Abstract
Purpose
Asymptomatic decline in left ventricular ejection fraction (LVEF) or heart failure (HF) occurs in up to 25% of patients treated with trastuzumab and can result in incomplete breast cancer therapy. The cardiac safety of continuing trastuzumab in patients with asymptomatic LVEF decline is unknown. We report the cardiac outcomes of patients treated with trastuzumab after a significant asymptomatic LVEF decline.
Methods
Patients with HER2-positive breast cancer and asymptomatic LVEF decline to < 50% during trastuzumab were identified from an institutional echocardiogram database. Patients who received trastuzumab with a LVEF < 50% were classified as the continued group, whereas patients who had trastuzumab held until LVEF improved to ≥ 50% or who had trastuzumab permanently discontinued were classified as the interrupted group. Cardiac events were defined as HF (New York Heart Association class III–IV) or cardiovascular death.
Results
Sixty patients were included; the median age was 54 years. In 23 patients who continued trastuzumab, 14 (61%) tolerated trastuzumab without a cardiac event, 6 (26%) developed worsening LVEF (range 25–42%) leading to trastuzumab discontinuation, and three (13%) developed a cardiac event (1 HF, 2 possible/probable cardiovascular deaths). In 37 patients with interrupted trastuzumab, 15 (41%) were re-challenged with trastuzumab after LVEF improved to > 50%, 21 (57%) were not re-challenged, and one (3%) developed HF. More patients in the continued trastuzumab group had metastatic disease (39% vs. 5%, p = 0.002). The final LVEF after median follow-up of 633 days was similar between patients with trastuzumab continuation versus interruption (54% vs. 56%, p = 0.29).
Conclusion
Continuation of trastuzumab after an asymptomatic LVEF decline to < 50% in patients who are expected to benefit from additional anti-HER2 therapy is a promising approach that warrants further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Up to 25% of invasive breast cancers over-express the human epidermal growth factor receptor-2 (HER-2) oncogene, which is associated with a more aggressive cancer phenotype and poor prognosis [1, 2]. Treatment with trastuzumab, a humanized monoclonal antibody against the extracellular domain of HER2, has improved disease-free survival and overall survival [3,4,5]. However, cardiotoxicity is a well described adverse effect associated with trastuzumab, and can manifest as an asymptomatic decline in left ventricular ejection fraction (LVEF) or symptomatic heart failure (HF).
Concerns over the cardiac safety of trastuzumab led to the development of cardiac surveillance guidelines which recommend that a LVEF assessment be performed at baseline and at regular intervals during trastuzumab therapy [6, 7]. If a patient develops a significant LVEF decline during treatment, trastuzumab should be interrupted based on current FDA recommendations [8]. Treating oncologists are faced with the dilemma of balancing the risk of worsening cardiac function or possible progression to HF and the benefit of improved cancer outcomes with continuation of trastuzumab [9]. This clinical decision is further complicated by the fact that trastuzumab cardiotoxicity often develops early in the course of treatment, at a time when patients may have received 50% or less of the intended trastuzumab course for breast cancer treatment [10, 11].
While the clinical significance or long-term sequelae of an asymptomatic LVEF decline remains poorly understood in the cardio-oncology setting, it is a leading cause of premature interruption of trastuzumab [11]. The rationale to withhold trastuzumab is based on the premise that an asymptomatic LVEF decline is a precursor that can lead to HF if additional trastuzumab is administered. A potential harm of this approach is that patients who develop an asymptomatic LVEF decline may receive an incomplete course of trastuzumab, thereby compromising the full oncologic benefit of anti-HER2 therapy.
Long-term follow-up of up to 10 years or more from four large adjuvant trials of trastuzumab have provided reassuring data that the rate of HF does not increase beyond the initial trastuzumab treatment period [12,13,14,15]. Additional findings from a retrospective study suggest that continuation of trastuzumab may be safe in asymptomatic patients who have a LVEF that decreases by ≥ 10% to below the lower limit of normal but remains ≥ 50% during trastuzumab therapy [16]. However, whether trastuzumab can be safely administered to patients who develop an asymptomatic LVEF decline to < 50% remains poorly understood. We performed a single-center retrospective study to evaluate the cardiac safety of trastuzumab in patients who develop an asymptomatic LVEF decline to < 50% during trastuzumab therapy.
Methods
Study population
All patients with HER2-positive breast cancer treated with trastuzumab between 2005 and 2016 were screened, of which 1582 underwent at least one echocardiogram at Memorial Sloan Kettering Cancer Center. The medical records of patients with a LVEF < 50% during the trastuzumab treatment period were retrospectively reviewed. Patients found to have symptomatic HF (New York Heart Association [NYHA] class III–IV) at the time of initial detection of LVEF decline to < 50% were excluded, given that continuation of trastuzumab is contraindicated in this treatment setting. Patients with a LVEF < 50% who had no symptoms of HF were subdivided into two groups. Patients with LVEF < 50% who received trastuzumab were classified in the continuous group, whereas patients who had trastuzumab interrupted until LVEF improved to ≥ 50% or who had trastuzumab permanently discontinued were classified in the interrupted group. The following baseline information was collected from the electronic medical record: age, body mass index (BMI), cardiovascular risk factors (i.e. hypertension, diabetes, smoking status, hyperlipidemia, coronary artery disease), cancer treatment details, and tumor characteristics including histological subtype, grade, hormone receptor status and metastasis. Baseline treatment with cardiac medications was determined. LVEF measurements were collected at baseline and throughout the trastuzumab treatment period. The nadir LVEF represents the minimum LVEF during trastuzumab treatment, and final LVEF represents the last available LVEF within the electronic medical record (which may have occurred during or after completion of trastuzumab treatment).
Outcomes
The primary endpoint of this study was the occurrence of a cardiac event, defined as symptomatic HF (NYHA class III or IV) or possible/probable cardiovascular death. The diagnosis of HF was confirmed by a cardiologist through review of the patient’s medical records, in accordance with the Heart Failure Society of America practice guidelines [17].
Statistical analysis
Patient characteristics were classified using descriptive statistics. Means were used for continuous measures and percentages used for categorical variables. Comparisons between continuous and interrupted groups were made using Fisher’s exact tests for categorical variables and Wilcoxon rank sum tests for continuous variables. In the group of 23 patients who received continued trastuzumab with a LVEF < 50%, associations between clinical characteristics and categorical differences in cardiac outcomes after continued trastuzumab (i.e., recurrent LVEF decline, HF, or cardiovascular death) were assessed with Fisher’s exact tests for categorical clinical characteristics and Wilcoxon rank sum tests for continuous clinical characteristics. p values < 0.05 were considered statistically significant. All statistical analyses were performed with GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA) and SPSS version 25 (SPSS, Chicago, IL).
Results
Patient characteristics
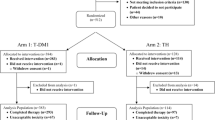
Of 1582 patients, 90 with a LVEF < 50% on an echocardiogram performed during trastuzumab treatment at our institution were identified. Of those, 30 patients had symptomatic HF at the time of initial LVEF decline and were excluded (Fig. 1). A total of 60 patients with HER2-positive breast cancer who developed an asymptomatic fall in LVEF to < 50% during trastuzumab therapy were studied: 49 with early-stage breast cancer and 11 with metastatic breast cancer. Forty-five of 49 patients with early-stage breast cancer were treated with anthracycline-based chemotherapy prior to trastuzumab, and 6 of 11 patients with metastatic breast cancer received anthracycline-based chemotherapy prior to the diagnosis of metastatic disease. After developing a decline in LVEF to < 50%, trastuzumab was continued in 23 patients: 14 of 49 (29%) in the early-stage group compared to 9 of 11 (82%) in the metastatic group (p = 0.002). Trastuzumab was temporarily or permanently interrupted in 37 patients: 35 of 49 (71%) in the early-stage group and 2 of 11 (18%) in the metastatic group. Excluding stage of disease, there was no significant difference in clinical characteristics or cardiovascular risk profiles of patients that received continued versus interrupted trastuzumab (Table 1).
Change in LVEF during trastuzumab
At baseline prior to initiation of trastuzumab, the median (interquartile range, IQR) LVEF was 59% (55.5, 63.5), and all but two patients had a LVEF ≥ 50%. The median (IQR) LVEF decline from baseline to nadir LVEF was 17% (12, 22) in the continued group versus 16% (13, 21) in the interrupted group. The nadir LVEF was not significantly different for patients receiving continued versus interrupted trastuzumab (43% vs. 43%, respectively, p = 0.72) (Table 2). The change in LVEF during trastuzumab is depicted in Fig. 2. The length of follow-up (from the nadir LVEF to final LVEF) was similar in the continued versus interrupted groups (570 vs. 701.5 days, p = 0.43) and no significant difference was seen in the final LVEF (54% vs. 56%, p = 0.29). At last follow-up, 16 of 23 (70%) in the continued group and 32 of 37 (86%) in the interrupted group had a LVEF ≥ 50%.
Box-and-whisker plot depicting LVEF by continued versus interrupted trastuzumab therapy. Baseline left ventricular ejection fraction (LVEF) represents LVEF prior to trastuzumab. Nadir LVEF represents minimum LVEF during trastuzumab treatment. Final LVEF represents the last available LVEF during or after trastuzumab treatment. Upper and lower boundaries of each box represent the third (Q3) and first (Q1) quartiles, respectively. The median is indicated with a horizontal bar in the box. The whiskers of the boxes represent Q3 + (1.5 × IQR) and Q1 − (1.5 × IQR). Outliers beyond the lower whisker are represented by circles. p > 0.05 for comparison of continued versus interrupted groups at baseline LVEF, nadir LVEF, and follow-up LVEF
Cardiac outcomes after LVEF decline
All 23 patients who continued trastuzumab with a LVEF < 50% were followed by a cardiologist and 21 of 23 (91%) were treated with new or increased doses of cardiac medications (beta-blocker, angiotensin-converting enzyme inhibitor [ACE-I], and/or angiotensin receptor blocker [ARB]). The median (IQR) delay of trastuzumab treatment after detection of a LVEF < 50% was 42 days (21, 98). Fourteen (61%) patients tolerated trastuzumab without a cardiac event and 6 (26%) developed worsening LVEF decline (but without HF symptoms) leading to permanent discontinuation of trastuzumab. Three (13%) patients developed a cardiac event. The first patient was a 58-year-old woman with metastatic breast cancer, diabetes (non-insulin dependent), hypercholesterolemia, and prior history of anthracycline exposure (for early-stage breast cancer). She was treated with paclitaxel, trastuzumab, and pertuzumab, and on this regimen she developed an asymptomatic LVEF decline to 43% at month 6 of her treatment. She was treated by a cardiologist with carvedilol and enalapril, and 9 months later with a LVEF of 46% she was re-challenged with trastuzumab. She underwent routine LVEF monitoring every 3 months with no further worsening of LVEF. After 17 months of trastuzumab, the patient had a sudden cardiac arrest. No autopsy was performed, therefore, the cause of death (i.e., cardiovascular-related versus cancer-related) could not be confirmed. The second patient was a 46-year-old woman with early-stage breast cancer and family history of dilated cardiomyopathy. Her LVEF decreased from 53 to 49% after anthracycline-based chemotherapy. She was evaluated by a cardiologist and treated with a beta-blocker but no ACE-I/ARB due to low blood pressure. Three months after beginning trastuzumab she developed symptomatic HF (NYHA class III) with a LVEF of 35%, leading to permanent discontinuation of trastuzumab. The third patient was a 60-year-old woman with early-stage breast cancer and hypertension. She developed a LVEF decline from 59 to 50% after anthracycline-based chemotherapy, leading to a cardiology consultation and initiation of enalapril and carvedilol. Her LVEF remained mildly reduced at 49% on maximally tolerated doses of cardiac medications. Since she was asymptomatic from a cardiac standpoint, she was treated with trastuzumab. After receiving two doses of trastuzumab, the patient had a sudden cardiac arrest. An autopsy revealed cardiomegaly with concentric left ventricular hypertrophy and marked pulmonary edema with no evidence of myocardial infarction or pulmonary embolism.
Age, BMI, blood pressure, cancer stage, HTN, DM, or treatment with cardiac medications (i.e., beta-blocker or ACE-I/ARB) were not predictive of cardiac outcomes after continued trastuzumab in patients with a LVEF < 50%. Additional clinical details for all patients who received continued trastuzumab can be found in the Supplementary Table.
Among 37 patients who had trastuzumab interrupted after LVEF decline to < 50%, 15 patients were re-challenged with trastuzumab after LVEF improved to > 50%: 12 successfully completed the intended course of treatment, and 3 developed a recurrent LVEF decline < 50% resulting in permanent discontinuation. Twenty-one of 37 patients were not re-challenged with trastuzumab due to the following: 10 had persistent LVEF decline < 50% for ≥ 3 months, 5 were at/near the completion of the intended treatment course, 5 at the discretion of the treating oncologist despite a LVEF recovery to > 50% within 3 months, and 1 was lost to follow-up. One of 37 (3%) patients developed a cardiac event. She was a 44-year-old woman with early stage breast cancer and hypertension and was treated with anthracycline-based chemotherapy followed by trastuzumab. After 3 months of trastuzumab, treatment was interrupted due to an asymptomatic LVEF decline to 44%. Despite treatment with maximally tolerated doses of carvedilol and lisinopril, she developed HF (NYHA class III) with further LVEF decline to 34%.
Discussion
This study provides one of the first descriptions of the cardiovascular sequelae associated with continued trastuzumab treatment in patients with trastuzumab cardiotoxicity and LVEF < 50%. We found that more than 50% of patients with a LVEF < 50% who continued trastuzumab were able to complete the intended course of therapy without adverse cardiac events. Importantly, all patients who received additional trastuzumab with a LVEF < 50% were treated by a cardiologist, and nearly all patients were prescribed new or increased doses of cardiac medications. Findings from this study contribute to our growing understanding of the cardiovascular risk of trastuzumab and provide important preliminary data to inform clinical decision-making among patients who develop trastuzumab cardiotoxicity. First, if oncologists and their patients determine that continuing trastuzumab is appropriate despite a significant decline in LVEF, early involvement of a cardiologist to educate the patient about cardiac risks and the potential signs and symptoms of HF is critical. Furthermore, the cardiologist should direct cardiac surveillance, optimize treatment for cardiovascular risk factors, and initiate cardiac medications with the goal of mitigating the potential risk of further cardiovascular injury.
A prevailing concern within the cardio-oncology community is whether trastuzumab can be safely continued in patients who develop an asymptomatic LVEF decline during treatment. This concern is driven by the by the significant survival benefit of trastuzumab-containing regimens in the early-stage and metastatic settings [3,4,5, 18], as well as data from clinical trials that have failed to demonstrate the noninferiority of shorter durations of trastuzumab therapy (< 12 months). The PHARE trial is a randomized phase III study in 3384 women which failed to show that 6 months of trastuzumab was non-inferior to 12 months, with a 2-year disease free survival (DFS) rate of 91.1% in the 6-month group versus 93.8% in the 12-month group (HR 1.28, p = 0.29) [19]. Shorter durations of trastuzumab have also been investigated in the Short-HER and SOLD trials. Both failed to demonstrate that 9 weeks of trastuzumab was non-inferior to 12 months, with a hazard ratio for DFS of 1.15 (90% CI, 0.91–1.46) and 1.39 (90% CI 1.12–1.72), respectively [20, 21]. This evidence may encourage both oncologists and patients to favor continuation of trastuzumab in the early stage disease setting even after development of a significant asymptomatic LVEF decline, particularly in those with high-risk cancer features. It is important to note that these studies showed a higher number of cardiac events in the 12-month treatment arms. However, cardiac events were often defined by surrogate endpoints such as a decrease in LVEF to < 50% or an absolute decrease in LVEF of > 15% from baseline, and the short- and long-term clinical significance of these surrogates remain uncertain.
In this study, the observation of a cardiac event (NYHA class III–IV HF or possible/probable cardiovascular death) in 13% of patients and worsening LVEF decline in 26% of patients who received continued trastuzumab warrants further investigation. This finding must be considered within the limitations of a retrospective study design, small sample size, and potential confounding from more metastatic disease in the continued group. It is possible that one of the cardiovascular deaths observed in the continued group was caused by an acute venous thromboembolism event attributable to metastatic breast cancer rather than to an acute cardiac cause; however, definitive adjudication of this endpoint was not possible in the absence of an autopsy. It is noteworthy that two of three patients with a cardiac event were noted to have a LVEF decline after anthracycline chemotherapy, suggesting that anthracycline-induced myocardial injury likely plays an important role in the pathogenesis of trastuzumab cardiotoxicity. Prospective studies are needed to define the cardiac event rate of continued trastuzumab in patients who develop asymptomatic LVEF decline and to identify markers that predict which patients can safely tolerate additional trastuzumab. The SAFE-HEaRt study is one such study evaluating the cardiac safety of anti-HER2 therapy in asymptomatic patients with mildly reduced LVEF of 40–49% after optimization of cardiac therapy. Findings from this study are forthcoming and will provide important data to inform future studies aimed at improving outcomes of patients with HER2-positive breast cancer and reduced LVEF [22]. Until prospective data are available, our findings support the need for cardiologists and oncologists to carefully consider the risks and benefits of additional trastuzumab and to coordinate cardiovascular care and monitoring if the decision is made to continue trastuzumab in patients with an asymptomatic LVEF decline.
It is important to highlight that 82% of patients who continued trastuzumab after a LVEF decline had metastatic disease, which suggests that stage of disease is an important variable within this complex clinical decision. In the metastatic setting, trastuzumab improves progression-free and overall survival, with reports of durable responses to trastuzumab without evidence of disease progression for as many as 9 years [18, 23,24,25,26,27]. When the goal of breast cancer treatment is palliative rather than curative, the impetus for continuation, rather than interruption, of life-extending therapy may become more important despite the potential for increased cardiovascular risk. Despite current recommendations for routine LVEF monitoring during trastuzumab [8, 28, 29], the optimal frequency remains unclear particularly in the metastatic setting when patients may be treated with trastuzumab for several years. Findings from a study by Mescher et al. also suggest that continuation of trastuzumab may be safe among patients with metastatic HER2-positive breast cancer who develop an asymptomatic LVEF decline [30].
A limitation of this retrospective study is the small sample size. However, as current practice guidelines recommend interruption of trastuzumab for patients with reduced LVEF,[8] we present novel preliminary cardiac outcomes data in a patient population that has not previously been well described in the literature. Given the retrospective study design, echocardiograms were performed at the discretion of the clinician and LVEFs were not centrally adjudicated. We attempted to identify clinical predictors of cardiac outcome in the group that received continued trastuzumab with a LVEF < 50%, although this analysis was limited by the small sample size. We cannot exclude the possibility of selection bias in this retrospective study between patients that received continued versus interrupted trastuzumab. However, comparisons between the two groups demonstrated similar baseline characteristics, cardiovascular risk factors, median follow-up duration, and LVEF throughout treatment. Given that 85% of patients in this study were previously treated with anthracyclines, findings from this study may not be applicable to patients that are treated with non-anthracycline trastuzumab-based regimens. Finally, although the final LVEF was not significantly different between the two groups, it is possible that differences may be identified with longer follow-up.
In conclusion, the optimal clinical management of patients with trastuzumab-induced asymptomatic LVEF decline remains unknown. Our results highlight that many patients with a LVEF < 50% tolerated additional trastuzumab while being treated with beta-blockers, ACE-Is, or ARBs. However, a decision to continue trastuzumab can be made only after carefully considering the cardiovascular and oncologic risks and benefits and requires a team-based approach between the cardiologist and oncologist to coordinate subsequent cardiac treatment and monitoring. Continued investigation is needed to identify predictors of favorable cardiovascular outcomes in patients with reduced LVEF receiving trastuzumab. With significantly improved survival for patients with HER2-positive breast cancer receiving trastuzumab-based therapy, both in the early and metastatic setting, it is crucial for our cardiology and oncology communities to closely collaborate in the clinical and research arenas to optimize both oncologic and cardiovascular outcomes.
References
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A et al (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244(4905):707–712
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA et al (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353(16):1673–1684
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C et al (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353(16):1659–1672
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M et al (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365(14):1273–1283
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. Version 1. 2012. http://www.nccn.org. Accessed June 30 2015
Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M et al (2017) Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 35(8):893–911
Genentech: Herceptin (trastuzumab): highlights of prescribing information, 04/2015 update. http://www.gene.com/download/pdf/herceptin_prescribing.pdf
Dang CT, Yu AF, Jones LW, Liu J, Steingart RM, Argolo DF, Norton L, Hudis CA (2016) Cardiac surveillance guidelines for trastuzumab-containing therapy in early-stage breast cancer: getting to the heart of the matter. J Clin Oncol 34(10):1030–1033
Seferina SC, de Boer M, Derksen MW, van den Berkmortel F, van Kampen RJ, van de Wouw AJ, Joore M, Peer PG, Voogd AC, Tjan-Heijnen VC (2016) Cardiotoxicity and cardiac monitoring during adjuvant trastuzumab in daily dutch practice: a study of the Southeast Netherlands Breast Cancer Consortium. Oncologist 21(5):555–562
Yu AF, Yadav NU, Lung BY, Eaton AA, Thaler HT, Hudis CA, Dang CT, Steingart RM (2015) Trastuzumab interruption and treatment-induced cardiotoxicity in early HER2-positive breast cancer. Breast Cancer Res Treat 149(2):489–495
Romond EH, Jeong JH, Rastogi P, Swain SM, Geyer CE Jr, Ewer MS, Rathi V, Fehrenbacher L, Brufsky A, Azar CA et al (2012) Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 30(31):3792–3799
Advani PP, Ballman KV, Dockter TJ, Colon-Otero G, Perez EA (2016) Long-term cardiac safety analysis of NCCTG N9831 (Alliance) Adjuvant Trastuzumab Trial. J Clin Oncol 34(6):581–587
Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, Castro G Jr, Untch M, Smith I, Gianni L et al (2017) 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 389(10075):1195–1205
Slamon DJ, Eierman W, Robert NJ, Giermek J, Martin M, Jasiowka M, Mackey JR, Chan A, Liu MC, Pinter T, Valero V, Falkson C, Fornander T, Shiftan TA, Bensfia S, Hitier S, Xu N, Bee-Munteanu V, Drevot P, Press MF, Crown J (2015) Ten year follow-up of the BCIRG-006 trial comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC-T) with doxorubicin plus cyclophosphamide followe by docetaxel and trastuzumab (AC-TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer patients [abstract]. In: Proceedings of the 38th Annual Meeting of the CTRC-AACR San Antonio Breast Cancer Symposium Dec 8–12; San Antonio, TX. Philadelphia (PA); 2016. Abstract S5-04
Yu AF, Yadav NU, Eaton AA, Lung BY, Thaler HT, Liu JE, Hudis CA, Dang CT, Steingart RM (2015) Continuous trastuzumab therapy in breast cancer patients with asymptomatic left ventricular dysfunction. Oncologist 20(10):1105–1110
Heart Failure Society of A, Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK et al (2010) HFSA 2010 comprehensive heart failure practice guideline. J Card Fail 16(6):e1–e194
Balduzzi S, Mantarro S, Guarneri V, Tagliabue L, Pistotti V, Moja L, D’Amico R (2014) Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev 2014(6):CD006242
Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, Lortholary A, Espie M, Fumoleau P, Serin D et al (2013) 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol 14(8):741–748
Joensuu H, Fraser J, Wildiers H, Huovinen R, Auvinen P, Utriainen M, Nyandoto P, Villman KK, Halonen P, Granstam-Bjorneklett H et al (2018) Effect of adjuvant trastuzumab for a duration of 9 weeks vs 1 year with concomitant chemotherapy for early human epidermal growth factor receptor 2-positive breast cancer: The SOLD Randomized Clinical Trial. JAMA Oncol 4(9):1199–1206
Conte P, Frassoldati A, Bisagni G, Brandes AA, Donadio M, Garrone O, Piacentini F, Cavanna L, Giotta F, Aieta M et al (2018) Nine weeks versus 1 year adjuvant trastuzumab in combination with chemotherapy: final results of the phase III randomized Short-HER studydouble dagger. Ann Oncol 29(12):2328–2333
Lynce F, Barac A, Tan MT, Asch FM, Smith KL, Dang C, Isaacs C, Swain SM (2017) SAFE-HEaRt: rationale and design of a pilot study investigating cardiac safety of HER2 targeted therapy in patients with HER2-positive breast cancer and reduced left ventricular function. Oncologist 22(5):518–525
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792
von Minckwitz G, Schwedler K, Schmidt M, Barinoff J, Mundhenke C, Cufer T, Maartense E, de Jongh FE, Baumann KH, Bischoff J et al (2011) Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3–05 phase III study in HER2-positive breast cancer. Eur J Cancer 47(15):2273–2281
Cancello G, Montagna E, D’Agostino D, Giuliano M, Giordano A, Di Lorenzo G, Plaitano M, De Placido S, De Laurentiis M (2008) Continuing trastuzumab beyond disease progression: outcomes analysis in patients with metastatic breast cancer. Breast Cancer Res 10(4):R60
Badulescu F, Badulescu A, Paul D, Popescu CF, Florescu C (2014) More than 9 years of continuous trastuzumab treatment in metastatic breast cancer without cardiac toxicity: a case report and literature review. Onco Targets Ther 7:1911–1917
Niikura N, Shimomura A, Fukatsu Y, Sawaki M, Ogiya R, Yasojima H, Fujisawa T, Yamamoto M, Tsuneizumi M, Kitani A et al (2018) Durable complete response in HER2-positive breast cancer: a multicenter retrospective analysis. Breast Cancer Res Treat 167(1):81–87
National Comprehensive Cancer Network (2016) Breast Cancer - NCCN Clinical Practice Guidelines in Oncology (Version 1.2016). https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed April 7 2016
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP et al (2014) Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 27(9):911–939
Mescher C, Ding C, Defor T, Konety S, Blaes A (2017) Left ventricular ejection fraction screening and clinical decision-making in metastatic HER2-positive breast cancer. Anticancer Res 37(7):3751–3755
Funding
Dr. Yu is supported by the National Institutes of Health/National Cancer Institute Grant K23 CA218897 and NIH/NCATS grant UL1 TR-002384. This work was funded in part through the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Dang receives research funding from Roche/Genentech and GlaxoSmithKline. Dr. Steingart is a consultant for Pfizer and Celgene. Dr. Yu is a consultant for Glenmark Pharmaceuticals. All remaining authors have declared no conflict of interest.
Ethical approval
All procedures were performed in accordance with the ethical standards of the Memorial Sloan Kettering Cancer Center institutional review board and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hussain, Y., Drill, E., Dang, C.T. et al. Cardiac outcomes of trastuzumab therapy in patients with HER2-positive breast cancer and reduced left ventricular ejection fraction. Breast Cancer Res Treat 175, 239–246 (2019). https://doi.org/10.1007/s10549-019-05139-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05139-6