Abstract
Purpose
Beginning in 2018, biomarkers including estrogen receptor (ER) status were incorporated in the 8th AJCC staging system. ER expression levels were not considered in these changes. We hypothesized that the levels of ER expression could affect the prognosis of breast cancer.
Methods
A retrospective review was conducted to identify all female patients with invasive breast cancer between 2003 and 2012. ER negative (group I), weakly ER-positive (group II), and strongly ER-positive (group III) were defined as Allred total scores of 0–2, 3–5, and 6–8, respectively. We examined a multigene panel, designated the BCT score, which is a newly developed prognostic model for predicting the risk of a distant metastasis.
Results
Among the 4949 patients enrolled in this study, 1310 (26.5%), 361 (7.3%), and 3277 (66.2%) were categorized as group I, II, and III, respectively. Median F/U duration was 57.8 months. Compared to group III, patients in group II were younger, had larger tumors, and were also more likely to have PR-negative tumors, HER-2 amplification, high Ki-67, and high nuclear grade. Between group II and III, there was a significant difference in OS (P = 0.0764, 0.909, and 0.010, respectively). After adjusting for additional factors that may affect OS, the HR for OS showed higher in group II than in group III. The baseline median BCT score indicated that lower ER expression was associated with significantly higher BCT score (P < 0.0001) and significantly more likely to have high risk group (P < 0.0001) relative to higher levels of ER expression group.
Conclusion
ER expression levels affect the prognosis of breast cancer. The risk for patients with weakly ER-positive breast cancer should not be underestimated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estrogen receptor (ER) status is one of the most important predictive and prognostic biomarkers in breast cancer [1,2,3]. ER-positive tumors are associated with better survival than ER negative tumors [4,5,6].
As understanding of the biologic markers ER, progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2) and their impacts on prognosis has grown, these biomarkers were incorporated in the eighth edition of the primary tumor, lymph node, and metastasis (TNM) classification of the American Joint Commission of Cancer (AJCC) for breast cancer [7,8,9]. Patients with hormone receptor-positive and/or HER-2-positive tumors who undergo appropriate targeted therapy can be down-staged according to these guidelines. In addition, for those with pT1 or pT2, pN0, M0, ER-positive, and HER-2-negative cancers combined with a low risk of multigene panels are expected to be categorized as stage IA.
However, the level of ER expression status is not considered in these changes. We hypothesized that the level of ER expression could affect prognosis as well as the risk score from the multigene panel, which predicts distant metastasis. In this study, based on the level of ER expression, we analyzed the characteristics and prognosis of patients, and examined the results of a multigene panel that predicts distant metastasis.
Methods
Data collection
A retrospective review was conducted to identify all female patients with invasive breast cancer who underwent surgery at Samsung Medical Center (SMC) between January 2003 and December 2012. We excluded patients who underwent neoadjuvant chemotherapy, had distant metastases or inflammatory breast cancer on presentation, or had other histopathology except for invasive ductal or lobular carcinoma. We also excluded patients who lacked immunohistochemistry (IHC) data (ER, PR, HER-2, and Ki-67) or had short follow-up durations (< 12 months). We collected the following variables: age at operation, family history of breast cancer, type of operation, pathologic stage according to 7th edition of AJCC classification [10], histopathology, nuclear grade (NG), lymphovascular invasion (LVI), ER, PR, HER-2 status, type of adjuvant treatment such as chemotherapy (CTx), radiotherapy (RTx), and anti-endocrine therapy (AET).
The dates of recurrence and death were collected by review of electronic medical charts, and the date of death also was collected from the Korean National Statistical Office database. Overall survival (OS) was defined as the time between the date of operation and the date of death from any cause. Disease-free survival (DFS) was defined as the time between the date of operation and the date of any recurrence because of breast cancer. Distant metastasis was defined as recurrence in any other area not included in locoregional recurrence. Distant metastasis free survival (DMFS) was defined as the time between the date of operation and the date of distant metastasis.
ER, PR, and HER-2 methodology
We used anti-ER (clone 6F11, 1:200 dilution, Novocastra, Bond-Max system) and anti-PgR (clone 16, 1:800 dilution, Novocastra, Bond-Max system) monoclonal antibodies on 10% neutral-buffered formalin fixed and paraffin embedded tissue. Only nuclear (not cytoplasmic) staining was scored.
ER and PR expression are measured primarily by IHC. Any staining of 1% of cells or more is considered positive for ER and PR [6]. We used the combined report with the Allred score interpretation system including intensity score (0–3) and proportion score (0–5) [11, 12]. The ER-negative (group I) was defined as having a total score (TS) of 0 and 2, weakly ER-positive (group II) was defined as a TS of 3–5, and the strongly ER-positive (group III) was defined as a TS of 6–8.
For Her-2 staining, we used anti-HER2 (4B5, Ventana, BenchMark XT) monoclonal antibody on 10% formalin fixed and paraffin-embedded tissue. Only the membrane staining intensity and pattern were evaluated using the new recommendations of the 2013 American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guideline [13]. Circumferential membrane staining that is complete, intense, and within > 10% of tumor cells results in a score of “3 +”. Circumferential membrane staining that is incomplete and/or weak/moderate and within > 10% of tumor cells or complete and circumferential membrane staining that is intense, and within ≥ 10% of tumor cells is scored 2 +. Incomplete membrane staining that is faint/barely perceptible and within > 10% of tumor cells is scored 1 +. When no staining is observed or membrane staining is incomplete and is faint/barely perceptible and within ≥ 10% of tumor cells the assigned score is 0. A positive test is defined as staining resulting in a 3 + score. The score of 2 + is interpreted as equivocal. Additionally, a negative test is defined as staining assigned a 0/1 + score. We considered positive results to be “amplification,” and equivocal and negative tests were considered to be “not amplification.”
Multigene panels
We examined a newly developed prognostic model (BCT score) for predicting the risk of distant metastasis. The BCT score assay is a molecular prognostic signature used to predict the risk of distant metastasis in patients with pN0-N1, HR+/HER2− breast cancer [14]. Among patients who underwent curative resection of a primary breast tumor at the SMC, the BCT scores were retrospectively obtained from 386 patients with pN0-N1, HR+/HER2- breast cancer. The BCT score assay was performed as previously described [14]. In brief, the BCT score assay is based on the quantification of six prognostic genes (UBE2C, TOP2A, RRM2, FOXM1, and MKI67) and three reference genes (CTBP1, CUL1, and UBQLN1) in FFPE tissue sections. All FFPE samples were collected at the time of surgical resection. Total RNA was isolated from the FFPE samples using a Tissue Preparation System device (Siemens AG, Munich, Germany), and qRT-PCR was performed using a QuantiFast Multiplex RT-PCR Kit (Qiagen, Hilden, Germany) and a LightCycler 480 system (Roche Applied Science, Mannheim, Germany). Relative expression of each prognostic gene was calculated as difference between target Cq value and average Cq value of three reference genes.
The BCT score was derived from relative expression values for the six prognostic genes and two clinical variables [tumor size (cm), and pN status (0 or 1)] and assessed on a scale from 0 to 10. The patients were categorized as being in either the high risk or low risk group according to a pre-specified cutoff BCT score of 4. Patients were classified as low risk for distant metastasis if BCT score was < 4, whereas patients with BCT score ≥ 4 were stratified as high risk. To assess different recurrence risk by ER status, the BCT scores were sub-classified according to levels of ER expression.
Statistical analysis
Patient characteristics were compared using Kruskal–Wallis test or the analysis of variance (ANOVA) test for continuous variables and the Chi square or Fisher’s exact test for categorical variables. Values are reported as mean ± standard deviation (SD) or median with ranges. Kaplan–Meier curves with corresponding results of log-rank tests were constructed for DFS, DMFS, and OS. Univariate and multivariate analyses for OS were conducted with a Cox regression and proportional hazard model to estimate the hazard ratio (HR) and 95% confidence interval (CI). Patients with any missing or unknown data were excluded from the analysis using the Cox model. All tests were two sided, and P < 0.05 was considered significant. All statistical analyses used SAS version 9.4 (SAS Institute, Cary, NC, USA) and R3.4.0 (Vienna, Austria; http://www.R-project.org). The need for informed consent was waived because of the low risk posed by this investigation. This study adhered to the ethical tenets of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of SMC in Seoul, Korea (IRB No.: 2017-06-130).
Results
A schematic diagram of patient selection is shown in Fig. 1. Overall, 4949 patients were enrolled in this study. Among them, 1310 (26.5%) were categorized in the ER-negative group (group I; Allred score 0, 2), 361 (7.3%) were categorized in the weakly ER-positive group (group II; Allred score 3–5), and 3277 (66.2%) were categorized in the strongly ER-positive group (group III; Allred score 6–8).
Patient characteristics
The clinicopathologic characteristics of patients in the three groups are shown in Table 1. The mean age was 48.6 ± 9.2 and the median follow-up duration was 57.8 (range 12.0–136.4) months. Compared with group III, patients in group II were younger (mean age, 46.5 vs. 48.4, P < 0.0001), and had larger tumors (T2/3 44.3% vs. 35.4%, P = 0.008). They were also more likely to have tumors that were PR-negative (28.5% vs. 5.6%, P < 0.0001), weakly PR-positive (31.0% vs. 14.8%, P < 0.0001), HER-2-amplified (40.4% vs. 10.6%, P < 0.0001), high Ki-67 (64.0% vs. 38.6%, P < 0.0001), and high NG (57.6% vs. 21.8%, P < 0.0001). Compared with patients in group II, patients in group I had larger tumors (T2/3 47.1% vs. 44.3%, P = 0.043), and were more likely be younger than 35 years old (8.7% vs. 5.8%, P < 0.0001), and to have tumors that were PR-negative (96.3% vs. 28.5%, P < 0.0001), high Ki-67 (85.0% vs. 64.0%, P < 0.0001), and high NG (79.9% vs. 57.6%, P < 0.0001).
Treatment characteristics
The types of surgery, adjuvant treatment, and clinical outcomes among the three groups are summarized in Table 2. In total, 3453 (69.8%) patients underwent breast conserving surgery (BCS) and 2510 (50.7%) patients underwent sentinel lymph node biopsy (SLNB). Compared to patients in group III, fewer patients in group II underwent anti- hormonal therapy (97.0% vs. 98.9%,P = 0.0009), BCS (60.7% vs. 68.3%, P < 0.0001), adjuvant radiotherapy (RTx) (69.0% vs. 78.8%, P < 0.0001), and more patients underwent adjuvant chemotherapy (CTx) (78.8% vs. 68.9%, P < 0.0001). Compared with patients in group I, fewer patients in group II underwent CTx (84.4% vs. 78.7%, P < 0.0001) and RTx (74.0% vs. 69.0%, P < 0.0001).
Oncologic outcome
The clinical outcomes, including recurrence, distant metastasis, and death, are summarized in Table 2. Patients in group II were significantly more likely to die than those in group III (4.7% vs. 2.0%; P = 0.001). There was no significant difference in recurrence between patients in groups II and III (9.4% vs. 7.9%; P = 0.316).
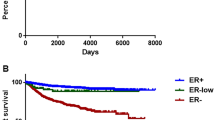
Between groups II and III, there were no significant difference in DFS and DMFS, but there was a significant difference in OS (P = 0.0764, 0.909, and 0.010, Fig. 2). Patients in group III were more likely to have bone metastasis, lowKi-67, and > 3-year distant metastasis-free interval (DMFI) than patients in groups I and II (Table 3). In the univariate analysis, patients in group II were shown to have significantly higher HRs for OS than patients in group III (HR 2.051; 95% CI 1.202–3.500; Table 4). After additional adjustments for pathologic stage, NG, LVI, Ki-67, PR, and HER-2 status, the HR for OS was higher in group II than in group III (group II: HR 1.773; 95% CI 1.002–3.137, group I: HR 1.868; 95% CI 1.002–3.481, Table 5).
BCT score according to the level of ER status
We examined BCT scores as an independent cohort in SMC. The baseline median BCT score showed that low ER expression was associated with a significantly higher risk score (4.87 vs. 4.63 vs. 3.54; P < 0.0001) and patients with this score were significantly more likely be in a high risk group (79.2% vs. 72.3% vs. 39.0%; P < 0.0001) compared to those with higher levels of ER expression (Fig. 3).
Discussion
Compared with the strongly ER-positive group, the weakly ER-positive group showed more unfavorable characteristics including: younger age, larger tumor size, and were more likely to be PR negative, have HER-2 amplification, higher Ki-67, and higher NG. DFS and DMFS were not significantly different between groups II and III, but patients in the strongly ER-positive group were more likely to have bone metastasis and a more than 3-year DMFI. The weakly ER-positive group had significantly worse OS than the strongly ER-positive group and had significantly higher HR for OS than the strongly ER-positive group after adjusting for factors that could affect OS. The median BCT risk score was significantly higher and patients were more likely to be in the high risk group compared to patients with higher levels of ER expression.
With the increased understanding of how biomarkers affect the prognosis of breast cancer, most ER-positive tumors have been down-staged in the 8th AJCC TNM staging. Since the Oncotype DxR (Genomic Health, Redwood City, CA) recurrence score (RS) assay was shown to be useful in predicting the benefits of adjuvant CTx, many early breast cancer patients with the luminal subtype don’t have to undergo CTx [15, 16]. Several algorithms for predicting the Oncotype DxR RS have been developed using clinicopathologic data. However, none of these algorithms take into consideration levels of the ER expression [17,18,19,20,21], despite several studies reporting that RS increases as ER expression decreases (RS 0–17, 18–30, and > 30; mean ER Allred score 7.78, 7.27, and 5.80) [20]. This trend could result in the underestimation of the risk for patients with weakly ER-positive tumors. In our study, we observed higher median BCT scores were more likely to result in a higher risk compared to higher levels of ER expression.
Furthermore, tumors with ER positivity of 1–9% on IHC have been shown to be ER-negative by mRNA gene expression; this could result in the misclassification of tumor subtype [22,23,24]. Iwamoto et al. [23] reported on 25 patients who were identified as being ER-positive with 1–9% staining by IHC. Among these patients, 19 (76.0%) were reported to be ER negative based on mRNA expression. They reported that tumors with 1–9% positivity by IHC result from a testing artifact, and CTx as well as AET is recommended for this group. Deyarmin et al. [24] also reported that 88.0% of weakly ER-positive tumors identified by IHC were classified as basal-like or HER-2 enriched subtype, and only 10.0% of those patients were classified as having the luminal A subtype by the intrinsic molecular subtype.
Lastly, in concurrent with our results, several studies have reported that ER expression levels can affect clinical outcomes [25,26,27,28,29,30]. Zhang et al. [25] analyzed the clinicopathological features in 1700 consecutive invasive breast cancer patients based on four subgroups based on ER expression levels: ER < 1%, 1–10%, 11–70% and > 70%. As the ER expression level lessened, there were significantly more unfavorable clinicopathological features, such as Nottingham grade, NG, and PR, and significantly worse survival in DFS. Bouchard-Fortier et al. [27] evaluated the effect of AET according to ER expression levels. They identified 2221 breast cancer patients who had been ER tested using a ligand-based assay (LBA) between 1976 and 1995. They subgrouped patients by ER expression level: 0–3, 4–9, 10–19, 20–49, and 50 fmol/mg or more cytosolic protein. Among the 661 patients treated with AET, 20-year BCSS were 41%, 41%, 77%, 68%, and 61% respectively. In the adjusted analysis for age, body mass index, tumor size, axillary lymph node involvement, surgery, RTx, and CTx, levels of ER expression were associated with a HR for lowered breast cancer mortality: 1.00 (reference), 0.59 (P = 0.09), 0.19 (P < 0.0001), 0.26 (P < 0.0001), and 0.31 (P < 0.0001), respectively.
Several studies have addressed some factors associated with survival among breast cancer patients with distant metastasis, and the preferred distant metastasis site by molecular subtype and have found that ER-positive tumors are more likely to result in bone metastases while ER-negative tumors are more likely to result in visceral or brain metastases [31]. Commonly, hormone receptor negative, high Ki-67 level, metastasis sites such as brain and liver, more than two organs metastases, and short duration of DMFI were independent predictors of poor OS after distant metastasis [32,33,34]. Our study demonstrated that strongly ER-positive tumors are more likely to result in bone metastasis, have lower Ki-67 and result in long periods of DMFI, compared to weakly ER-positive tumors with de novo distant metastasis. These differences may be associated with the differences in OS despite DFS and DMFS not being significantly different between groups II and III.
There are a few limitations associated with this study. First, there was no centralized IHC testing in our study. This could possibly have resulted in subjectivity in the interpretation of results, which could, accordingly, have resulted in different categorization. Second, we defined patients as having weakly ER-positive tumors when the Allred score was 3–5. Total score was consistent with the intensity score and proportion score. However, since 2010, ER positivity has been defined as having more than 1% of cells stained positive for the estrogen receptor. Consequently, some patients could have been misclassified. Third, we retrospectively collected data at a single institute and treatment was not assigned in a randomized method. Lastly, the follow-up duration was 57.8 months which is a relative short follow-up period.
In conclusion, weakly ER-positive tumors resulted in worse OS than strongly ER-positive tumors and this higher HR for OS after adjusting for other factors could affect prognosis. Weakly ER-positive tumors had significantly higher BCT scores and were more likely to be found in the high risk group compare strongly ER-positive tumors. We should not underestimate the risk for patients with weakly ER-positive tumors.
References
Aaltomaa S, Lipponen P, Eskelinen M, Kosma VM, Marin S, Alhava E, Syrjanen K (1991) Hormone receptors as prognostic factors in female breast cancer. Ann Med 23:643–648
Dunnwald LK, Rossing MA, Li CI (2007) Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 9:R6
Robertson JF, Bates K, Pearson D, Blamey RW, Nicholson RI (1992) Comparison of two oestrogen receptor assays in the prediction of the clinical course of patients with advanced breast cancer. Br J Cancer 65:727–730
Clark GM, Osborne CK, McGuire WL (1984) Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J Clin Oncol 2:1102–1109
Bagaria SP, Ray PS, Sim MS, Ye X, Shamonki JM, Cui X, Giuliano AE (2014) Personalizing breast cancer staging by the inclusion of ER, PR, and HER2. JAMA Surg 149:125–129
Barnes DM, Harris WH, Smith P, Millis RR, Rubens RD (1996) Immunohistochemical determination of oestrogen receptor: comparison of different methods of assessment of staining and correlation with clinical outcome of breast cancer patients. Br J Cancer 74:1445–1451
Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, Weaver DL, Winchester DJ, Hortobagyi GN (2017) Breast cancer-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 67:290–303
Weiss A, Chavez-MacGregor M, Lichtensztajn DY, Yi M, Tadros A, Hortobagyi GN, Giordano SH, Hunt KK, Mittendorf EA (2018) Validation study of the American Joint Committee on Cancer eighth edition prognostic stage compared with the anatomic stage in breast cancer. JAMA Oncol 4:203–209
American Joint Committee on Cancer (2016) AJCC cancer staging manual, 8th edn. Springer, Chicago
Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A (2010) AJCC cancer staging manual, 7th edn. Springer, New York
Harvey JM, Clark GM, Osborne CK, Allred DC (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17:1474–1481
Allred DC (2010) Issues and updates: evaluating estrogen receptor-alpha, progesterone receptor, and HER2 in breast cancer. Mod Pathol 23(Suppl 2):S52–S59
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013
Gong G, Kwon MJ, Han J, Lee HJ, Lee SK, Lee JE, Lee SH, Park S, Choi JS, Cho SY, Ahn SH, Lee JW, Cho SR, Moon Y, Nam BH, Nam SJ, Choi YL, Shin YK (2017) A new molecular prognostic score for predicting the risk of distant metastasis in patients with HR+/HER2- early breast cancer. Sci Rep 7:45554
Paik S, Tang G, Shak S, Kim C, Bake rJ, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE Jr, Wickerham DL, Wolmark N (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24:3726–3734
Lee MH, Han W, Lee JE, Kim KS, Park H, Kim J, Bae SY, Shin HJ, Lee JW, Lee ES (2015) The clinical impact of 21-gene recurrence score on treatment decisions for patients with hormone receptor-positive early breast cancer in Korea. Cancer Res Treat 47:208–214
Klein ME, Dabbs DJ, Shuai Y, Brufsky AM, Jankowitz R, Puhalla SL, Bhargava R (2013) Prediction of the Oncotype DX recurrence score: use of pathology-generated equations derived by linear regression analysis. Mod Pathol 26:658–664
Gage MM, Rosman M, Mylander WC, Giblin E, Kim HS, Cope L, Umbricht C, Wolff AC, Tafra L (2015) A validated model for identifying patients unlikely to benefit from the 21-gene recurrence score assay. Clin Breast Cancer 15:467–472
Orucevic A, Bell JL, McNabb AP, Heidel RE (2017) Oncotype DX breast cancer recurrence score can be predicted with a novel nomogram using clinicopathologic data. Breast Cancer Res Treat 163:51–61
Tang P, Wang J, Hicks DG, Wang X, Schiffhauer L, McMahon L, Yang Q, Shayne M, Huston A, Skinner KA, Griggs J, Lyman G (2010) A lower Allred score for progesterone receptor is strongly associated with a higher recurrence score of 21-gene assay in breast cancer. Cancer Invest 28:978–982
Turner B, Tang P, Hicks D (2017) The value of algorithms predicting the Oncotype DX recurrence score should not be underestimated!Breast Cancer Res Treat 164:249–250
Sheffield BS, Kos Z, Asleh-Aburaya K, Wang XQ, Leung S, Gao D, Won J, Chow C, Rachamadugu R, Stijleman I, Wolber R, Gilks CB, Myles N, Thomson T, Hayes MM, Bernard PS, Nielsen TO, Chia SK (2016) Molecular subtype profiling of invasive breast cancers weakly positive for estrogen receptor. Breast Cancer Res Treat 155:483–490
Iwamoto T, Booser D, Valero V, Murray JL, Koenig K, Esteva FJ, Ueno NT, Zhang J, Shi W, Qi Y, Matsuoka J, Yang EJ, Hortobagyi GN, Hatzis C, Symmans WF, Pusztai L (2012) Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1–10% ER-positive by immunohistochemistry. J Clin Oncol 30:729–734
Deyarmin B, Kane JL, Valente AL, van Laar R, Gallagher C, Shriver CD, Ellsworth RE (2013) Effect of ASCO/CAP guidelines for determining ER status on molecular subtype. Ann Surg Oncol 20:87–93
Zhang Z, Wang J, Skinner KA, Shayne M, Hajdu SI, Bu H, Hicks DG, Tang P (2014) Pathological features and clinical outcomes of breast cancer according to levels of oestrogen receptor expression. Histopathology 65:508–516
Balduzzi A, Bagnardi V, Rotmensz N, Dellapasqua S, Montagna E, Cardillo A, Viale G, Veronesi P, Intra M, Luini A, Pruneri G, Mastropasqua G, Goldhirsch A, Colleoni M (2014) Survival outcomes in breast cancer patients with low estrogen/progesterone receptor expression. Clin Breast Cancer 14:258–264
Bouchard-Fortier A, Provencher L, Blanchette C, Diorio C (2017) Prognostic and predictive value of low estrogen receptor expression in breast cancer. Curr Oncol 24:e106–e114
Hill DA, Barry M, Wiggins C, Nibbe A, Royce M, Prossnitz E, Lomo L (2017) Estrogen receptor quantitative measures and breast cancer survival. Breast Cancer Res Treat 166:855–864
Raghav KP, Hernandez-Aya LF, Lei X, Chavez-Macgregor M, Meric-Bernstam F, Buchholz TA, Sahin A, Do KA, Hortobagyi GN, Gonzalez-Angulo AM (2012) Impact of low estrogen/progesterone receptor expression on survival outcomes in breast cancers previously classified as triple negative breast cancers. Cancer 118:1498–1506
Yi M, Huo L, Koenig KB, Mittendorf EA, Meric-Bernstam F, Kuerer HM, Bedrosian I, Buzdar AU, Symmans WF, Crow JR, Bender M, Shah RR, Hortobagyi GN, Hunt KK (2014) Which threshold for ER positivity? A retrospective study based on 9639 patients. Ann Oncol 25:1004–1011
Kast K, Link T, Friedrich K, Petzold A, Niedostatek A, Schoffer O, Werner C, Klug SJ, Werner A, Gatzweiler A, Richter B, Baretton G, Wimberger P (2015) Impact of breast cancer subtypes and patterns of metastasis on outcome. Breast Cancer Res Treat 150:621–629
Lee ES, Jung SY, Kim JY, Kim JJ, Yoo TK, Kim YG, Lee KS, Lee ES, Kim EK, Min JW, Han W, Noh DY, Moon HG (2016) Identifying the potential long-term survivors among breast cancer patients with distant metastasis. Ann Oncol 27:828–833
Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH (2010) Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol 21:2169–2174
Savci-Heijink CD, Halfwerk H, Hooijer GK, Horlings HM, Wesseling J, van de Vijver MJ (2015) Retrospective analysis of metastatic behaviour of breast cancer subtypes. Breast Cancer Res Treat 150:547–557
Acknowledgements
This research was supported by Samsung Medical Center Grant (SMO1170021) and by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI17C1142).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JK and BK are employees of Gencurix. The other authors have no competing interests to declare.
Rights and permissions
About this article
Cite this article
Ryu, J.M., Choi, H.J., Kim, I. et al. Only estrogen receptor “positive” is not enough to predict the prognosis of breast cancer. Breast Cancer Res Treat 172, 627–636 (2018). https://doi.org/10.1007/s10549-018-4948-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4948-y