.7 months; log rank p < 0.001; Fig. 4D).
Unadjusted overall survival for untreated patients with de novo metastatic breast cancer from the National Cancer Database, diagnosed 2010–2016, stratified by: A tumor subtype (HR + /HER2- vs HER2 + vs TNBC), B number of metastatic sites (= number of organ systems involved), C location of metastatic site/involvement, and D stage (IVA vs IVB vs IVC). HR + : hormone receptor positive. HER2: human epidermal growth factor receptor-2. TNBC: triple-negative breast cancer (estrogen receptor negative, progesterone receptor negative, and HER2 negative)
After adjusting for select covariates, factors associated with worse survival outcomes included older age, non-Hispanic White race/ethnicity, higher comorbidity scores, having government insurance or no insurance, higher tumor grade, location of metastatic site (bone, brain, liver, and lung), and TNBC tumor subtype (all p ≤ 0.01; Table 2). Notably, the number of metastatic sites was not significantly associated with survival in the adjusted analysis. Similar findings were noted when disease stage (A/B/C) was used instead of the individual disease characteristics, and a higher disease stage was found to be associated with worse outcomes (p < 0.001; Table 2). After excluding patients who died or were lost to follow-up within 1 month of diagnosis, factors associated with worse survival outcomes included: older age, higher comorbidity scores, having government insurance or no insurance, tumor grade, metastatic site (brain and liver), and TNBC tumor subtype (all p ≤ 0.002; Supplemental Table 2). As previously observed, using disease stage instead of individual disease characteristics again demonstrated an association with higher disease stage and worse outcomes (p < 0.001; Supplemental Table 2).
Discussion
De novo MBC has a poor prognosis, and few studies have delineated factors that lead to differences in OS among patients with untreated dnMBC. To fill this knowledge gap, our study identified factors that impact survival in this patient population. Overall, patients with untreated dnMBC were more likely to be older, have multiple comorbidities, were disproportionately of non-White race and/or Hispanic ethnicity, lower income, lower education, uninsured, had triple-negative disease, and higher disease burden. After adjustment, we continued to see that older age, higher comorbidity scores, higher tumor grade, and triple-negative disease were still associated with a worse OS in untreated dnMBC patients compared to treated dnMBC patients. Interestingly, extent of disease (or number of metastatic sites involved) was not associated with survival, after adjusting for known covariates, suggesting that the presence of any distant metastasis is a sufficiently poor prognostic indicator for patients who do not receive treatment. These results contribute to our understanding of patients with untreated dnMBC by demonstrating that a combination of disease-specific and patient-related factors impacts OS. While the prognosis is generally poor for all patients with dnMBC, survival is particularly dismal for those who do not receive any treatment.
Other national registry studies have also shown that survival for patients with treated MBC is influenced by a combination of patient and disease-specific factors. For example, a Brazilian population-based study identified histologic grade and initial site of metastases as key prognostic factors [12]. In this study, median survival was 20 months, and insurance status significantly impacted OS (public 19.7 months vs private 27.2 months). The authors further recognized that socioeconomic constraints, which limited access to treatment, contributed to lower OS when compared to MBC patients in the US [12]. Even when health-care access was accounted for in a study from New Zealand, which has a national health-care system, dnMBC was shown to be associated with older age, living in more deprived areas and HER2 + disease [13]. Similarly, recent studies of patients with dnMBC in SEER demonstrated that worse outcomes were related to both disease factors, such as tumor phenotype and disease burden, but also race/ethnicity and insurance status [3]. Notably, all of these studies were largely examining patients who received at least some treatment for their disease. However, our study suggests that survival is dependent on similar factors whether treatment is received or not.
Many studies have confirmed that systemic therapy can improve survival outcomes for patients with dnMBC [14]. This is particularly true for patients with HER2 + disease, where targeted therapies have revolutionized the treatment paradigm for these patients, and new regimens are on the horizon [15,16,17,18]. However, it is less clear if surgery for the primary breast tumor is an important component of treating dnMBC. Several retrospective studies of large national tumor registries have suggested that surgery may improve survival [19,20,21], yet prospective randomized controlled trials have yielded conflicting findings [22,23,24]. Notably, the only trial performed in the US did not demonstrate an improved survival for patients with dnMBC who underwent early local–regional therapy for the primary site [24]. In contrast, the study by Soran et al. demonstrated a higher OS with longer follow-up (10 years) in patients who underwent local–regional therapy followed by systemic therapy, compared to those who only received systemic therapy [23]. Taken together, it may be possible that there are smaller subgroups of patients with dnMBC that may benefit from surgery [25], and further stratification of these patients may help reveal those subgroups. For example, our group has previously stratified patients into proposed stage IV subgroups (A/B/C) and noted that those with dnMBC and the most favorable tumor biology and least disease burden may benefit the most from surgery, assuming that there is a subgroup that benefits from resection of the primary tumor [26]. Our prior work has also shown that isolated nodal metastases in particular are sites of metastases that often portend a better prognosis, and as such, supraclavicular metastases are now considered N3 disease with better survival outcomes than those with dnMBC [27], while recent studies suggest a similar shift for patients with contralateral axillary metastases [28]. Similar to the surgical question, radiation therapy is also controversial [29, 30], and more studies are needed to confirm or refute the findings of prior work.
Another interesting finding from our study is the significant difference in survival for patients aged < 40 years old compared to the other subgroups (ages 40–70 or ≥ 70). Unfortunately, the comparatively younger cohort of untreated patients was quite small (only 102 patients). However, it appears that many of the deaths in the age groups 40–70 and ≥ 70 years old occurred in the 1st year, compared to those in the youngest cohort, which occurred steadily over time. This is likely multifactorial, but it could be related to younger patients having less advanced disease at diagnosis, more aggressive treatment because of their young age, and more willingness to accept any/all treatment because of their young age. Furthermore, we have previously shown that patients in a similar age group (although all stages) have a higher proportion of those with HER2 + disease [31], which is often more responsive to systemic therapies.
One surprising finding from our study was the lack of association between number of sites involved and survival outcomes. However, the specific site involved (bone, liver, lung, etc.) was significantly associated with outcomes. This is in contrast with the treated group, where the number of sites involved (or extent of disease) was shown to be a significant factor associated with outcomes in other studies from some of the authors in our group [4, 32]. It is unclear why this difference exists, but it could be related to the effectiveness of select treatments at some anatomic sites compared to others (more or less treatment responsive due to uptake/exposure, such as activity in the brain), whereas without treatment, this becomes irrelevant because no sites are being treated.
Another interesting finding from our study was the improved survival outcomes observed in minority populations. This is in contrast with other studies, where minority patients are often found to have worse survival outcomes. For example, the 2022 Breast Cancer Statistics report noted a lower incidence rate in Black versus White women, yet substantial racial disparities persisted [33]. Although likely multifactorial, the authors postulated that some of this difference may be related to variations in tumor subtype [33], which could be a similar confounder for the current study. Unfortunately, treatment documentation in the NCDB is not always available or accurate [34, 35]. Furthermore, it could be that the minority patients were more likely to receive care at multiple facilities (instead of no treatment, as documented by one facility), and this potential care fragmentation could have impacted our findings. Others have shown that care fragmentation is common among some minority populations [36], and this may be associated with survival outcomes [37].
While our study is one of the largest studies and most contemporary to review survival outcomes for patients with dnMBC that goes untreated, there are some limitations. The NCDB captures > 80% of breast cancer patients in the US [38], but the reported data have notable biases inherent to national registries [34, 39]. Specifically, there is no information on frailty, functional status, clinical presentation, symptoms, and/or results from biopsies of distant metastases, available in the NCDB. Furthermore, information regarding treatment intent could have been mis-interpreted and incorrectly coded in the NCDB as palliative when it was intended as therapeutic. In addition, there is a lack of granularity related to patient comorbidities, treatments received, and treatment adherence, as well no record of why certain treatments were or were not given [39]. Unfortunately, there were some missing data in the dataset, which was often more common for the untreated group, which may be related to patients not completing recommended testing (due to illness or patient preference, etc.). We have previously shown that missing data are associated with worse outcomes [34], suggesting that outcomes could be even worse for those with untreated disease than our study findings suggest. As a retrospective study, it is also not possible to control for the biases that led to treatments being offered for select patients and not others. More specifically, patients presenting with dnMBC who were younger, with less comorbidities, and more favorable tumor biology, were notably more likely to receive systemic therapy. Regardless, our study provides contemporary insight into the patient and disease-related factors that contribute to outcomes in patients with dnMBC who do not receive treatment, thus providing a background against which the benefits of treatment can be compared.
Conclusion
Overall, our study showed that patients with untreated dnMBC tended to be older, have more comorbid conditions, and presented with more clinically aggressive disease compared to those patients treated with systemic and/or local–regional therapies. Similar to those who do receive treatment, survival in an untreated population, irrespective of the reason, is associated with select patient and disease characteristics. However, the prognosis for untreated dnMBC is dismal.
Data availability
All data used for this study are from the National Cancer Data Base, which is freely available to hospitals with CoC status.
References
Cancer Facts and Figures 2020. In.; 2020
Katanoda K, Matsuda T (2014) Five-year relative survival rate of breast cancer in the USA, Europe and Japan. Jpn J Clin Oncol 44(6):611
Taskindoust M, Thomas SM, Sammons SL, Fayanju OM, DiLalla G, Hwang ES, Plichta JK (2021) Survival outcomes among patients with metastatic breast cancer: review of 47,000 patients. Ann Surg Oncol 28(12):7441–7449
Plichta JK, Thomas SM, Sergesketter AR, Greenup RA, Rosenberger LH, Fayanju OM, Kimmick G, Force J, Hyslop T, Hwang ES (2020) A novel staging system for de novo metastatic breast cancer refines prognostic estimates. Ann Surg
Afifi AM, Saad AM, Al-Husseini MJ, Elmehrath AO, Northfelt DW, Sonbol MB (2020) Causes of death after breast cancer diagnosis: a US population-based analysis. Cancer 126(7):1559–1567
Galmarini CM, Tredan O, Galmarini FC (2015) Survivorship in untreated breast cancer patients. Med Oncol 32(2):466
Phillips AJ (1959) A comparison of treated and untreated cases of cancer of the breast. Br J Cancer 13(1):20–25
Bloom HJ, Richardson WW, Harries EJ (1962) Natural history of untreated breast cancer (1805–1933). Comparison of untreated and treated cases according to histological grade of malignancy. Br Med J 2(5299):213–221
WHO/IARC Classification of Tumours (2012) vol. 4, 4 edn. World Health Organization
AJCC Cancer Staging Manual (2016) 8th edn. Springer International Publishing, New York, NY
National Cancer Database Participant User File: 2021 Data Dictionary (2023) In., 9/2023 edn: American College of Surgeons: Cancer Programs
Soares LR, Freitas-Junior R, Curado MP, Paulinelli RR, Martins E, Oliveira JC (2020) Low overall survival in women with de novo metastatic breast cancer: Does This reflect tumor biology or a lack of access to health care? JCO Glob Oncol 6:679–687
Lao C, Kuper-Hommel M, Elwood M, Campbell I, Edwards M, Lawrenson R (2021) Characteristics and survival of de novo and recurrent metastatic breast cancer in New Zealand. Breast Cancer 28(2):387–397
Wang R, Zhu Y, Liu X, Liao X, He J, Niu L (2019) The Clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer 19(1):1091
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792
Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Antón A, Lluch A et al (2005) Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 23(19):4265–4274
Martínez-Sáez O, Prat A (2021) Current and future management of HER2-positive metastatic breast cancer. JCO Oncol Pract 17(10):594–604
Lebert J, Lilly EJ (2022) Developments in the management of metastatic HER2-positive breast cancer: a review. Curr Oncol 29(4):2539–2549
Khan SA, Stewart AK, Morrow M (2002) Does aggressive local therapy improve survival in metastatic breast cancer? Surgery 132(4):620–626; discussion 626–627
Thomas A, Khan SA, Chrischilles EA, Schroeder MC (2016) Initial surgery and survival in stage IV breast cancer in the United States, 1988–2011. JAMA Surg 151(5):424–431
Lane WO, Thomas SM, Blitzblau RC, Plichta JK, Rosenberger LH, Fayanju OM, Hyslop T, Hwang ES, Greenup RA (2019) Surgical resection of the primary tumor in women with de novo stage IV breast cancer: contemporary practice patterns and survival analysis. Ann Surg 269(3):537–544
Badwe R, Hawaldar R, Nair N, Kaushik R, Parmar V, Siddique S, Budrukkar A, Mittra I, Gupta S (2015) Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. The LancetOncology 16(13):1380–1388
Soran A, Ozmen V, Ozbas S, Karanlik H, Muslumanoglu M, Igci A, Canturk NZ, Utkan Z, Evrensel T, Sezgin E (2021) Primary surgery with systemic therapy in patients with de novo stage IV breast cancer: 10-year follow-up; protocol MF07-01 randomized clinical trial. J Am Coll Surg 233(6):742-751.e745
Khan SA, Zhao F, Solin LJ, Goldstein LJ, Cella D, Basik M, Golshan M, Julian TB, Pockaj BA, Lee CA et al (2020) A randomized phase III trial of systemic therapy plus early local therapy versus systemic therapy alone in women with de novo stage IV breast cancer: A trial of the ECOG-ACRIN Research Group (E2108). In: 2020 ASCO Virtual Scientific Program: 2020: ASCO Meeting Library
Wang K, Shi Y, Li ZY, Xiao YL, Li J, Zhang X, Li HY (2019) Metastatic pattern discriminates survival benefit of primary surgery for de novo stage IV breast cancer: a real-world observational study. Eur J Surg Oncol 45(8):1364–1372
Marks CE, Thomas SM, Fayanju OM, DiLalla G, Sammons S, Hwang ES, Plichta JK (2021) Metastatic breast cancer: Who benefits from surgery? Am J Surg
Tamirisa NP, Ren Y, Campbell BM, Thomas SM, Fayanju OM, Plichta JK, Rosenberger LH, Force J, Hyslop T, Hwang ES et al (2021) Treatment patterns and outcomes of women with breast cancer and supraclavicular nodal metastases. Ann Surg Oncol 28(4):2146–2154
Nash AL, Thomas SM, Plichta JK, Fayanju OM, Hwang ES, Greenup RA, Rosenberger LH (2021) Contralateral axillary nodal metastases: stage IV disease or a manifestation of progressive locally advanced breast cancer? Ann Surg Oncol 28(10):5544–5552
Soran A, Dogan L, Isik A, Ozbas S, Trabulus DC, Demirci U, Karanlik H, Soyder A, Dag A, Bilici A et al (2021) The effect of primary surgery in patients with de novo stage IV breast cancer with bone metastasis only (protocol BOMET MF 14–01): a multi-center, prospective registry study. Ann Surg Oncol 28(9):5048–5057
Chmura SJ, Winter KA, Woodward WA, Borges VF, Salama JK, Al-Hallaq HA, Matuszak M, Milano MT, Jaskowiak NT, Bandos H et al (2022) NRG-BR002: a phase IIR/III trial of standard of care systemic therapy with or without stereotactic body radiotherapy (SBRT) and/or surgical resection (SR) for newly oligometastatic breast cancer (NCT02364557). J Clin Oncol 40(16_suppl):1007–1007
Plichta JK, Thomas SM, Vernon R, Fayanju OM, Rosenberger LH, Hyslop T, Hwang ES, Greenup RA (2020) Breast cancer tumor histopathology, stage at presentation, and treatment in the extremes of age. Breast Cancer Res Treat 180(1):227–235
Plichta JK, Thomas SM, Hayes DF, Chavez-MacGregor M, Allison K, de Los SJ, Fowler AM, Giuliano AE, Sharma P, Smith BD et al (2023) Novel Prognostic Staging System for Patients With De Novo Metastatic Breast Cancer. J Clin Oncol 41(14):2546–2560
Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, Jemal A, Siegel RL (2022) Breast Cancer Statistics, 2022. CA Cancer J Clin 72(6):524–541
Plichta JK, Rushing CN, Lewis HC, Rooney MM, Blazer DG, Thomas SM, Hwang ES, Greenup RA (2023) Implications of missing data on reported breast cancer mortality. Breast Cancer Res Treat 197(1):177–187
Mallin K, Palis BE, Watroba N, Stewart AK, Walczak D, Singer J, Barron J, Blumenthal W, Haydu G, Edge SB (2013) Completeness of American Cancer Registry Treatment Data: implications for quality of care research. J Am Coll Surg 216(3):428–437
Doose M, Sanchez JI, Cantor JC, Plascak JJ, Steinberg MB, Hong CC, Demissie K, Bandera EV, Tsui J (2021) Fragmentation of care among black women with breast cancer and comorbidities: the role of health systems. JCO Oncol Pract 17(5):e637–e644
Gamboa Ó, Buitrago G, Patiño AF, Agudelo NR, Espinel LS, Eslava-Schmalbach J, Guevara Ó, Caycedo R, Junca E, Bonilla C et al (2023) Fragmentation of care and its association with survival and costs for patients with breast cancer in Colombia. JCO Glob Oncol 9:e2200393
Mallin K, Browner A, Palis B, Gay G, McCabe R, Nogueira L, Yabroff R, Shulman L, Facktor M, Winchester DP et al (2019) Incident cases captured in the national cancer database compared with those in U.S. Population Based Central Cancer Registries in 2012–2014. Ann Surg Oncol
Boffa DJ, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, Thoburn K, Gress D, McKellar DP, Shulman LN et al (2017) Using the national cancer database for outcomes research: a review. JAMA Oncol 3(12):1722–1728
Acknowledgements
The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC's NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Funding
Dr. Plichta is supported by the National Institutes of Health Office of Women’s Research Building Interdisciplinary Research Careers in Women’s Health K12AR084231 (PI: Amundsen). This work was in part supported by Duke Cancer Institute through NIH grant P30CA014236 (PI: Kastan) for the Biostatistics Core.
Author information
Authors and Affiliations
Contributions
Jennifer K. Plichta helped in conceptualization, methodology, data analysis, writing (original draft, review, and editing), and project administration. Samantha Thomas helped in methodology, resources, data curation, formal analysis, and writing (review and editing). Xuanji Wang worked in data review and writing (original draft, review, and editing). Sarah Sammons worked in data analysis and writing (review and editing). Susan McDuff worked in data analysis and writing (review and editing). Gretchen Kimmick worked in data analysis and writing (review and editing). E. Shelley Hwang worked in resources, data analysis, writing (review, and editing), and project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article. Dr. J. Plichta is a recipient of research funding by the Color Foundation and Earlier.org (PI: Plichta), unrelated to the submitted work. JP serves on the Editorial Committee for the American Joint Committee on Cancer (AJCC), Breast Cancer panel for the AJCC, and the National Comprehensive Cancer Network (NCCN) Breast Cancer Screening and Diagnosis panel. JP is on the editorial board for the journal (Breast Cancer Research and Treatment), but she was not involved in the editorial process for this manuscript. Dr. E.S. Hwang serves on the NCCN Breast Cancer Risk Reduction panel. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Plichta, J.K., Thomas, S.M., Wang, X. et al. Survival among patients with untreated metastatic breast cancer: “What if I do nothing?”. Breast Cancer Res Treat 205, 333–347 (2024). https://doi.org/10.1007/s10549-024-07265-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-024-07265-2