Abstract
Purpose
Oxidative stress-responsive kinase 1 (OSR1) plays a crucial role in regulating diverse cellular pathophysiologic functions, including ion homeostasis, development, differentiation, angiogenesis, invasive migration, and metastasis. Regardless, the clinical significance of OSR1 in breast cancer is scarce. The current study was conducted to evaluate the effect of OSR1 on the prognosis of patients with breast cancer with a long-term follow-up.
Methods
OSR1 expression in formalin-fixed and paraffin-embedded tissue specimens was analyzed. These specimens were collected from 551 evaluable breast cancer cases by immunohistochemistry (IHC). OSR1 expression was dichotomized based on the H-score in IHC. The effects of OSR1 levels on the clinicopathological attributes and survival prediction in patients with breast cancer were explored.
Results
Among 551 specimens, 183 (33.2%) exhibited high expression of OSR1 in tumor cells. High OSR1 levels were markedly correlated with histologic grade (P = 0.035), ER (P < 0.001) and PgR (P = 0.043) expression, lymph node involvement (P < 0.001), TNM stage (P < 0.001), and axillary surgery procedures (P = 0.003). Univariate analysis results indicate that patients with high OSR1 expression had significantly poor overall survival (P < 0.001), distant disease-free survival (P < 0.001), and breast cancer-specific survival (P < 0.001). Multivariable Cox regression analyses suggest that OSR1 expression was an independent predictive marker of poor prognosis and lymph node metastasis (HR 3.224, 95% CI 1.182–8.702, P = 0.023) in patients with breast cancer.
Conclusions
Our findings indicate that OSR1 is a significantly independent prognosis index for patients with breast cancer with respect to distant disease-free survival, overall survival, and breast cancer-specific survival. High OSR1 expression caused an increase in deaths specifically attributed to breast cancer and was related to increased lymph node metastasis. However, the precise cellular mechanisms for OSR1 in breast cancer require further research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Progress in incipient detection, correct diagnosis, and therapeutic surveillance has led to improved results for aggressive breast carcinoma; however, relapse and distant metastasis still occur in roughly 20% of patients with breast cancer [1,2,3]. Determination of biomarkers markedly related to tumor advancement and latent clinical effects remains as one of the primary targets of breast cancer study.
Oxidative stress-responsive kinase 1 (OSR1), a serine–threonine protein kinase encoded by the OXSR1 gene in humans, belongs to the STE20/germinal center kinase subfamily [4]. OSR1 protein is responsible for the regulation of ion homeostasis and downstream signaling in response to environmental stress [5]. OSR1 that binds to and phosphorylates p21-activated protein kinase PAK1, which collaborates with chloride channel Na+-K+-2Cl+ cotransporter isoform 1 (NKCC1) by interacting with the R/K-F-X-V/1 motif, may be forming sensor-signaling elements that launch the cellular reaction to environmental stressors [6]. Increasing evidence has suggested that OSR1 is a fundamental transcription intermediary involved in biological events, including embryonic development, autophagy, cellular differentiation, and angiogenesis [7,8,9]. Results from pertinent research have also reported that OSR1 may be potentially implicated in the aggravation of diverse malignant tumors [10,11,12,13,14]. OSR1 promotes angiogenesis by interacting with lysine-deficient protein kinase 1 (WNK1) in virtue of regulatorily conserved regions in the C-terminal of cervical carcinoma [15]. NKCC1 activity is decreased in response to the knockout of upstream kinases of WNK1 and OSR1 in glioblastoma multiforme cells, and the WNK1/OSR1/NKCC1 signal transduction path plays a vital role in improving cancer cell migration [16]. A retrospective study including 54 hematuria specimens from patients with urothelial malignant tumors indicated that OSR1 was an independent and sensitive biomarker for the detection and prediction of urothelial malignant tumors [17]. The considerable roles of OSR1 in initiating and accelerating a malignant tumor manifested in in vivo and in vitro studies render OSR1 potentially useful for cancer screening, systemic therapy selection, and survival prediction. However, despite such potential, the role of OSR1 in breast cancer thus far remains unclear.
The present study was conducted to evaluate the expression of OSR1 protein levels in formalin-fixed, paraffin-embedded specimens of breast cancer. We estimated the correlation between OSR1 expression and clinicopathological characteristics and survival events on the basis of cohorts with large-scale characterized populations.
Materials and methods
Patient population and clinical information
The retrospective study included 551 pathologically confirmed patients with primary breast cancer who underwent surgical resection in Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine from January 2003 to December 2007. The available primary tumor samples with clinicopathologic data and follow-up information were retrospectively analyzed. The original patient follow-up survey was obtained from the Registry Center of the Department of General Surgery at Shanghai General Hospital and the outpatient doctor of the patients.
We retrieved information on patients from their clinical history and pathology reports, including surgical strategies for initial tumor and axillary lymph nodes, systemic adjuvant treatment, clinical data, and histopathological parameters. Patients were staged in accordance with the American Joint Committee on Cancer (AJCC) for breast cancer staging, eighth edition [18, 19]. Hematoxylin and eosin staining and immunohistochemistry (IHC) were performed to verify the presence of isolated tumor cells, micrometastasis, and macrometastasis, as well as to update the N classification in our cohort. The estrogen receptor (ER) and the progesterone receptor (PgR) were regarded as positive cases by a cut-off with more than 10% of tissue staining positive. IHC was performed to determine HER2 expression levels, which was regarded as positive when stained 3 + or negative for 1 + and 0. Tumors with 2 + moderate staining was classified as HER2-positive only if positive results were obtained in alternative fluorescence in situ hybridization (FISH). Ki67 levels were considered high with a cut-off of ≥ 14% positive staining.
Tissue specimens and histopathological information were retrieved in accordance with the regulatory framework of information security laws concerning specific moral standards and patient privacy. The research protocol was reviewed and authorized by the Ethics Committee of the Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine. Written informed consent for voluntary participation was obtained from each patient during the first interview. No animal experiments were conducted in this study.
OSR1 protein expression
Antigen retrieval in 4 μm paraffin-cut whole-tissue sections was conducted using the heat-induced recovery method by using Cell Conditioning Solution (Ventana Medical Systems, Tucson, USA). Antigen preservation was also verified before OSR1 analysis by immunohistochemistry with positive internal anatomic controls. This procedure prevented the proteolytic degradation of the samples because formalin-fixed tissues within paraffin blocks are known to maintain intact protein structures for more than 20 years, unlike old slides in which proteolytic degradation may occur after several years. The primary rabbit-anti-human polyclonal OSR1 antibody (1:100 dilution, Abcam, Cambridge, UK) was applied for immunohistochemistry. Immunostaining was automatically completed using the BenchMark XT immunostainer (Ventana Medical Systems, Tucson, USA) and detected with the ultraView Universal DAB Detection Kit (Ventana Medical Systems, Tucson, USA) as instructed by the manufacturer. Counterstaining was conducted using the hematoxylin solution for 5 min.
OSR1 expression was semi-quantitatively evaluated based on the improved histochemical scoring (H-score), which consists of an estimate of the staining intensities and percentages of positive cells [20]. The score for staining intensity was assigned as follows: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. The percentage of stained cells (0–100%) was calculated using the relevant intensity score to obtain the percentage–intensity score. OSR1 H-scores were determined by the summation of 4 percentage–intensity scores. Thus, the minimal H-score was 0, whereas the maximum H-score was 300. Several thresholds were used for evaluation as high OSR1 expression levels were examined. The threshold yielding the maximum prognostic implication was determined as the optimal cut-off point. Receiver operating characteristic curve was analyzed to calculate the candidate cut-off scores for high OSR1 expression. Univariate analysis of the data was conducted for overall survival by using the top 10 OSR1 H-score cut-off points of 74 to 229 with high Youden indexes. The highest seven cut-off points were selected by univariate analysis in accordance with lower P values and higher hazard ratio (HR) and then a multivariate analysis for overall survival was performed (Table 1). The highest HR and the lowest P value were achieved using the OSR1 H-score cut-off point of 127. Specimens with H-score ≥ 127 were thus determined as having high OSR1 expression. Two breast tumor pathologists performed the IHC H-scoring calculation and excellent agreement was achieved for defining OSR1 high expression with the H-score ≥ 127 (Cohen’s kappa = 0.85).
Follow-up
The median follow-up time was 10.3 years from the date of surgery as treatment for primary breast cancer. The median follow-up time for the subgroup of patients with low OSR1 expression was 10.3 years, whereas that for the subgroup of patients high OSR1 expression was 9.6 years. The patients were evaluated once every 3 months from the date of surgery to the first three years; once every 6–12 months from the fourth year to the fifth year after surgery; annually from the sixth year after surgery. The evaluation mainly consisted of a physical exam, blood testing including tumor markers, ultrasonography of breast and axillary lymph nodes, and enhanced magnetic resonance imaging (MRI) of bilateral breast. Whole-body bone isotope scanning and computerized tomography (CT) of the chest were conducted as needed. Breast cancer can recur; thus, patients were referred to the Shanghai General Hospital for further clinical diagnosis and therapy.
Statistical analysis
Patients and histopathologic features distributed between two groups were evaluated using the independent two-sample t test and Pearson’s chi-squared under specific conditions. The groups were the high-OSR1-expression and low-OSR1-expression groups. Distant disease-free survival was analyzed from the date of the primary operation to the date of the first distant metastasis of breast cancer, excluding locoregional recurrence. Patients who were alive without distant metastasis on the last follow-up visit or with the event of death were censored. Overall survival was analyzed from the date of the primary operation to the date of death due to any condition. Patients who are still alive on the last follow-up visit were censored. Breast cancer-specific survival was analyzed from the date of the primary operation to the date of death specifically caused by breast cancer. Patients who were alive on the last follow-up visit or died from any other cause were censored. Locoregional relapse-free survival was analyzed from the date of the primary operation to the date of relapse of ipsilateral breast tumor or first local lymph node. Patients with no locoregional relapse on the last follow-up visit or died were censored.
Life–table analysis was conducted using the Kaplan–Meier estimator. Differences in survival between groups were determined using the log-rank test. The Cox proportional hazard regression model was used to evaluate the effect of independent risk factors on survival. Variables with P < 0.1 in univariable survival analysis were subjected to multiple stepwise regression analysis. Logistic regression analysis was conducted to evaluate the independent variables that determine lymph node involvement. P < 0.05 with two sides was considered statistically significant. Statistical evaluation was performed using SPSS version 20 (IBM SPSS software, Chicago, USA) and GraphPad Prism 8.0 (GraphPad Software, San Diego, USA).
Results
OSR1 protein expression and correlation with clinicopathological characteristics
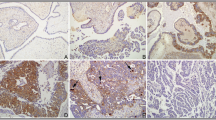
OSR1 in breast cancer cells was almost completely expressed relative to that in neighboring cells in the tissue specimens. OSR1 expression was localized in the cytoplasm of the breast cancer cells. Focal weak-to-moderate nuclear immunoreactivity, together with cytoplasmic staining, was observed in the tumor cells. Stain intensity was classified into negative, weak, moderate, and strong (Fig. 1). OSR1 expression was dichotomized into high and low—that is, characterizing 368 (66.8%) and 183 (33.2%) patients, respectively—with an H-score of 127 as the cut-off point. The median follow-up time in patients with low OSR1 expression was longer than that in patients with high OSR1 expression (10.3 vs. 9.6 years; P = 0.037).
Correlations between OSR1 protein levels and clinicopathologic parameters were assessed. Patients with the following characteristics showed high OSR1 levels: a high histologic grade (P = 0.035), negative ER (P < 0.001) and PgR (P = 0.043) expression, lymph nodes involvement (P < 0.001), advanced TNM stage (P < 0.001), and sentinel node biopsy with axillary lymph node dissection (P = 0.003) (Table 2). No correlation was found between OSR1 expression level and age at diagnosis, menopausal status, diameter, HER2 status, molecular subtype, histological subtype, and postoperative therapy (P > 0.05).
Association between OSR1 protein expression and clinical outcome
Distant metastasis occurred in 57 (10.3%) patients during follow-ups. These patients included 30 (8.2%) of the 368 cases with low OSR1 expression and 27 (14.8%) of the 183 cases with high OSR1 expression. The 10-year distant disease-free survival was 82.7% in the low-OSR1-expression group and 73.9% in the high-OSR1-expression group (univariable Cox regression HR 2.680, 95% CI 1.458–4.925; P < 0.001; Fig. 2a, Table 3). When all eligible prognostic variables were subjected to multivariable Cox regression analysis, high OSR1 expression was considered as an independent prognostic factor for distant metastasis (HR 2.032, 95% CI 1.063–3.885; P = 0.033) coupled with ER expression, lymph node involvement, and TNM stage (Table 3).
Within the follow-up period, 37 (6.7%) patients died—16 (4.3%) of the 368 cases from the low-OSR1-expression group and 21 (11.5%) of the 183 cases from the high-OSR1-expression group. The 10-year overall survival was 94.7% in the low-OSR1-expression group, and 86.0% in the high-OSR1-expression group (univariable Cox regression HR 2.707, 95% CI 1.572–4.674; P < 0.001; Fig. 2b, Table 4). Cox proportional hazards model multivariable analysis indicated that in addition to lymph node involvement, high OSR1 expression was recognized as an independent prognostic factor for overall survival (HR 2.420, 95% CI 1.316–4.463; P = 0.005).
Only 25 (66.7%) of the 37 deaths were confirmed to have been specifically caused by breast cancer (8 deaths from the low-OSR1-expression group and 17 death from the high-OSR1-expression group). The 10-year breast cancer-specific survival was 90.9% in the low-OSR1-expression group and 82.1% in the high-OSR1-expression group (univariable Cox regression HR 3.791, 95% CI 1.926–7.250; P < 0.001; Fig. 2c). Cox multivariable regression analysis identified high OSR1 expression as an independent prognosis risk factor for breast cancer-specific survival (HR 2.637, 95% CI 1.754–9.355, P = 0.001).
Local relapse was identified in 35 (6.4%) cases consisting of 25 (6.8%) cases in the low-OSR1-expression group and 10 (5.5%) cases in the high-OSR1-expression group. The 10-year locoregional recurrence-free survival was 84.6% in the low-OSR1-expression group and 81.7% in the high-OSR1-expression group (log-rank P = 0.803; Fig. 2d).
Correlation of OSR1 expression with lymph node metastasis
We analyzed OSR1 expression and lymph node involvement by using the chi-squared test. Patients with high OSR1 expression showed higher incidence of lymph node involvement than did patients with low OSR1 expression (48.1% vs. 34.0%; P = 0.001). OSR1 expression and stages N1–N3 were analyzed using the chi-squared test. Patients with high OSR1 expression had lower incidence of stages N1 and N2 than did patients with low OSR1 expression (44.3% vs. 52.8% for N1; 21.6% vs. 29.6% for N2); however, no significant difference was determined (P = 0.223 for N1; P = 0.191 for N2). Meanwhile, among patients with high OSR1 expression, a significantly higher incidence of massive lymph node spread was observed (≥ 10 positive lymph nodes, N3) (34.1% vs. 17.6%; P = 0.006).
The outcomes of univariable and multivariable logistical regression analysis of variables for lymph node involvement are listed in Table 5. The clinicopathologic characteristics were statistically associated with lymph node involvement in univariable analysis: higher histological grade (P = 0.019), negative ER (P = 0.037), and high OSR1 expression (P = 0.001). Multivariable regression analysis determined that high OSR1 expression was an independent risk factor for lymph node involvement (HR 3.224, 95% CI 1.182–8.702, P = 0.023). The aforementioned findings suggest that high OSR1 expression is a risk factor to identify the high-risk population with potential lymph node metastasis.
Correlation of OSR1 expression with the outcome by AJCC stage classification
Patients were stratified using the AJCC staging system, subgrouping patients into stages I–II and stages III–IV. The correlation of OSR1 expression with the results for the two subgroups was analyzed by Kaplan–Meier analysis (Fig. 3). With regard to distant disease-free survival, 273 (93.4%) of the 292 cases with low OSR1 levels and stages I–II showed no distant metastasis at the 10-year follow-up; meanwhile, 54 (50.7%) of the 107 cases with high OSR1 expression and stages I–II showed no distant metastasis at the 10-year follow-up (P < 0.001). Patients with low OSR1 expression and stages III–IV had a longer distant disease-free survival than those with high OSR1 expression and stages I–II (P = 0.002) and were in a considerably better condition than the cases with high OSR1 expression and stages III–IV (P = 0.001). With regard to overall survival, 283 (96.8%) of the 292 cases with low OSR1 expression and stages I–II survived at 10-year follow-up; meanwhile, 79 (74.0%) of the 107 cases with high OSR1 expression and stages I–II survived at 10-year follow-up (P < 0.001). Patients with low OSR1 expression and stages III–IV had a considerably higher overall survival than patients with high OSR1 expression and stages III–IV (P = 0.001). However, no significant difference was determined between patients with low OSR1 expression and stages III–IV and those with high OSR1 expression and stages I–II (P = 0.060). Taken together, the above findings suggest that the combination of high OSR1 expression and lymph node metastasis status serves as predictive biomarkers to classify patients into two groups with highly distinct prognostic features.
Discussion
In the present study, we demonstrated that high OSR1 expression was highly correlated with tumor aggressiveness, namely, high histological grade, negative ER and PgR expression, lymph node involvement, and advanced TNM stage, indicating that breast cancer cells with high OSR1 expression exhibit more aggressive characteristics. OSR1 expression exhibited no correlation with age, tumor size, menstrual status, HER2 status, molecular subtype, histological subtype, Ki67, and postoperative therapy.
Our results showed that OSR1 overexpression is an independent prognostic biomarker for distant relapse in patients with breast cancer. The function is consistent with the mainly selected terminal point of survival, for instance, distant disease-free survival, overall survival, or breast cancer-specific survival. However, OSR1 expression did not exhibit the prediction for local relapse. OSR1 overexpression was correlated with poor prognosis in univariate analysis and multivariate regression analysis. No matter whether systemic adjuvant treatments performed were contained in the multivariate regression analysis or not. Patients with low OSR1 expression had higher 10-year distant disease-free and breast cancer-specific survival rates. These findings were consistent with previous research. Among patients with glioblastoma multiforme in tissue-microarray assays analysis, all biopsy specimens from patients with distant metastasis were stained highly for OSR1 [16]. Notably, the present study is the first to identify OSR1 as an independent predictor for the survival of patients with breast cancer.
The prognostic markers ER, PgR, and HER2 were not highly prognostic in this cohort, which may be attributed to several factors. First, the number of cases is limited in the present study. Second, one-third of breast cancers change their hormone receptor status, and 15% of patients experience a change in HER2 status during tumor progression and adjuvant treatments [21, 22]. Linda et al. revealed that the proportion of patients losing ER, PgR, and HER2 was highest in the groups treated with hormonal therapy alone and in hormonal therapy combined with chemotherapy [23]. Loss of hormone receptors and HER2 implies resistance to endocrine therapy and trastuzumab, respectively. Thus, hormonal therapy and/or chemotherapy affect the status of hormone receptor and HER2, which may reduce the prognostic values of ER, PgR, and HER2 detected in the primary breast cancer specimens. Third, Engstrøm et al. showed that they found a poorer prognosis for HER2 + and triple-negative subtypes the first five years after diagnosis and for those who survived five years, the prognosis did not differ among the subtypes [24]. The time from diagnosis may be crucial for the prognostic value of hormonal receptors and HER2 throughout the follow-up period.
Notably, high OSR1 expression in breast cancer could act as an independent risk factor for predicting potential lymph node involvement. The results also suggested that high OSR1 expression was significantly correlated with the increased extent of lymph node metastasis (N3). From the perspective of molecular mechanisms, OSR1 interacts with and activates cation chloride cotransporters to maintain fluid/ion homeostasis during osmotic and oxidative stress and has been indicated in the regulation of cancer cell chemoresistance [15]. OSR1 partly mediates cytoskeleton rearrangement and facilitates cancer cell migration [25]. Some patients who were misdiagnosed for various reasons showed preoperative axillary lymph node involvement in breast cancer [26]. Consequently, quantitative analysis of OSR1 expression by histopathologic biopsy examination and comprehensively preoperative evaluation to exclude lymph node involvement could help perform mini-invasive treatment in the earlier period of breast cancer. Notably, giant cell tumor (GCT) patients with distant metastasis exhibited distinctly high OSR1 expression in the serum, as detected using the hydrogel nanoparticle method; high OSR1 expression in the serum of GCT patients was associated with the increased probability of pulmonary metastasis in Kaplan–Meier analysis [27]. High OSR1 expression might suggest aggressive biology of cancer, indicating that in addition to metastatic axilla lymph nodes, micro-metastases might have been seeded to other sites of the body. Such micro-metastases may develop as definite metastases after a different incubation period in a suitable environment. If the supposition is verified, patients with high OSR1 expression may benefit more from adjuvant systemic treatment as well as radical axillary lymphadenectomy.
Another important finding suggested that OSR1 expression levels were classified based on the AJCC staging system (stages I–II vs. stages III–IV) into two subgroups to observe patients with different prognosis in the identical AJCC staging subgroup. In the present study, we found that high OSR1 expression markedly worsened the prognosis of patients in the same AJCC stage. Stages III–IV patients and patients with low OSR1 expression achieved better outcomes than those of stages I–II patients with high OSR1 expression in both overall survival and distant disease-free survival. Therefore, the results of the present study indicate that OSR1 expression appears to exceed the strength of AJCC stage in determining patients’ outcome, and that tumor biological features such as OSR1 expression may represent an even robust determinant of survival than the traditional tumor stage. High OSR1 expression could ease the identification of high-risk populations and help develop an individualized treatment. One recommended adaptation for systemic adjuvant therapy is that the risk of relapse exceeds more than 10% during the 10-year follow-up period [28, 29]. In the current study, 17 (9.3%) of the 183 cases with high OSR1 expression died particularly from breast cancer; in addition, distant metastasis was reported in 27 (14.8%) cases, accumulating to more than 20% risk of relapse during a median 9.6 years. The data obtained demonstrate that high OSR1 expression may function as a determinant to individualize systemic treatment for patients with postoperative breast cancer. Future studies with a large sample size are warranted to allow us to validate the prognostic and predictive role of OSR1 in breast cancer patients.
Positive ion–chloride cotransporters relate to high-speed cell volume regulation and maintaining homeostasis in the intracellular environment [30]. The results of the coordination of cytoskeletal reorganization and changes in cell volume explain the stretching and constriction of the pseudopods during cell migration [31]. OSR1 is involved in the functional accommodation of transporters by phosphorylating specific serine–threonine sites [32]. The protein kinase PAK1 is activated by OSR1 phosphorylation [33]. Actin protein is then phosphorylated by PAK1 kinase, leading to the decomposition of stress fibers and the redistribution of tubulin proteins [34]. PAK1 activation by OSR1 phosphorylation renders PAK1 insensitive to small G protein activation. Thus, OSR1 slows actin protein proteolysis in response to pressure in the microenvironment [33]. Moreover, NKCC1 could be activated by OSR1 to regulate cell size and shape by excreting intracellular potassium and chloride ions as well as osmotically endocellular fluid. Such activation is important for glioma cells to migrate via the narrow extracellular space [16]. On the basis of these findings, OSR1 can be regarded as a potential target for therapeutic strategies, apart from its prognostic significance. Tumor necrosis factors (TNFs) are involved in tumor cell death, proliferation, migration, apoptosis, inflammation, and stress response [35]. Cusick et al. also concluded that OSR1 is responsible for the interaction and phosphorylation of the complex of the TNF receptor expressed in lymphoid tissues (RELT), RELL1, and RELL2 [36]. Thus, phosphorylation of the activation of OSR1 has a high probability of negatively influencing prognosis by activating the nuclear factor-κB signal pathway possibly by adjusting a complex of RELL1, RELL2, and RELT [10]. The OSR1 gene and protein levels were increased in osteosarcoma specimens; in addition, OSR1 silencing inhibited the growth and suppressed the invasive ability of osteosarcoma cells. OSR1 knockdown also led to the inhibition of Smad2/3 phosphorylation, indicating that OSR1 suppression exerted an antagonistic effect on the neoplasm formation of TGF-β signaling in osteogenic sarcoma. This finding suggested that OSR1 can aggravate oncogenesis and tumor progression by activating the TGF-β pathway [37]. Rauch et al. found that the CpG islands associated with the OSR1 gene were methylated in both adenocarcinomas and squamous cell carcinomas at a frequency exceeding 95% [11]. However, Stransky et al. indicated that the CpG islands within the OSR1 promoters exhibited no DNA methylation in either bladder tumor cells or tumor samples [38]. These contradictory findings implied that OSR1 can potentially exert different effects on tumor progression in different tumor types. High OSR1 expression resulting in more aggressive characteristics in breast cancer is involved in complex molecular pathways, which requires further research.
The present study has several limitations and deficiencies related to the results. First, this study is its retrospective nature. Despite the relatively large sample content, the prognosis of the study population is significantly favorable. The low number of terminal events of survival analysis might restrict the reliability of the statistical results to a certain extent. Moreover, the heterogeneity of adjuvant chemotherapy regimens should also be acknowledged as a limiting factor for this study although most were anthracyclines followed by a taxane. Second, the reliability and accuracy of OSR1 immunohistochemical staining have been a concern because of the absence of a standard staining protocol and an analytical method as well as diverse antibodies. The commercially available rabbit-anti-human OSR1 polyclonal antibody used in our study was validated using the human small intestine myenteric plexus tissue as a positive control. Using the same antibody, Zhu et al. detected OSR1 expression in 205 glioblastoma multiforme tissue-microarray samples and found a correlation between OSR1 high expression and shortened time to recurrence [16]. Third, heterogenous OSR1 expression was found in several sections of breast cancer tissue samples. OSR1 positivity was observed in the cytoplasm of breast cancer cells. Although occasional cases exhibited nuclear staining, the number was insufficient for reliable statistical analysis and the expression was weak to moderate and observed simultaneously with cytoplasmic staining.
To conclude, the aforementioned finding that high OSR1 expression in breast cancer tissue samples correlates to a positive lymph node status and more aggressive tumors suggests that activation of the OSR1 signaling pathway may help these tumor cells respond to stress in the microenvironment, thus migrating and rapidly spreading. Moreover, higher OSR1 expression was found to correlate with worse outcome in patients with breast cancer, indicating that OSR1 is an innovative prognostic indicator. Regardless, large and well-defined studies are needed to confirm the clinical and prognostic significance of OSR1 in patients with breast cancer. Further research to explore the molecular pathways of OSR1 in cancer cell progression is warranted.
References
Gorbounov M, Carleton NM, Asch-Kendrick RJ, Xian L, Rooper L, Chia L, Cimino-Mathews A, Cope L, Meeker A, Stearns V, Veltri RW, Bae YK, Resar LMS (2019) High mobility group A1 (HMGA1) protein and gene expression correlate with ER-negativity and poor outcomes in breast cancer. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-019-05419-1
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34. https://doi.org/10.3322/caac.21551
Wu Q, Li J, Sun S, Zhu S, Chen C, Wu J, Liu Q, Wei W, Sun S (2017) Breast carcinoma in situ: an observational study of tumor subtype treatment and outcomes. Oncotarget 8:2361–2371. https://doi.org/10.18632/oncotarget.13785
Tamari M, Daigo Y, Nakamura Y (1999) Isolation and characterization of a novel serine threonine kinase gene on chromosome 3p22-21.3. J Hum Genet 44:116–120. https://doi.org/10.1007/s100380050121
Pedro NF, Biselli JM, Maniglia JV, de Santi-Neto D, Pavarino ÉC, Goloni-Bertollo EM, Biselli-Chicote PM (2018) Candidate biomarkers for oral squamous cell carcinoma: differential expression of oxidative stress-related genes. APJCP 19:1343. https://doi.org/10.22034/APJCP.2018.19.5.1343
Piechotta K, Lu J, Delpire E (2002) Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J Biol Chem 277:50812–50819. https://doi.org/10.1074/jbc.m208108200
Mazorra Z, Popa X, Garcia B, Huerta V, Viada C, Neninger E, Rodriguez P, Gonzalez Z, Gonzalez A, Crombet T (2018) PO-486 Surrogate biomarkers of clinical efficacy in stage IIIB/IV non-small-cell lung cancer patients treated with an optimised EGF-based vaccination schedule. BMJ Publishing Group Limited, London
Tahmasbpour E, Ghanei M, Qazvini A, Vahedi E, Panahi Y (2016) Gene expression profile of oxidative stress and antioxidant defense in lung tissue of patients exposed to sulfur mustard. Mutat Res Genet Toxicol Environ Mutagen 800:12–21. https://doi.org/10.1016/j.mrgentox.2016.03.006
Gallolu Kankanamalage S, Lee A-Y, Wichaidit C, Lorente-Rodriguez A, Shah AM, Stippec S, Whitehurst AW, Cobb MH (2017) WNK1 is an unexpected autophagy inhibitor. Autophagy 13:969–970. https://doi.org/10.1080/15548627.2017.1286431
Burington B, Barlogie B, Zhan F, Crowley J, Shaughnessy JD (2008) Tumor cell gene expression changes following short-term in vivo exposure to single agent chemotherapeutics are related to survival in multiple myeloma. Clin Cancer Res 14:4821–4829. https://doi.org/10.1158/1078-0432.ccr-07-4568
Rauch TA, Wang Z, Wu X, Kernstine KH, Riggs AD, Pfeifer GP (2012) DNA methylation biomarkers for lung cancer. Tumor Biol 33:287–296. https://doi.org/10.1016/b978-0-12-801899-6.00013-9
Selvan L, Danda R, Madugundu A, Puttamallesh V, Sathe G, Krishnan U, Khetan V, Rishi P, Prasad T, Pandey A (2018) Phosphoproteomics of retinoblastoma: a pilot study identifies aberrant kinases. Molecules 23:1454. https://doi.org/10.3390/molecules23061454
Treiber JM, Steed TC, Brandel MG, Patel KS, Dale AM, Carter BS, Chen CC (2018) Molecular physiology of contrast enhancement in glioblastomas: an analysis of the cancer imaging archive (TCIA). J Clin Neurosci 55:86–92. https://doi.org/10.1016/j.jocn.2018.06.018
Both J, Krijgsman O, Bras J, Schaap GR, Baas F, Ylstra B, Hulsebos TJ (2014) Focal chromosomal copy number aberrations identify CMTM8 and GPR177 as new candidate driver genes in osteosarcoma. PLoS ONE 9:e115835. https://doi.org/10.1371/journal.pone.0115835
Anselmo AN, Earnest S, Chen W, Juang Y-C, Kim SC, Zhao Y, Cobb MH (2006) WNK1 and OSR1 regulate the Na+, K+, 2Cl− cotransporter in HeLa cells. Proc Natl Acad Sci 103:10883–10888. https://doi.org/10.1073/pnas.0604607103
Zhu W, Begum G, Pointer K, Clark PA, Yang S-S, Lin S-H, Kahle KT, Kuo JS, Sun D (2014) WNK1-OSR1 kinase-mediated phospho-activation of Na+-K+-2Cl− cotransporter facilitates glioma migration. Mol Cancer 13:31. https://doi.org/10.1186/1476-4598-13-31
Du E, Lu C, Sheng F, Li C, Li H, Ding N, Chen Y, Zhang T, Yang K, Xu Y (2018) Analysis of potential genes associated with primary cilia in bladder cancer. Cancer Manag Res 10:3047. https://doi.org/10.2147/cmar.s175419
Hortobagyi GN, Connolly JL, D'Orsi CJ, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, Weaver DL, Winchester DJ, Giuliano AE (2017) American Joint Committee on Cancer (AJCC). AJCC cncer staging manual, 8th edn. Springer, Chicago
Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, Weaver DL, Winchester DJ, Hortobagyi GN (2017) Breast cancer-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 67:290–303. https://doi.org/10.3322/caac.21393
Grunkin M, Raundahl J, Foged NT (2011) Practical considerations of image analysis and quantification of signal transduction IHC staining. Signal Transduction Immunohistochemistry. Springer, New York, pp 143–154
Thompson AM, Jordan LB, Quinlan P, Anderson E, Skene A, Dewar JA, Purdie CA (2010) Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS). Breast Cancer Res 12:R92. https://doi.org/10.1186/bcr2771
Wilking U, Karlsson E, Skoog L, Hatschek T, Lidbrink E, Elmberger G, Johansson H, Lindström L, Bergh J (2011) HER2 status in a population-derived breast cancer cohort: discordances during tumor progression. Breast Cancer Res Treat 125:553–561. https://doi.org/10.1007/s10549-010-1029-2
Lindström LS, Karlsson E, Wilking UM, Johansson U, Hartman J, Lidbrink EK, Hatschek T, Skoog L, Bergh J (2012) Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol 30:2601–2608. https://doi.org/10.1200/jco.2011.37.2482
Engstrøm MJ, Valla M, Bofin AM (2017) Basal markers and prognosis in luminal breast cancer. Breast Cancer Res Treat 163:207–217. https://doi.org/10.1007/s10549-017-4182-z
Delpire E, Gagnon KB (2006) SPAK and OSR1, key kinases involved in the regulation of chloride transport. Acta Physiol 187:103–113. https://doi.org/10.1111/j.1748-1716.2006.01565.x
Zavagno G, De Salvo GL, Scalco G, Bozza F, Barutta L, Del Bianco P, Renier M, Racano C, Carraro P, Nitti D (2008) A randomized clinical trial on sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer: results of the Sentinella/GIVOM trial. Ann Surg 247:207–213. https://doi.org/10.1097/sla.0b013e31812e6a73
Conti A, Luchini A, Benassi MS, Magagnoli G, Pierini M, Piccinni-Leopardi M, Quattrini I, Pollino S, Picci P, Liotta LA (2018) Circulating candidate biomarkers in giant cell tumors of bone. Proteom Clin Appl 12:1800041. https://doi.org/10.1002/prca.201800041
Goldhirsch A, Winer EP, Coates A, Gelber R, Piccart-Gebhart M, Thürlimann B, Senn H-J, Members P, Albain KS, André F (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24:2206–2223. https://doi.org/10.1159/000351222
Group EBCTC (2011) Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378:1707–1716. https://doi.org/10.1016/j.breastdis.2012.06.011
Villa F, Goebel J, Rafiqi FH, Deak M, Thastrup J, Alessi DR, van Aalten DM (2007) Structural insights into the recognition of substrates and activators by the OSR1 kinase. EMBO Rep 8:839–845. https://doi.org/10.2210/pdb2v3s/pdb
Stossel TP (1993) On the crawling of animal cells. Science 260:1086–1094. https://doi.org/10.1126/science.8493552
Gagnon KB, Rios K, Delpire E (2011) Functional insights into the activation mechanism of Ste20-related kinases. Cell Physiol Biochem 28:1219–1230. https://doi.org/10.1159/000335854
Chen W, Yazicioglu M, Cobb MH (2004) Characterization of OSR1, a member of the mammalian Ste20p/germinal center kinase subfamily. J Biol Chem 279:11129–11136. https://doi.org/10.1074/jbc.m313562200
Papakonstanti EA, Stournaras C (2002) Association of PI-3 kinase with PAK1 leads to actin phosphorylation and cytoskeletal reorganization. Mol Biol Cell 13:2946–2962. https://doi.org/10.1091/mbc.02-01-0599
Locksley RM, Killeen N, Lenardo MJ (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487–501. https://doi.org/10.1016/s0092-8674(01)00237-9
Cusick JK, Xu L-G, Bin L-H, Han K-J, Shu H-B (2006) Identification of RELT homologues that associate with RELT and are phosphorylated by OSR1. Biochem Biophys Res Commun 340:535–543. https://doi.org/10.1016/j.bbrc.2005.12.033
Barrios-Rodiles M, Brown KR, Ozdamar B, Bose R, Liu Z, Donovan RS, Shinjo F, Liu Y, Dembowy J, Taylor IW (2005) High-throughput mapping of a dynamic signaling network in mammalian cells. Science 307:1621–1625. https://doi.org/10.1126/science.1105776
Stransky N, Vallot C, Reyal F, Bernard-Pierrot I, De Medina SGD, Segraves R, De Rycke Y, Elvin P, Cassidy A, Spraggon C (2006) Regional copy number–independent deregulation of transcription in cancer. Nat Genet 38:1386. https://doi.org/10.1038/ng1923
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81670595, 81970568) and Excellent Youth Medical Talents Program of Shanghai General Hospital (06N1702011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no conflict of interest or other disclosures.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. The research protocol was reviewed and authorized by the Ethics Committee of the Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Qin, J., Wu, J. et al. High expression of OSR1 as a predictive biomarker for poor prognosis and lymph node metastasis in breast cancer. Breast Cancer Res Treat 182, 35–46 (2020). https://doi.org/10.1007/s10549-020-05671-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05671-w