Abstract
Purpose
In order to better define the breast cancer (BC) genetic risk factors in men, a germline investigation was carried out on 81 Male BC cases by screening the 24 genes involved in BC predisposition, genome stability maintenance and DNA repair mechanisms by next-generation sequencing.
Methods
Germline DNAs were tested in a custom multi-gene panel focused on all coding exons and exon–intron boundaries of 24 selected genes using two amplicon-based assays on PGM-Ion Torrent (ThermoFisher Scientific) and MiSeq (Illumina) platforms. All variants were recorded and classified by using a custom pipeline.
Results
Clinical pathological data and the family history of 81 Male BC cases were gathered and analysed, revealing the average age of onset to be 61.3 years old and that in 35 cases there was a family history of BC. Our genetic screening allowed us to identify a germline mutation in 22 patients (23%) in 4 genes: BRCA2, BRIP1, MUTYH and PMS2. Moreover, 12 variants of unknown clinical significance (VUS) in 9 genes (BARD1, BRCA1, BRIP1, CHEK2, ERCC1, NBN, PALB2, PMS1, RAD50) were predicted as potentially pathogenic by in silico analysis bringing the mutation detection rate up to 40%.
Conclusion
As expected, a positive family history is a strong predictor of germline BRCA2 mutations in male BC. Understanding the potential pathogenicity of VUS represents an extremely urgent need for the management of BC risk in Male BC cases and their own families.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Male breast cancer (Male BC) is a rare condition representing 0.5–1% of all BC cases [1]. Although, epidemiologic data regarding female BC is extensive, relatively little is known about Male BC. Male BC cases tend to occur in patients between the ages of 60 and 70 years and often expressing an oestrogen receptor (ER) and progesterone receptor (PR) (ER > 90%, PR > 75%) [2]. Subsequently, the most common phenotype is the luminal subtype (ER+ and/or PR+) with an occasional HER2 amplification (generally < 10%) [3, 4].
The lifetime risk of BC for men is about 1 in 833 [5]. Although a viral origin for BC was suggested [6], a relevant genetic component underlies the pathogenesis of the disease. In general, BC family history among first-degree relatives confers a 2–3-fold increase in Male BC risk [2]. Since the main BC susceptibility gene, BRCA1, was identified in 1994 [7], strong evidence indicates that other than this gene also the BRCA2 confers a high Male BC risk [8].
The lifetime risk of BC for BRCA2-mutation male carriers at the age of 70 is 6.8% and for BRCA1-mutation male carriers it is 1.2% [9]. BRCA2 mutations are estimated to be responsible for 60–76% of Male BC occurring in high-risk BC families, whereas the frequency rate of BRCA1 mutations ranges from 10 to 16% [10, 11]. An Italian multi-centre study reports BRCA2 mutations in 12% and BRCA1 mutations in 1% of Male BC cases [12].
PALB2 might act as a moderate-penetrance gene in Male BC since pathogenic variants have a higher prevalence in families with both female and Male BC cases (6.7%) than in families with only female BC cases (1%) [13]. Recently, CHEK2 and BRIP1 were associated with the moderately increased risk of Male BC; but in a less consistent manner than PALB2 [14].
Despite the increase in the use of multi-gene panel testing, to date, a limited number of studies have investigated Male BC susceptibility genes. Most studies performed multi-gene panel testing on a limited number of Male BC patients, ranging from 22 to 102 [15,16,17,18]. Few studies assessed multi-gene panel testing on more than 500 Male BC patients [14, 19].
Since genetic predisposition continues to be scarcely understood in Male BC, our main goal was to carry out a germline investigation on Male BC cases to better define genetic risk factors. The coding sequence and the exon–intron boundary regions of 24 genes involved in breast and ovarian cancer predisposition, maintenance of genome stability and DNA repair mechanisms (BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, ERCC1, MLH1, MSH2, MSH6, MRE11, MUTYH, NBN, PALB2, PARP1, PMS1, PMS2, PTEN, RAD50, RAD51C, RAD52, STK11, TP53, TP53BP1) were analysed by next-generation sequencing (NGS).
Materials and methods
Patients
Overall, 81 Male BC cases were admitted to the University Hospital of Pisa (AOUP) and the Tuscan Regional Discharge System database thanks to the collaboration with the Institute for Cancer Research, Prevention and Clinical Network (ISPRO) in Florence. For each patient, a blood sample, clinical information, family history and a written information consent were obtained. The study was approved by the Local Ethical Research Committee (Florence Health Unit).
Mutational screening
A NGS custom panel was designed using AmpliSeq™Designer (https://www.ampliseq.com/) (ThermoFisher Scientific) and DesignStudio (https://designstudio.illumina.com/) (Illumina) software to cover > 90% of the interested region of 24 genes (Supplementary Table 1). DNA was extracted from blood samples (QIAamp DNA Blood Midi Kit, Qiagen). Sequencing Library preparation was performed according to the manufacturer's protocols on the PGM-Ion Torrent (AmpliSeq™Library, OneTouch™200 Template, Sequencing200 Kits v2, ThermoFisher Scientific) and MiSeq Illumina (TruSeq Custom Amplicon Low-Input LibraryPrep, MiSeq ReagentNano Kits v2, Illumina) platforms. Raw data were analysed by using Torrent Suite™ (ThermoFisher Scientific) and VariantStudio™ (Illumina) software.
Genetic variants were filtered using MAF < 1% in 1000 Genomes Project as a cut off. Variants were classified by following the 5-tier International Agency for Research on Cancer (IARC) system, as recommended by the IARC and the American College of Medical Genetics (ACMG) [20, 21]. The potential functional impact of Class 3 VUS was assessed by four bioinformatics algorithms: SIFT, PolyPhen-2 (PP-2), Mutation Taster, and Human Splicing Finder (HSF). VUS were considered “potentially pathogenic” if simultaneously classified as deleterious by all tools applied. Pathogenic and “potentially pathogenic” variants were confirmed by capillary sequencing (BigDye® Terminator v3.1-ABI3730; ThermoFisher Scientific). BRCA1/2 chromosomal rearrangements were excluded by the MLPA (P002-P045, MRC-Holland) and Coffalyser.NET™ software (MRC-Holland).
Results
Patients
Overall, 81 Male BC patients were admitted. The age of BC diagnosis ranged from 38 to 88 years old (mean age = 61.30, SD = 11.26, 95% CI = 58.28–63.81). Invasive carcinoma of no special type (NST) was the most common phenotype (87%) even though a small percentage of papillary phenotype was reported (7.4%). They were predominantly grades 2–3, and luminal was the most common subtype with high percentages of ER and PR expression in tumour tissue (ER+ = 95% and PR+ = 85%). 35 cases had positive family history for breast/ovarian/prostate/pancreatic cancer. 12 Patients developed BC before the age of 50, 10 had a diagnosis of another primitive cancer, 2 had a relapse and 1 had a bilateral BC. The most common additional cancer was prostate cancer, with a 40% (4/10) frequency rate.
Mutational screening
In 71 patients, 75 heterozygous rare variants were identified in 20 genes (on average 1.06 variants for each patient with MAF < 1%). 15 out of 75 variants were classified as pathogenic in 4 genes (BRCA2, BRIP1, MUTYH, PMS2). BRCA2 accounted for the highest percentage of pathogenic variants (73.3%, 11/15): 5 frameshifts, 4 splice-sites, 1 nonsense and 1 missense variants were found in 18/81 patients (22.2%). In BRIP1 a total of 2 truncating mutations (1 nonsense and 1 frameshift mutation) were detected in 2 patients (2.5%). One patient carried a MUTYH pathogenic missense mutation and one other patient carried a PMS2 truncating mutation (Table 1). In patients tested for variants in 24 genes involved in DNA repair mechanism, the mutation detection rate was 27.1% (22/81). BC family history was referred in 16 cases.
Overall, 39 variants in 20 genes were reported as Class 3: 37 missense, 1 splice-site, and 1 in-frame deletion variants. 11 Missense and 1 splice-site variants in 9 genes (BARD1, BRCA1, BRIP1, CHEK2, ERCC1, NBN, PALB2, PMS1, RAD50) (Table 1) were considered as “potentially pathogenic” by all in silico tools. Each variant was found in one patient and did not co-occur with other pathogenic mutations. BC family history was referred in six cases.
A total of 48 variants in 19 genes were predicted as tolerated or benign by at least one in silico tool and/or reported as “benign/likely benign” in the literature and clinical databases, thus excluded from further analysis (Supplementary Tables 2, 3, 4). No rare variants were found in PTEN, RAD51C, RAD52, and TP53. Overall, a pathogenic or a “potentially pathogenic” variant was identified in 34 cases (34/81, 42%).
Discussion
Male BC accounts for ≈ 1% of all BC cases with an increasing incidence rate. Despite its rarity, here we present a cohort of 81 patients. As reported in the literature, NST was the most common phenotype (87%) even though a small percentage of other phenotypes were reported (7.4%); most of them were grade 3 carcinomas (52%) and luminal was the most common subtype in our study. High ER/PR expressions were observed, as reported in many studies [2, 3]. Approximately 20% of Male BC patients report a family history of breast or ovarian cancer [22]. In this cohort, 37% (30/81) reported to have breast and ovarian cancer history among first-degree relatives. As this is a retrospective study on men selected from genetic counselling, this cohort may over-represent Male BC cases in a setting of cancer family history.
In this study, a germline investigation was performed by NGS focusing on coding and intron–exon regions of 24 cancer predisposition genes in a well-characterized series of 81 Male BC cases. In total, we detected 75 rare variants in 20 genes. 15 Variants in 4 genes were previously classified as pathogenic, and 12 variants in 9 genes were predicted as “potentially pathogenic” by a custom pipeline.
As expected, BRCA2 harboured the highest number of pathogenic variants (73.3%, 11/15): 18/81 patients (22.2%) carried pathogenic variants in BRCA2.
In our cohort the most common deleterious variant is the nonsense c.289G>T (p.Glu97Ter) in BRCA2, detected in four unrelated patients. This nonsense was identified for the first time in a Dutch family with history of breast and ovarian cancer [23].
Although its frequency rate is extremely low worldwide, in the families we gathered information on over the past 20 years was often found: 31 BC patients (male and female) were carriers of this variant, accounting for ≈ 20% of all BRCA2-mutation carriers as in the Male BC cases analysed (25%) here. This supports a different allelic distribution in Italy. In fact, evidence of founder BRCA1/2 mutations in geographically restricted areas was reported [24,25,26,27].
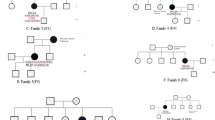
The Ashkenazi Jews founder mutation c.5946delT (p.Ser1982ArgfsTer22) was found in two cases. Segregation analysis in one of the two families revealed the presence of the same mutation in the proband’s 25-year-old son affected by pilocytic astrocytoma (Fig. 1). The co-occurrence of brain and breast cancers was observed in many families with carriers of BRCA2 mutations. A previous case report described a high-grade glioma in a 19-year-old BRCA2-mutation carrier (c.2808_2811delACAA) [28]. Biallelic BRCA2 mutations were identified in glioblastoma multiforme cases [29,30,31].
The c.631G>A (p.Val211Ile) and c.7008-2A>T were found in co-occurrence in two unrelated patients. Both mutations alter normal mRNA splicing, leading to the expression of a truncated protein [32]. Their co-occurrence was reported in a number of early onset and bilateral breast and ovarian cancers cases [33, 34]. Segregation analysis showed that both mutations affected the same allele [33]. However, the origin of this unusual BRCA2 allele remains unexplained.
Recent studies identified the 24 naturally occurring alternate splicing events associated with normal BRCA2 mRNA processing [35, 36], and a functional study demonstrated that variant alleles producing only transcripts lacking exon 3 should be considered to be pathogenic [37]. The c.316+5G>A is reported to be responsible for a nearly complete exon 3 skipping (95%), as quantified by fluorescent RT-PCR [37].
The c.8754+4A>G produced an aberrant transcript containing a 46-nt insertion of intron 21 [38], which was predicted to disrupt the protein function in splicing the assay in a minigene, and thus classified as pathogenic [39].
In our results, truncating mutations in BRIP1 represent about 15% of all pathogenic mutations.
Germline mutations (c.1372G>T, p.Glu458Ter and c.2684_2687delCCAT, p.Ser895Ter) found in BRIP1 lead to truncated proteins lacking a BRCA1-interacting region. Recently, BRIP1 was considered as a moderate-penetrance BC susceptibility gene. Truncations in BRIP1 double the risk of developing BC [40], and events of loss of heterozygosity were reported in female BC [41, 42], therefore, its role in Male BC requires further evaluation.
A single case of heterozygous for the pathogenic variant c.1187G>A was found in MUTYH. A high frequency rate of monoallelic MUTYH mutations in families with both breast and colorectal cancer is reported compared to the general population [43]. Recently, monoallelic pathogenic variants were identified in 2.5% Male BC patients [44].
To our knowledge, this is the first report of a truncating mutation in PMS2 in a man affected by BC and kidney cancer. Germline mutations in PMS2 cause susceptibility to HNPCC-related tumours, but an increased incidence for cancers of small bowel, ovaries, breast and renal pelvis was observed [45]. Functional assays in yeast support the indication that MSH2 mutations contribute to the development and progression of breast and ovarian cancer by modulating BRCA1-driven tumorigenesis [46]. One primary Male BC was reported in a subject who also had colon cancer and MLH1 mutation [47].
While loss-of-function variants are easily considered pathogenic, the association with the disease for missense variants is much more difficult to assess. In order to indicate the clinical utility of VUS, bioinformatics tools were applied: 12 variants in 9 genes were considered as “potentially pathogenic” thus classified as deleterious by all tools. Each variant was found in a single patient and all of them did not co-occur with other pathogenic mutations, giving evidence of their potential role in cancer predisposition as a genetic risk factor.
Segregation analysis was performed for BRCA1 c.2018A>G (p.Glu673Gly) because the missense was absent from all the database interrogated. The results supported its pathogenicity. The index case and his daughter inherited the same variant; she was affected by BC at the age of 49 years old (Fig. 2).
Pathogenic variants were not identified in TP53 or PTEN. Since Male BC is not associated with mutation in these genes, it is possible that men with clinical histories indicative of Li–Fraumeni syndrome or Cowden syndrome could benefit from single gene testing, potentially introducing ascertainment bias. There are some limitations to this study; the segregation analysis in families with “potentially pathogenic” variants was rarely applicable. The segregation data could clarify the association between Male BC and the “potentially pathogenic” variants identified in these families. In addition, the analysis of personal and familial cancer history may be limited according to the accuracy of the data provided.
Family Pedigree of 41-year-old Male BC patient carrying the missense variant c.2018A>G (p.Glu673Gly) in BRCA1. The variant was absent from all the database interrogated. The patient had early onset breast cancer (41 years old), and a strong positive family history for breast cancer (his daughter, his sister and her daughter). The segregation analysis, practicable only in his daughter, had revealed that she inherited the same pathogenic variant
In conclusion the results from this study revealed ~ 22% of Male BC patients carried mutations in BRCA2, according to the literature. Our screening allowed us to identify a pathogenic mutation in genes other than BRCA2 (BRIP1, PMS2, MUTYH) in an additional 5% of cases. Moreover 12 VUS were identified in 9 genes that might have a role in BC susceptibility.
These results support our choice to perform a multi-gene panel testing in Male BC patients regardless of one’s age at diagnosis, history of multiple primary cancers, and breast/ovarian cancer family history.
Understanding the role and the potential pathogenicity of VUS in high- and moderate-penetrance genes represents an exciting research challenge. In clinical settings, a VUS diagnosis raises so many questions, particularly in healthy carriers. With the increase in the use of multi-gene panels, comprehensive genetic counselling is essential in allowing the right management of a VUS carrier. In our experience, since variant classification evolves, VUS in moderate-penetrance genes is not used in clinical decision-making. Reclassification is to be communicated to carriers only when a VUS is reclassified as more pathogenic than previously. Surveillance examinations and screening programs are advised for high-penetrance VUS gene carriers only.
References
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29. https://doi.org/10.3322/caac.20138
Ottini L, Palli D, Rizzo S et al (2010) Male breast cancer. Crit Rev Oncol Hematol 73:141–155. https://doi.org/10.1016/j.critrevonc.2009.04.003
Giordano SH, Cohen DS, Buzdar AU et al (2004) Breast carcinoma in men: a population-based study. Cancer 101:51–57. https://doi.org/10.1002/cncr.20312
Severson TM, Zwart W (2017) A review of estrogen receptor/androgen receptor genomics in male breast cancer. Endocr Relat Cancer 24:R27–R34. https://doi.org/10.1530/ERC-16-0225
Howlader N, Noone AM, Krapcho M (2013) Lifetime risk of developing or dying from cancer. SEER Cancer Statistics Review 1975–2011
Mazzanti CM, Lessi F, Armogida I et al (2015) Human saliva as route of inter-human infection for mouse mammary tumor virus. Oncotarget. https://doi.org/10.18632/oncotarget.4567
Miki Y, Swensen J, Shattuck-Eidens D et al (1994) A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266:66–71. https://doi.org/10.1126/science.7545954
Stratton MR, Ford D, Neuhausen S et al (1994) Familial male breast cancer is not linked to the BRCA1 locus on chromosome 17q. Nat Genet 7:103–107. https://doi.org/10.1038/ng0594-103
Tai YC, Domchek S, Parmigiani G, Chen S (2007) Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst 99:1811–1814. https://doi.org/10.1093/jnci/djm203
Rizzolo P, Silvestri V, Tommasi S et al (2013) Male breast cancer: genetics, epigenetics, and ethical aspects. Ann Oncol 24:viii75–viii82. https://doi.org/10.1093/annonc/mdt316
Frank TS, Deffenbaugh AM, Reid JE et al (2002) Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol 20:1480–1490
Ottini L, Silvestri V, Rizzolo P et al (2012) Clinical and pathologic characteristics of BRCA-positive and BRCA-negative male breast cancer patients: results from a collaborative multicenter study in Italy. Breast Cancer Res Treat 134:411–418. https://doi.org/10.1007/s10549-012-2062-0
Silvestri V, Rizzolo P, Zanna I et al (2010) PALB2 mutations in male breast cancer: a population-based study in central Italy. Breast Cancer Res Treat 122:299–301. https://doi.org/10.1007/s10549-010-0797-z
Pritzlaff M, Summerour P, McFarland R et al (2017) Male breast cancer in a multi-gene panel testing cohort: insights and unexpected results. Breast Cancer Res Treat 161:575–586. https://doi.org/10.1007/s10549-016-4085-4
Fostira F, Saloustros E, Apostolou P et al (2018) Germline deleterious mutations in genes other than BRCA2 are infrequent in male breast cancer. Breast Cancer Res Treat 169:105–113. https://doi.org/10.1007/s10549-018-4661-x
Nielsen FC, van Overeem Hansen T, Sørensen CS (2016) Hereditary breast and ovarian cancer: new genes in confined pathways. Nat Rev Cancer 16:599–612. https://doi.org/10.1038/nrc.2016.72
Tung N, Battelli C, Allen B et al (2015) Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel: mutations in BRCA1/2-tested patients. Cancer 121:25–33. https://doi.org/10.1002/cncr.29010
Easton DF, Pharoah PDP, Antoniou AC et al (2015) Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med 372:2243–2257. https://doi.org/10.1056/NEJMsr1501341
Rizzolo P, Zelli V, Silvestri V et al (2019) Insight into genetic susceptibility to male breast cancer by multigene panel testing: results from a multicenter study in Italy: multigene panel testing for male breast cancer predisposition. Int J Cancer. https://doi.org/10.1002/ijc.32106
Plon SE, Eccles DM, Easton D et al (2008) Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat 29:1282–1291. https://doi.org/10.1002/humu.20880
Richards S, on behalf of the ACMG Laboratory Quality Assurance Committee, Aziz N et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–423. https://doi.org/10.1038/gim.2015.30
Korde LA, Zujewski JA, Kamin L et al (2010) Multidisciplinary Meeting on Male Breast Cancer: summary and research recommendations. J Clin Oncol 28:2114–2122. https://doi.org/10.1200/JCO.2009.25.5729
van der Hout AH, van den Ouweland AMW, van der Luijt RB et al (2006) A DGGE system for comprehensive mutation screening of BRCA1 and BRCA2: application in a Dutch cancer clinic setting. Hum Mutat 27:654–666. https://doi.org/10.1002/humu.20340
Marroni F, Cipollini G, Peissel B et al (2008) Reconstructing the genealogy of a BRCA1 founder mutation by phylogenetic analysis. Ann Hum Genet 72:310–318. https://doi.org/10.1111/j.1469-1809.2007.00420.x
Cipollini G (2004) Genetic alterations in hereditary breast cancer. Ann Oncol 15:i7–i13. https://doi.org/10.1093/annonc/mdh651
Malacrida S, Agata S, Callegaro M et al (2008) BRCA1 p.Val1688del is a deleterious mutation that recurs in breast and ovarian cancer families from northeast Italy. J Clin Oncol 26:26–31. https://doi.org/10.1200/JCO.2007.13.2118
Palmieri G (2002) BRCA1 and BRCA2 germline mutations in Sardinian breast cancer families and their implications for genetic counseling. Ann Oncol 13:1899–1907. https://doi.org/10.1093/annonc/mdf326
Wilson BT, Douglas SF, Polvikoski T (2010) Astrocytoma in a breast cancer lineage: part of the BRCA2 phenotype? J Clin Oncol 28:e596–e598. https://doi.org/10.1200/JCO.2010.28.9173
Offit K, Levran O, Mullaney B et al (2003) Shared genetic susceptibility to breast cancer, brain tumors, and Fanconi anemia. J Natl Cancer Inst 95:1548–1551. https://doi.org/10.1093/jnci/djg072
Reid S (2005) Biallelic BRCA2 mutations are associated with multiple malignancies in childhood including familial Wilms tumour. J Med Genet 42:147–151. https://doi.org/10.1136/jmg.2004.022673
Dodgshun AJ, Sexton-Oates A, Saffery R, Sullivan MJ (2016) Biallelic FANCD1/BRCA2 mutations predisposing to glioblastoma multiforme with multiple oncogenic amplifications. Cancer Genet 209:53–56. https://doi.org/10.1016/j.cancergen.2015.11.005
Gaildrat P, Krieger S, Di Giacomo D et al (2012) Multiple sequence variants of BRCA2 exon 7 alter splicing regulation. J Med Genet 49:609–617. https://doi.org/10.1136/jmedgenet-2012-100965
Colombo M, Ripamonti CB, Pensotti V et al (2009) An unusual BRCA2 allele carrying two splice site mutations. Ann Oncol 20:1143–1144. https://doi.org/10.1093/annonc/mdp241
Pensabene M, Spagnoletti I, Capuano I et al (2009) Two mutations of BRCA2 gene at exon and splicing site in a woman who underwent oncogenetic counseling. Ann Oncol 20:874–878. https://doi.org/10.1093/annonc/mdn724
Fackenthal JD, Yoshimatsu T, Zhang B et al (2016) Naturally occurring BRCA2 alternative mRNA splicing events in clinically relevant samples. J Med Genet 53:548–558. https://doi.org/10.1136/jmedgenet-2015-103570
Gambino G, Tancredi M, Falaschi E et al (2015) Characterization of three alternative transcripts of the BRCA1 gene in patients with breast cancer and a family history of breast and/or ovarian cancer who tested negative for pathogenic mutations. Int J Mol Med 35:950–956. https://doi.org/10.3892/ijmm.2015.2103
Caputo SM, Léone M, Damiola F et al (2018) Full in-frame exon 3 skipping of brca2 confers high risk of breast and/or ovarian cancer. Oncotarget. https://doi.org/10.18632/oncotarget.24671
Bonatti F, Pepe C, Tancredi M et al (2006) RNA-based analysis of BRCA1 and BRCA2 gene alterations. Cancer Genet Cytogenet 170:93–101. https://doi.org/10.1016/j.cancergencyto.2006.05.005
Acedo A, Hernández-Moro C, Curiel-García Á et al (2015) Functional classification of BRCA2 DNA variants by splicing assays in a large minigene with 9 exons. Hum Mutat 36:210–221. https://doi.org/10.1002/humu.22725
Cantor SB, Guillemette S (2011) Hereditary breast cancer and the BRCA1-associated FANCJ/BACH1/BRIP1. Future Oncol 7:253–261. https://doi.org/10.2217/fon.10.191
Spugnesi L, Gabriele M, Scarpitta R et al (2016) Germline mutations in DNA repair genes may predict neoadjuvant therapy response in triple negative breast patients: DNA repair mutations in neoadjuvant TNBCS. Genes Chromosomes Cancer 55:915–924. https://doi.org/10.1002/gcc.22389
De Nicolo A, Tancredi M, Lombardi G et al (2008) A novel breast cancer-associated BRIP1 (FANCJ/BACH1) germ-line mutation impairs protein stability and function. Clin Cancer Res 14:4672–4680. https://doi.org/10.1158/1078-0432.CCR-08-0087
Wasielewski M, Out AA, Vermeulen J et al (2010) Increased MUTYH mutation frequency among Dutch families with breast cancer and colorectal cancer. Breast Cancer Res Treat 124:635–641. https://doi.org/10.1007/s10549-010-0801-7
Rizzolo P, Silvestri V, Bucalo A et al (2018) Contribution of MUTYH variants to male breast cancer risk: results from a multicenter study in Italy. Front Oncol. https://doi.org/10.3389/fonc.2018.00583
ten Broeke SW, Brohet RM, Tops CM et al (2015) Lynch syndrome caused by germline PMS2 mutations: delineating the cancer risk. J Clin Oncol 33:319–325. https://doi.org/10.1200/JCO.2014.57.8088
Maresca L, Spugnesi L, Lodovichi S et al (2015) MSH2 role in BRCA1-driven tumorigenesis: a preliminary study in yeast and in human tumors from BRCA1-VUS carriers. Eur J Med Genet 58:531–539. https://doi.org/10.1016/j.ejmg.2015.09.005
Boyd J, Rhei E, Federici MG et al (1999) Male breast cancer in the hereditary nonpolyposis colorectal cancer syndrome. Breast Cancer Res Treat 53:87–91
Acknowledgements
The authors would like to acknowledge all the patients that were involved in this study.
Funding
This study was supported by Grants from the Istituto Toscano Tumori (ITT) Grant 2010 and from the Fondazione Pisa Grant 2016 (prog.127/16 and prog.148/16), and from research funding Susan G. Komen Italia onlus 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M.A. Caligo was supported by Grant 2016 (prog.127/16) from the Fondazione Pisa and by research funding 2017 from the Susan G. Komen Italia onlus. A. G. Naccarato was supported by Grant 2016 (prog.148/16) from the Fondazione Pisa. D. Palli was supported by Grant 2010 from the Istituto Toscano Tumori (ITT). All authors declare that there are no conflicts of interest.
Ethical approval
All the procedures performed in studies involving human participants were in accordance with the Ethical Standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Scarpitta, R., Zanna, I., Aretini, P. et al. Germline investigation in male breast cancer of DNA repair genes by next-generation sequencing. Breast Cancer Res Treat 178, 557–564 (2019). https://doi.org/10.1007/s10549-019-05429-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05429-z