Abstract
Purpose
Breast cancer in men (BC-M) is almost exclusively hormone receptor positive. We conducted a large review of the SEER-Medicare linked database to compare endocrine therapy adherence, discontinuation, and survival outcomes of male versus female patients with breast cancer.
Methods
Study data were obtained through the SEER-Medicare linked database. The study included patients age ≥ 65 years-old diagnosed with breast cancer between 2007 and 2015. The primary endpoints were rates of adherence and discontinuation of endocrine therapy (ET). Adherence was defined as a gap of less than 90 days in-between consecutive Medicare prescriptions. Discontinuation was defined as a gap of greater than 12 months in-between Medicare prescriptions. Secondary endpoint was the association of use of ET with overall survival (OS).
Results
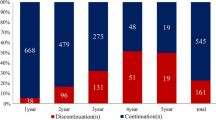
Of the 363 male patients on ET, 214 patients (59.0%) were adherent to the therapy, and 149 patients (41.0%) were nonadherent. Of the 20,722 females on ET, 10,752 (51.9%) were adherent to the therapy, and 9970 (48.1%) were nonadherent. 39 male patients (10.7%) discontinued therapy, while 324 (89.3%) did not discontinue therapy. 1849 female patients (8.9%) discontinued therapy, while 18,873 (91.1%) patients did not. Men were significantly more adherent than women (p = 0.008), but there was no significant difference in discontinuation among men and women (p = 0.228). Survival was significantly improved in both men (HR 0.77, 95% CI 0.60–0.99, p = 0.039) and women (HR 0.84, 95% CI 0.81–0.87, p < 0.001) on ET.
Conclusion
Identification of contributing factors impacting adherence and discontinuation is needed to allow physicians to address barriers to long term use of ET.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer in men (BC-M) is an uncommon malignancy, representing less than 1% of breast cancers; however, annual incidence of BC-M has been increasing over recent decades [1]. BC-M is almost exclusively estrogen receptor positive (ER +), and the most common pathologic subtype is overwhelmingly invasive ductal carcinoma (IDC) [2]. Thus, BC-M tends to resemble breast cancer in women (BC-W) that are primarily postmenopausal. There are very few randomized controlled trials involving BC-M due to its low incidence, and many older breast cancer trials explicitly excluded men from their cohorts. As a result, most treatment guidelines for BC-M are based on data extrapolated from BC-W [3], including indications for surgical management options, radiotherapy, chemotherapy, and hormone therapy.
Despite this approach, there is evolving evidence to suggest a difference in the underlying genetic and pathophysiology of BC-M from BC-W and improvement in the survival rate for men has lagged behind the improvement seen in women [4]. As BC-M is diagnosed at an older age and at a later stage, it is less likely to receive recommended treatment based on guidelines for BC-W [5]. Given that an overwhelming majority of BC-M patients is ER +, endocrine therapies such as tamoxifen have significant clinical utility in the management of BC-M. However, in Cardoso et al. showed that among male patients with breast cancer, despite being ER + in over 95% of cases, only 76% of patients were given adjuvant endocrine therapy (ET) [2]. Additionally, the authors found that adjuvant radiotherapy was not given to 45% of patients after breast conservation surgery and 30% of patients with node positive disease, which would be clinically indicated based on guidelines for BC-W.
There are high rates of tamoxifen discontinuation and problems with adherence to hormonal therapy, around 20% in men [6, 7]. Adverse events (AEs) that affect adherence and lead to discontinuation include hot flushes, decreased libido, weight gain, and general malaise. Tamoxifen discontinuation due to AEs has been associated with a worse prognosis [8], and it has been speculated that treatment with tamoxifen is less well-tolerated in men than women with breast cancer [3]. In this study, we present a large review of the Surveillance, Epidemiology, and End Results (SEER)-Medicare Registry to compare adherence, discontinuation, and survival outcomes of male versus female patients with breast cancer to identify any population-based trends.
Methods
Data collection
Study data were obtained through the SEER-Medicare registry. The SEER registry is supported by the National Cancer Institute (NCI) and contains demographics, cancer-related clinical information, and cause of death information for patients with cancer for up to 34% of the US population. The Medicare dataset, maintained and managed by the Centers for Medicare and Medicaid Services (CMMS), contains health care claims including prescription drug information for over 97% of the US population that is 65 years or older. The linkage of SEER database and Medicare dataset provides a unique population-based source of information for epidemiological cancer research [9].
Patient population
The study included patients age ≥ 65 years-old diagnosed with breast cancer between 2007 and 2015. The year 2007 was picked as a starting point of the data query due to the availability of SEER-Medicare Part D prescription drug coverage data. Patients were identified using the International Classification of Diseases for Oncology, third edition (ICD-O-3) codes from the SEER database. ICD 9 and 10 codes were used to identify patients with diagnosis of breast cancer who were prescribed endocrine therapy. National drug code (NDC) directory of the Federal Drug Administration (FDA) and Healthcare Common Procedure Coding System (HCPCS) were used to look up additional treatment details, including drug identification codes used for endocrine therapy in the patient population. Demographics data were collected for year of diagnosis, age, race, marital status, census poverty, comorbidity score, TNM stage, tumor grade, and for history of surgery or radiation therapy (Table 1).
Drug adherence and discontinuation
The primary endpoints of the analysis were drug adherence and discontinuation rates of ET as surrogate markers to evaluate gender specific practices, and to comment on any disparity in prescribing practices. Secondary endpoint was the correlation of ET with overall survival (OS). Drug adherence was defined as a gap of less than 90 days in-between Medicare prescriptions; 90 days was extrapolated from the standard of 80% optimal adherence [10] and rounded up to include full prescription cycles and to account for 90-day fills. Percentage of adherence was calculated as a proportion of patients with less than 90-day gap between refills and the entire gender specific cohort. Drug discontinuation was defined as a gap of greater than 12 months in-between Medicare prescriptions. Percentage of discontinuation was calculated as a proportion of patients with greater than 12-month gap in prescriptions and the entire gender specific cohort.
Statistical analysis
Patients with stage I through III breast cancer hormone receptor positive (HR +) were defined into two cohorts: male and female. Patient demographics, disease specific, and treatment-related variables were compared between the two cohorts using the Chi-square test. A two-proportion z-test was used to compare the proportions for drug adherence and drug discontinuation rate. We fitted multivariate logistic regression models to identify variables associated with adherence to or discontinuation of ET. Multivariate Cox proportional-hazards models were then constructed for each of the two cohorts to determine the relationship between use of hormone blockade therapy and OS, and was controlled for demographic variables, cancer stage, and grade, and history of surgery and radiation therapy. All statistical tests were two-sided and statistical significance was defined as p < 0.05. Analyses were conducted using SAS version 9.4 software (SAS institute).
Results
A total of 66,360 patients met the screening criteria of the study: 713 males and 65,647 females. Only 363 males (50.9%) and 20,722 females (31.6%) were on ET. Baseline characteristics for male and female cohorts are shown in Table 1. Of all the HR patients with breast cancer within the female cohort, a statistically significant proportion of them were not on ET, among all demographic and cancer-related variables. On the other hand, there was no statistically significant difference between the proportions of patients within the male cohort who were on ET versus not on ET, with the exception of history of surgery. Among patients who had surgery, more patients were on ET and among those who did not have surgery, more patients were not on ET (Table 1).
ET use pattern
Of the 363 male on ET, 214 patients (59.0%) were adherent to ET, and 149 patients (41.0%) were non-adherent. On the other hand, of the 20,722 female patients on ET, 10,752 (51.9%) were adherent to the therapy, and 9970 (48.1%) were non-adherent. There was significantly more adherence noted among the male cohort (p = 0.008) compared to the female cohort. Interestingly, of the 363 men on ET, only 39 patients (10.7%) discontinued therapy while 324 (89.3%) did not. Of the 20,722 women on ET, 1849 discontinued therapy (8.9%), while 18,873 (91.1%) did not. Hence, there was no difference in the discontinuation rate of hormone blockade therapy (p = 0.228) between men and women with breast cancer.
Among male patients on ET, majority of the patients (341, 93.9%) were on tamoxifen, 12 patients (3.3%) were on exemestane, 34 patients (9.4%) were on letrozole (data not shown).
Among female patients on ET, 11,375 patients (54.9%) were on letrozole, 8160 patients (39.4%) were on tamoxifen, 4858 patients (23.4%) were on exemestane, and 25 patients (0.1%) were on anastrozole (data not shown).
We conducted multivariate logistic regression analysis to identify variables associated with adherence to or discontinuation of ET among BC-M and BC-W. Within the male cohort, there was no difference between adherence to ET in terms of age, race, marital status, poverty status, geographic region, comorbidity score, grade, or history of radiation or surgery. Within the female cohort, higher rate of adherence was noted in women older than 80, and lower rate of adherence was seen in women of black ethnicity, in the 20–100% poverty range, 3 + comorbidity score, stage II or III cancers, or a positive history of radiation or chemotherapy (Fig. 1).
In terms of ET discontinuation, within the male cohort, significantly more discontinuation was seen in men older than 80, and less discontinuation seen in men with grade II tumors. Within the female cohort, more discontinuation was noted in women with increasing age, increasing comorbidity score, or positive history of chemotherapy. There was significantly less discontinuation of ET in white and Hispanic women, women who were married, or women who had surgery (Fig. 2).
Overall survival
Among the male cohort, being on ET was associated with a 23% risk reduction in mortality versus not being on ET (HR 0.77, 95% CI 0.60–0.99, p = 0.039). Among the female cohort, being on ET was associated with a 16% risk reduction in mortality versus not being on ET (HR 0.84, 95% CI 0.81–0.87, p < 0.001), data not shown. Using the multivariate cox regression analysis, we performed sub-group analysis, which showed that among the male cohort, being on ET was associated with a significant benefit in improved survival in patients who were white, married, in the 5- < 10% poverty range, lived in the Midwest, or had grade III tumors (Fig. 3). Among the female cohort, being on ET was associated with a significant benefit in improved survival in women older than 75, all races, regardless of marital status, any poverty range, any geographic region, any comorbidity score, regardless of stage I-III, grade I-III or history or surgery. There was also favorable survival for patients on ET in patients who did not receive radiation or chemotherapy (Fig. 3).
Discussion
BC-M is a rare disease making up for less than 1% of all cancers in men and about 1% of all breast cancers. Although the incidence of BC-M has increased to over 20% in the last 25 years, the majority of available data comes from observational studies due to a real dearth or focus in translational and clinical research [2]. Over time, there have been reports of improved age-corrected breast cancer mortality, with better OS and recurrence-free survival (RFS) noted in highly ER + and progesterone receptor positive (PR +), while no associated was noted with regards to Her-2 status, Ki67, or grade [2]. As majority of BC-M are IDCs with 80–90% expressing the estrogen receptor and 65–92% expressing the progesterone receptor, ET is the mainstay of treatment in this population [11].
Tamoxifen remains the gold standard adjuvant ET in early BC-M, as well as an important therapy in metastatic BC-M in absence of visceral crisis [12, 13]. Aromatase inhibitors (AIs) block the conversion of androstenedione to 17β-estradiol and are commonly used for HR + BC-W in postmenopausal women due to their documented superiority over tamoxifen in that age group [12]. However, in males, AIs lead to increased levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone (T). Increased T then counteracts the block imposed by AIs through an excess of substrate and directly stimulates cancer cells with androgen receptors. Therefore, gonadotropin releasing hormone (GnRH) analog should be used with AIs to achieve the deepest T suppression [14].
Tamoxifen and AIs both have toxicities that limit their use in patients with BC-M and BC-W, and only a few studies report the data on adherence or discontinuation in patients with BC-M. Visram et al. describe a retrospective report of 38 men with BC-M and note a 50% AE rate from tamoxifen, with toxicity largely being hot flashes (18.4%), decreased libido (13.2%), weight gain (13.2%), and malaise (13.2%). The authors also note a discontinuation rate of 23.7% due to AEs [11]. Xu et al. noted an adherence rate of 64.6% in the first year, 46.4% in the second year, and 28.7% in the third year, and the authors noted that low adherence (defined as medication possession ratio of 80% or less) was related to worse OS [15]. Cavazza et al. reported an adherence rate of 94% (at 1 year) to 58% (at 5 years) [16].
In this study, we utilize the SEER-Medicare database to focus on a larger cohort of patients to analyze the adherence and discontinuation practices. While SEER-Medicare database has been used to comment on the survival outcomes and biology of the cancers, the adherence and drug discontinuation data largely comes from retrospective reviews. We note a 59.0% adherence in patients with BC-M and 51.9% in patients with BC-W, and note that the difference is of significant value. Multivariate cox regression showed that there was no difference in adherence among men based on demographics, grade or stage, or cancer treatment. The lack of difference may in part be due to the small sample size. On the other hand, among women, we see lower adherence in patients with lower socioeconomic status, more comorbidities, and in women who had had radiation or chemotherapy likely accounting for previous cumulative toxicity from previous therapy.
We note that the adherence rate in this review is lower than the reported adherence rates as reported above by Xu et al. and Cavazza et al. [15, 16], and we attribute to several factors, such as undercapturing of the true adherence rate if patients are using private insurance as opposed to medicare alone for their prescription drug coverage. In the review by Cavazza et al. the authors report a mean age of 61–62 [16], and in Xu et al. the authors report a mean age of 62 for their patient population [15], whereas all of our patients by definition are > 65 years. Using SEER-Medicare dataset, we acknowledge that we are missing a subset of patients because of age eligibility requirement for Medicare, which makes it difficult to compare this adherence data to datasets, which include a younger subset of patients. We also acknowledge that as our data is largely focusing on an older patient population (> 65 years of age), this is a patient population with a high incidence of other non-cancer-related life-limiting comorbidities that may limit adherence to endocrine therapy.
We also observed that among all patients that met the screening criteria for this review, only 50.9% of eligible men and 31.6% of eligible women were on ET. We believe this is partially explained by the fact that patients were obtaining ET prescriptions from private insurances. We also recognize that this may identify a true deficiency and warrants further investigation into prescribing patterns. We also observed that up to 14% of male patients were on AIs but only 3% were on GnRH analogs, hence identifying another area of discordance and improvement as simultaneous use of GnRH analogs with AIs is key to achieve the deepest T suppression in men with breast cancer.
In terms of discontinuation rate, variables associated with increased discontinuation in men were age > 80, and in women, increasing age, stage III tumors and positive history of chemotherapy were associated with more discontinuation, again reflecting a patient population with increased propensity to drug-related toxicity. A lower rate of discontinuation of ET was noted in women that were black, married, or had history of surgery. We also note a much lower discontinuation rate in our study, which is 10.7% for men and 8.9% for women. This discontinuation rate was lower than 20–23% discontinuation rate of tamoxifen in men reported in retrospective studies where after discontinuing treatment patients presumably were not put back on it [6, 11, 17], although there is a report of 10% rate of tamoxifen withdrawal in men with breast cancer [18]. Our reported rate of discontinuation of ET was also lower than discontinuation rate in BC-W, where there are reported discontinuation rates in the 17–22% range, although the definitions of discontinuation vary (17% cumulative discontinuation at 1 year, up to 58% at 5 years [19] versus 22% self-reported discontinuation or use of ET < 5 years [20]). We define discontinuation as a gap in refill > 12 months, and that definition inherently overestimates the number of drug discontinuations if a fraction of patients were simply choosing to not fill their prescriptions through Medicare in favor of use of a private insurer. Because of this overestimation, we feel confident about the report of our discontinuation rate, which is lower than what is reported in literature. We also note that the lower discontinuation rate is in the context of a proportionately lower number of patient population on ET to begin with, hence, overall identifying an area of opportunity in prescribing practices and patient education in order to improve breast cancer-related outcomes.
Within this dataset, we compare the male and female adherence and discontinuation rates to control for patient selection bias, inherent in the retrospective design of this database driven study. For instance, the underestimation of number of patients not captured by the prescription fill data if they were obtaining hormone blockade therapy through a private insurer. We also note that accessing SEER-Medicare database gives us access to a larger set of data than what would not be attainable as a single institution retrospective review for the same duration.
There are several limitations to the study due to its inherent design. As SEER-Medicare database stores data for patients ≥ age 65 and BC-M tends to proportionately affect more men in older age, it is possible that our query shows a slight over-representation of breast cancer patients compared to their gender-controlled comparator cohort of females. As adherence and discontinuation data were obtained from the SEER registry based on prescription fill record, we are unable to comment on the exact AEs occurred that would have led up to the low adherence or discontinuation of ET. We did not query the SEER-Medicare database with the ICD9/10 diagnosis codes for the AEs, as the AEs seen with ET can be somewhat non-specific and hard to primarily attribute to ET in absence of patient report and physician documentation, as medical notes were not available to us.
As mentioned previously, due to the nature of the retrospective review from the SEER registry, we do believe this review underestimates the initiation and adherence rates of ET in both female and male patients in breast cancer, as we are unable to account for patients receiving drug coverage through private insurance. We recognize that we are not accounting for nuanced socioeconomic predictors of adherence such as, delivery of care in a tertiary care center with multidisciplinary teams, surgery performed in a high volume center, and concurrent treatment for mental health illnesses [16].
As there is clearly established and widely reported benefit of improved survival with use of ET in hormone positive breast cancer, as we show in this study, it is pertinent to identify population-based trends that contribute towards higher adherence and lower discontinuation rate of therapy. Identification of contributing factors can further allow physicians to note and address barriers to long term use of ET. Further larger database and ultimately prospective studies are desperately needed to compare adherence and discontinuation patterns among men and women with breast cancer.
References
Humphries MP, Jordan VC, Speirs V (2015) Obesity and male breast cancer: provocative parallels? BMC Med 13:134. https://doi.org/10.1186/s12916-015-0380-x
Cardoso F, Bartlett JMS, Slaets L, van Deurzen CHM, van Leeuwen-Stok E, Porter P, Linderholm B, Hedenfalk I, Schröder C, Martens J, Bayani J, van Asperen C, Murray M, Hudis C, Middleton L, Vermeij J, Punie K, Fraser J, Nowaczyk M, Rubio IT, Aebi S, Kelly C, Ruddy KJ, Winer E, Nilsson C, Lago LD, Korde L, Benstead K, Bogler O, Goulioti T, Peric A, Litière S, Aalders KC, Poncet C, Tryfonidis K, Giordano SH (2018) Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International male breast cancer program. Ann Oncol 29(2):405–417. https://doi.org/10.1093/annonc/mdx651
Losurdo A, Rota S, Gullo G, Masci G, Torrisi R, Bottai G, Zuradelli M, Gatzemeier W, Santoro A (2017) Controversies in clinicopathological characteristics and treatment strategies of male breast cancer: a review of the literature. Crit Rev Oncol Hematol 113:283–291. https://doi.org/10.1016/j.critrevonc.2017.03.013
Anderson WF, Jatoi I, Tse J, Rosenberg PS (2010) Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol 28(2):232–239. https://doi.org/10.1200/JCO.2009.23.8162
Jylling AMB, Jensen V, Lelkaitis G, Christiansen P, Nielsen SS, Lautrup MD (2020) Male breast cancer: clinicopathological characterization of a National Danish cohort 1980–2009. Breast Cancer 27(4):683–695. https://doi.org/10.1007/s12282-020-01066-3
Pemmaraju N, Munsell MF, Hortobagyi GN, Giordano SH (2012) Retrospective review of male breast cancer patients: analysis of tamoxifen-related side-effects. Ann Oncol 23(6):1471–1474. https://doi.org/10.1093/annonc/mdr459
Anelli TF, Anelli A, Tran KN, Lebwohl DE, Borgen PI (1994) Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer 74(1):74–77. https://doi.org/10.1002/1097-0142(19940701)74:1%3c74::aid-cncr2820740113%3e3.0.co;2-#
Dezentjé VO, van Blijderveen NJ, Gelderblom H, Putter H, van Herk-Sukel MP, Casparie MK, Egberts AC, Nortier JW, Guchelaar HJ (2010) Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early-stage breast cancer. J Clin Oncol 28(14):2423–2429. https://doi.org/10.1200/JCO.2009.25.0894
Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF (2002) Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 40(8 Suppl):IV-3–18. https://doi.org/10.1097/01.MLR.0000020942.47004.03
Chalela P, Munoz E, Inupakutika D, Kaghyan S, Akopian D, Kaklamani V, Lathrop K, Ramirez A (2018) Improving adherence to endocrine hormonal therapy among breast cancer patients: study protocol for a randomized controlled trial. Contemp Clin Trials Commun 12:109–115. https://doi.org/10.1016/j.conctc.2018.10.001
Visram H, Kanji F, Dent SF (2010) Endocrine therapy for male breast cancer: rates of toxicity and adherence. Curr Oncol 17(5):17–21. https://doi.org/10.3747/co.v17i5.631
Zagouri F, Sergentanis TN, Azim HA, Chrysikos D, Dimopoulos MA, Psaltopoulou T (2015) Aromatase inhibitors in male breast cancer: a pooled analysis. Breast Cancer Res Treat 151(1):141–147. https://doi.org/10.1007/s10549-015-3356-9
Hassett MJ, Somerfield MR, Baker ER, Cardoso F, Kansal KJ, Kwait DC, Plichta JK, Ricker C, Roshal A, Ruddy KJ, Safer JD, Van Poznak C, Yung RL, Giordano SH (2020) Management of male breast cancer: ASCO guideline. J Clin Oncol 38(16):1849–1863. https://doi.org/10.1200/JCO.19.03120
Di Lauro L, Pizzuti L, Barba M, Sergi D, Sperduti I, Mottolese M, Amoreo CA, Belli F, Vici P, Speirs V, Santini D, De Maria R, Maugeri-Saccà M (2015) Role of gonadotropin-releasing hormone analogues in metastatic male breast cancer: results from a pooled analysis. J Hematol Oncol 8:53. https://doi.org/10.1186/s13045-015-0147-z
Xu S, Yang Y, Tao W, Song Y, Chen Y, Ren Y, Liu J, Pang D (2012) Tamoxifen adherence and its relationship to mortality in 116 men with breast cancer. Breast Cancer Res Treat 136(2):495–502. https://doi.org/10.1007/s10549-012-2286-z
Cavazza M, Banks H, Ercolanoni M, Cukaj G, Bianchi G, Capri G, Longo F (2020) Factors influencing adherence to adjuvant endocrine therapy in breast cancer-treated women: using real-world data to inform a switch from acute to chronic disease management. Breast Cancer Res Treat 183(1):189–199. https://doi.org/10.1007/s10549-020-05748-6
Eggemann H, Bernreiter AL, Reinisch M, Loibl S, Taran FA, Costa SD, Ignatov A (2019) Tamoxifen treatment for male breast cancer and risk of thromboembolism: prospective cohort analysis. Br J Cancer 120(3):301–305. https://doi.org/10.1038/s41416-018-0369-2
Wibowo E, Pollock PA, Hollis N, Wassersug RJ (2016) Tamoxifen in men: a review of adverse events. Andrology 4(5):776–788. https://doi.org/10.1111/andr.12197
Kemp A, Preen DB, Saunders C, Boyle F, Bulsara M, Malacova E, Roughead EE (2014) Early discontinuation of endocrine therapy for breast cancer: who is at risk in clinical practice? Springerplus 3:282. https://doi.org/10.1186/2193-1801-3-282
Aiello Bowles EJ, Boudreau DM, Chubak J, Yu O, Fujii M, Chestnut J, Buist DS (2012) Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early-stage breast cancer. J Oncol Pract 8(6):e149-157. https://doi.org/10.1200/JOP.2012.000543
Disclaimers
Authors report that the views expressed in the submitted article represent their own and not the official position of the institution.
Funding
No institutional or industry support was obtained in the conduct of the work described in the article or the writing of the article itself.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval was waived by the local Ethics Committee in view of the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, A., Xie, Z., Stanko, L. et al. Endocrine adherence in male versus female breast cancer: a seer-medicare review. Breast Cancer Res Treat 192, 491–499 (2022). https://doi.org/10.1007/s10549-022-06536-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06536-0