Abstract
Purpose
The goal of this systematic review is to provide an update to the review by Pouwels et al. by conducting a systematic review and an assessment of the reporting quality of the economic analyses conducted since 2014.
Methods
This systematic review identified published articles focused on metastatic breast cancer treatment using the Medline/PubMed and Scopus databases and the following search criteria: (((cost effectiveness[MeSH Terms]) OR (cost effectiveness) OR (cost-effectiveness) OR (cost utility) OR (cost–utility) OR (economic evaluation)) AND ((“metastatic breast cancer”) OR (“advanced breast cancer”))). The reporting quality of the included articles was evaluated using the International Society of Pharmacoeconomics and Outcomes Research (ISPOR) Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist.
Results
Of the 256 identified articles, 67 of the articles were published after October 2014 when the prior systematic review stopped its assessment (Pouwels et al. in Breast Cancer Res Treat 165:485–498, 2017). From the 67 articles, we narrowed down to include 17 original health economic analyses specific to metastatic or advanced breast cancer. These articles were diverse with respect to methods employed and interventions included.
Conclusion
Although each of the articles contributed their own analytic strengths and limitations, the overall quality of the studies was moderate. The review demonstrated that the vast majority of the reported incremental cost-effectiveness ratios exceeded the typically employed willingness to pay thresholds used in each country of analysis. Only three of the reviewed articles studied chemotherapies rather than treatments targeting either HER2 or hormone receptors, demonstrating a gap in the literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An estimated 6–10% of all breast cancer cases diagnosed annually are predicted to be metastatic at diagnosis, and 20–30% of current breast cancer cases are estimated to become metastatic [1]. The treatment landscape for metastatic breast cancer (MBC) has evolved significantly over the past few decades. Metastatic breast cancer is incurable, but treatments may improve survival time, delay progression of disease, improve quality of life, and manage symptoms.
MBC treatment planning depends on hormone receptor (HR) status, human epidermal growth factor receptor 2 (HER2) status, patient performance status and organ function, sites of disease, patient preferences, and prior treatment, if relevant. Tumors that are HR positive (HR+) require the female hormones estrogen and/or progesterone to grow, and these cancer cells have hormone receptors to which estrogen or progesterone bind [2]. The National Comprehensive Cancer Network (NCCN) recommends the use of an endocrine therapy—such as a nonsteroidal aromatase inhibitor (AI) (e.g., anastrozole or letrozole) or anti-estrogen therapy (e.g., tamoxifen)—for the first-line treatment for patients with advanced stage HR+ tumors [3]. One of the limitations of endocrine therapy is that its usefulness decreases over time with changes in tumor biology and as endocrine resistance develops. In addition, HER2 is an important protein for cell growth and survival [2]. When a cancer is HER2-positive (HER2+), it over-expresses this protein. Targeted therapies such as trastuzumab, pertuzumab, and lapatinib are commonly used in the treatment of HER2+ cancers [4]. Because they target specific molecular pathways, these treatments typically do not impact other cells lacking these targets.
Breast cancers that are HR- and HER2− (triple negative) lack these molecular targets, so typical treatments include single agent or combination regimens of chemotherapeutic drugs [4]. Because conventional chemotherapeutic drugs target all dividing cells rather than specific molecular pathways, these drugs are associated with serious side effects that may negatively impact patients’ quality of life [5].
The incurable nature of MBC can contribute to high health care utilization and cost [6], since treatment typically continues over a period of years and serial treatments are employed for progressive disease. Further, new developments in the research and development of treatments for this advanced cancer also cause concerns related to costs and value, since new therapies are usually under patent protection and introduced at higher price points than older, generic options. It is not always clear what the optimal sequence of treatments should be in this complex decision-making environment. Cost-effectiveness studies play an important role in the economics of cancer drugs by investigating the value of an intervention as compared to another, weighing costs and outcomes together. These economic analyses are important to healthcare decision-making, both to payers, namely, for inclusion in formularies and reimbursement policies, and to society as a whole [7].
In 2017, Pouwels et al. conducted a review of economic analyses published between January 2000 and October 2014 for metastatic breast cancer treatments [8]. Since 2014, five new compounds have been approved for MBC and a number of studies have been published addressing the cost-effectiveness of new and existing regimens. The relative costs of multiple treatment options may also have changed due to the introduction of generic equivalents or other changes in pricing. The goal of this systematic review is to provide an update to the review by Pouwels et al. by conducting a systematic review and an assessment of the reporting quality of the economic analyses conducted since 2014.
Methods
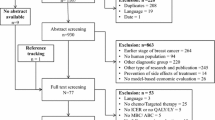
We conducted a systematic literature review using the NIH PubMed/Medline and Scopus databases. We used the following search criteria to query the database: (((cost effectiveness[MeSH Terms]) OR (cost effectiveness) OR (cost-effectiveness) OR (cost utility) OR (cost–utility) OR (economic evaluation)) AND ((“metastatic breast cancer”) OR (“advanced breast cancer”))). The search yielded 256 articles for review. We excluded studies published prior to October 2014 [8]. This narrowed the results to 67 articles, whose titles and abstracts were screened. Studies were included if they were original health economic studies specific to metastatic or advanced metastatic breast cancer (studies focused on local or regional disease were excluded). We also excluded reports or posters for which only abstracts were available; studies in languages other than English; analyses of diagnostic screening, imaging, and therapies for either palliative care or cancer-related osteoporosis; and studies relating to the use of bevacizumab for metastatic breast cancer (because of this treatment’s limited relevance in the United States in this indication during this time period) [9]. Ultimately, 17 articles were deemed appropriate for detailed review (Fig. 1).
Studies were grouped according to characteristics of the interventions of interest. This resulted in three categories: (1) treatments targeting HER2, (2) treatments targeting HRs, or (3) chemotherapy. Detailed information from each of the 17 studies was collected. The extraction checklist included title, authors, year of publication, line of treatment, country/setting, treatment and comparator(s), study design, perspective, and study outcomes. Study outcomes included quality-adjusted life years gained, incremental costs, and the incremental cost-effectiveness ratio (ICER). A quality-adjusted life year (QALY) is estimated as the time spent in each health state multiplied by the utility associated with the health state [10]. In each of the studies, the authors compared the ICER results against a willingness to pay threshold and then reported on the cost-effectiveness of the intervention(s) of interest. These thresholds vary across countries of analysis. For example, a threshold between $50,000 and $100,000 per QALY gained is typically referenced in the United States [11, 12]. The UK uses a threshold of £20,000 to 30,000 per QALY gained and Canada uses a threshold of $20,000 CAD per QALY gained [13, 14]. The model characteristics and study outcomes are presented in Tables 1 and 2, respectively.
Further, we assessed the quality of each reviewed study using the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist [32]. This checklist comprises 24 items that should be included when reporting economic evaluations of healthcare interventions. For each item, the studies received a score of 0 (item is absent), 1 (item is present), or 0.5 (item is partially fulfilled). Items reviewed for quality assessment and results are presented in Table 3.
Results
Overview of studies
Eight of the studies were performed in North America, five in Asia, and four in Europe. Seven studies looked at 1st line treatments, two for 1st or 2nd line treatments, six for 2nd or 3rd line treatments, and two for multiple lines of treatments. The studies analyzed interventions for various tumor characteristics, in terms of HR and HER2 status (Table 1).
The majority of the studies used a Markov disease-state transition model design (N = 12), two studies used a discrete event simulation design (DES), two used partitioned survival analyses, and one was a nonmodel-based analysis of costs and outcomes. Of the studies employing a Markov model, most models had either three health states (progression-free, progressive disease, death) or four health states (progression-free, progressive disease, hospice, death). The one study that was not model-based employed data from a meta-analysis of ten clinical trials. Nine of the studies took a payer perspective, four took a health system perspective, three took a societal perspective, and one took both the payer and societal perspectives. Several studies used a lifetime horizon (N = 9) and the rest varied (15, 10, or 5 years, for example). Model cycle length varied between one week and one year (Table 1). Extrapolation methods were described in nine studies, whereas the remainder used shorter time horizons or made other assumptions about model parameters. The studies using extrapolation methods assumed that data followed various parametric survival distributions including Weibull, log-logistic, nonlinear least-squares regression, exponential, log-normal, and gamma. Results of each cost-effectiveness study are summarized in Table 2.
Treatments targeting HER2
Eight articles estimated the cost-effectiveness of treatments targeting HER2 receptors. Of the eight studies, all but two concluded that the interventions of interest were not cost-effective. The two studies in which certain interventions of interest were deemed cost-effective are described in further detail, below [15, 17].
Beauchemin et al. developed a global economic Markov model for MBC treatments [15]. The global model was tested through an assessment of the cost-effectiveness of lapatinib plus letrozole compared with other first-line therapies for post-menopausal women with HR+, HER2+ cancer. The analysis was conducted from the perspective of the Canadian healthcare system over a lifetime horizon. Lapatinib plus letrozole was associated with higher total costs and QALYs relative to all other comparators in this study. The incremental cost-effectiveness ratios were $131,811 CAD per QALY when compared to letrozole alone, CA$56,211 per QALY when compared to trastuzumab plus anastrozole, and CA$102,477 per QALY when compared to anastrozole alone. In the base-case, only one of the three comparisons was cost-effective at a willingness to pay threshold of CA$100,000. Deterministic sensitivity analyses suggested that cost of treatments under evaluation, the discount rate, and the utility values associated with each health state had the greatest impact on the base-case results. Probabilistic sensitivity analyses show that the lapatinib plus letrozole have a 24% probability of being cost-effective when compared to letrozole alone, 86% compared to trastuzumab plus anastrozole, and 43% compared to anastrozole alone. Model testing resulted in similar results to a previously conducted cost-effectiveness analysis of lapatinib plus letrozole in HR+/HER2+ MBC [33].
Diaby et al. considered the cost-effectiveness of 1st through 3rd lines of treatment for HER2+ MBC from the perspectives of 3 public and 1 private payer in Mexico [17]. The model evaluated the cost-effectiveness of four targeted treatment sequences for HER2+ MBC over a lifetime horizon. From the perspective of the public payers, sequences with pertuzumab or trastuzumab emtansine were not cost-effective when compared to sequences not including those drugs. From the private payer perspective, a sequence containing trastuzumab emtansine without pertuzumab was considered cost-effective but at a lower clinical effectiveness than sequences containing pertuzumab.
Treatments targeting HRs
Six articles estimated the cost-effectiveness of treatments targeting hormone receptors. Of the six studies, all but two found that the intervention of interest was not cost-effective. The two studies in which the interventions of interest were deemed cost-effective are described in further detail, below [28, 31].
Sabale et al. compared fulvestrant 500 mg to generic aromatase inhibitors (letrozole, anastrozole, and exemestane) for patients with HR+ metastatic or locally advanced breast cancer [28]. Authors used a three-state partitioned survival model from the Swedish national payer perspective over a lifetime horizon. In base-case results, the incremental cost per QALY gained of fulvestrant 500 mg compared to anastrozole, letrozole, and exemestane were €33,808, €33,883, and €49,225, respectively. Sensitivity analyses demonstrate that Fulvestrant 500 mg had a 70% probability of being cost-effective at a willingness to pay threshold of €100,000/QALY.
Xie et al. compared the cost-effectiveness of everolimus with exemestane versus endocrine monotherapies (exemestane, fulvestrant, tamoxifen) for HR+/HER2− metastatic breast cancer treatment [31]. The study was conducted from the US third-party payer perspective over a 10-year time horizon. In base-case analysis, the authors found that the incremental cost per QALY was $139,740 when compared to exemestane alone, $157,749 when compared to fulvestrant alone, and $115,624 when compared to tamoxifen alone. Everolimus plus exemestane was found to be the most cost-effective treatment option at willingness to pay thresholds of $130,000 or higher.
Chemotherapy
Three articles estimated the cost-effectiveness of chemotherapeutic agents [19,20,21]. Unlike the ICER results for targeted treatments, the majority (2/3) of ICER results for chemotherapeutic agents were cost-effective. Greenhalgh et al. conducted the single study in this treatment category which concluded that the intervention of interest was not cost-effective. In the analysis, the authors evaluated eribulin versus treatment of physician’s choice (TPC) for locally advanced or metastatic breast cancer in the 3rd line of treatment. This analysis was conducted from the UK national payer (National Health Service and Personal Social Services in England and Wales) perspective over a lifetime horizon [21]. The base-case ICER for eribulin versus TPC was £76,110 per QALY, ultimately resulting in the Appraisal Committee’s decision not to recommend the use of eribulin in this patient population.
Quality assessment
The results of the reporting quality assessment based on the ISPOR CHEERS Checklist are presented in Table 3. The articles by Squires et al. and Greenhalgh et al. were not scored based on this checklist because they are Health Technology Assessments prepared for the express purpose of reimbursement review by a national review agency, and with their own set of requirements and regulations, rather than an independent economic analyses [21, 29]. The remaining 15 articles were reviewed using the CHEERS Checklist.
The majority of the studies did not include the intervention of comparison in the title (N = 10, 66.67%). Nine of the studies sufficiently described the healthcare system and reimbursement status of the drugs (N = 9, 60%). One study incorrectly failed to consider indirect costs after specifying a societal perspective [25]. Most of the studies clearly justified why the comparisons were chosen for analysis (N = 14, 93.3%). Six of the studies did not describe why a given time horizon was appropriate (N = 6, 40%). An even larger portion of the studies provided no justification as to the discount rate selected (N = 10, 66.67%). A few of the studies failed to describe either why a single study was appropriate as the source of the effectiveness data or the methods used to identify and synthesize studies (N = 5, 33.3%). Utility weights were described in all studies, but only two studies elicited preferences for these outcomes rather than referencing external sources for utility data (N = 2, 13.3%). Another three studies did not clearly describe methods used to estimate healthcare resources and their unit costs. Two studies did not report the dates of the estimated resource quantities and unit costs (N = 2, 13.3%) [18, 27]. Seven studies included a figure of the model but no justification for the analytic approach (N = 7, 46.67%) and four studies included neither a figure nor a justification (N = 4, 26.67%). Two studies failed to describe all the structural assumptions going into the model (N = 2, 13.3%). Five studies did not describe any of the analytic methods supporting the evaluation such as dealing with skewed, missing, censored data, or extrapolation methods (N = 5, 33.33%).
In reporting the parameters, three studies provided incomplete details. One study did not include the source information in the input parameters table [18]. Another study did not include the ranges used in sensitivity analysis in their input parameters table [31]. The third study did not justify why they varied model parameters using 95% confidence interval ranges for the probabilistic sensitivity analysis [20]. Three studies did not report the incremental cost differences between the interventions in the table of results. Of these three studies, one did not provide a table of the base-case cost-effectiveness results [18]. Two studies had incomplete descriptions of the sensitivity analyses conducted. Two studies failed to include figures of the ICER scatterplot, tornado chart, or a cost-effectiveness acceptability curve [19, 25]. Six articles did not describe the extent of funder involvement in the studies (N = 6, 40%). One study did not describe the potential for conflicts of interest among study contributors [28].
Discussion
This study reviewed 17 recently published cost–utility analyses of drugs for metastatic breast cancer. The quality of the included studies was moderate based on the CHEERS checklist total scores. The average reporting quality score of the 15 articles reviewed was 19.4, with a highest possible score of 24. Only 41% (approximately 6.5 studies) found that the intervention of interest was cost-effective at the willingness to pay threshold for the country of analysis. Three studies contribute a 0.5-score because they were either analyzing multiple combinations of interventions in which one or more were not cost-effective or the analyses used an unconventionally high willingness to pay threshold for the given country.
The overall cost-effectiveness results present a challenge to treatment in the MBC setting because both private insurance plans and single-payer national healthcare systems may not be willing to accept such high ICERs and therefore may not grant access to these drugs on formularies. One of the consequences of this globally is that there will be large differences in patterns of care due to varying levels of decision-making power by payers. As such, it may become even more difficult to define the standard of care for future clinical trials if access to treatments varies based on cost and setting. More importantly, high ICERs represent high opportunity cost. Both within cancer and across disease areas, dollars allocated to drugs that show minimal benefit are not being spent on gains elsewhere. This review highlights the challenge in the metastatic breast cancer setting, where very few published studies since 2014 have demonstrated cost-effectiveness at commonly accepted willingness to pay thresholds.
Although economic analyses from the societal perspective are considered best practice, this review finds that only four studies employed this perspective [34]. The majority of the included analyses employed a payer perspective, suggesting that they were conducted for regulatory purposes. Unlike a payer perspective, a societal perspective would consider all stakeholders impacted by an intervention regardless of who incurs the costs or experiences the outcomes [35]. Indirect costs, such as those associated with lost productivity due to illness, are an important component to the societal perspective and are needed in more published CEAs.
Only three of the reviewed articles studied chemotherapies rather than treatments targeting either HER2 or hormone receptors. In other words, the reviewed articles did not study interventions which address the common problem of acquired endocrine resistance, by which a tumor stops responding to a therapy to which it was initially responsive [36]. In the absence of a target or when endocrine resistance develops and targeted therapies are no longer viable options as in TNBC, taxane-based and anthracycline-based chemotherapies are the recommended treatments [37]. The publication bias towards expensive new targeted therapies creates the false impression that there are no moderately priced chemotherapeutic treatment options for endocrine-refractory breast cancer. This review also brings to attention a lack of evidence to inform the cost-effectiveness of newer treatments for metastatic TNBC [38]. This is an important area for future research.
The results of this systematic review confirm several of the points made by Pouwels et al. [8]. One of the main takeaways from the Pouwels et al. study was that treatments for MBC did not provide good value for money and that ICERs did not meet typical willingness to pay thresholds. This review, much like the one by Pouwels et al., found that most of the reviewed articles employed Markov models with three health states but that the studies varied with respect to the time horizons, cycle lengths, utility weights, and adverse events that were included. In order to improve consistency and quality of economic analyses for MBC moving forward, the authors suggested the development of a disease-specific reference model that is not limited to a setting or patient population, as is one of the included studies in this review [15]. This model was designed based on a Canadian context, and will need to be adapted prior to use in the United States.
There are a few limitations to this analysis. In the selection of articles for analyses, we excluded reports or posters for which only abstracts were available. This may have led to an omission of relevant work. Reports or posters of this nature lack details on the methodological approach which would make quality assessments a challenge to conduct. We did not conduct a meta-analysis because of the heterogeneity of model assumptions, outcomes, and other study features. Another limitation of reviewing studies that were conducted in various countries is that it is difficult to compare ICER results when they are evaluated against different willingness to pay thresholds.
Despite these limitations, this analysis contributes to the literature because it consists of a thorough review and quality assessment for most of the recently published cost-effectiveness studies for MBC. A major strength of this systematic review is that the quality assessment was conducted using a validated instrument for reporting on health economic evaluations [32]. By identifying informational gaps in the literature, this review also provides directions for future research.
Conclusion
We identified several economic analyses of treatments for metastatic breast cancer published since October 2014. Although each of the studies contributed its own range of incremental cost-effectiveness ratios and study limitations, the review demonstrates that the vast majority exceeded the typical willingness to pay thresholds for the countries in which the analyses were conducted. This review also uncovers a gap in the literature regarding the cost-effectiveness of treatments for endocrine-refractory and triple-negative metastatic breast cancers.
References
Most Common Statistics Cited for MBC. Metastatic breast cancer network. http://www.mbcn.org/most-common-statistics-cited-for-mbc/
Tumor Characteristics. Susan G, Komen. http://ww5.komen.org/BreastCancer/TumorCharacteristics.html. Published 2016
NCCN. NCCN clinical practice guidelines in oncology (NCCN Guidelines®) breast cancer. Version 12016. 2016
NCCN. NCCN Guidelines® for patients metastatic breast cancer. 2018
Li N, Hao Y, Xie J et al (2015) Everolimus-based therapy versus chemotherapy among patients with HR. Int J Breast Cancer. https://doi.org/10.1155/2015/240750
Foster TS, Miller JD, Boye ME, Blieden MB, Gidwani R, Russell MW (2011) The economic burden of metastatic breast cancer: a systematic review of literature from developed countries. Cancer Treat Rev 37(6):405–415. https://doi.org/10.1016/j.ctrv.2010.12.008
Siegel JE (2005) Cost-effectiveness analysis in US healthcare decision-making: where is it going? Med Care 43(7):II1–II4. http://www.jstor.org/stable/3768423
Pouwels XGLV, Ramaekers BLT, Joore MA (2017) Reviewing the quality, health benefit and value for money of chemotherapy and targeted therapy for metastatic breast cancer. Breast Cancer Res Treat 165:485–498. https://doi.org/10.1007/s10549-017-4374-6
Sasich LD, Sukkari SR (2012) The US FDAs withdrawal of the breast cancer indication for Avastin (bevacizumab). Saudi Pharm J 20(4):381–385. https://doi.org/10.1016/j.jsps.2011.12.001
Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL (2005) Methods for the economic evaluation of health care programmes. Oxford University Press, Oxford
Neumann PJ, Cohen JT, Weinstein MC (2014) Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. https://doi.org/10.1056/NEJMp1405158
Chapman RH, Berger M, Weinstein MC, Weeks JC, Goldie S, Neumann PJ (2004) When does quality-adjusting life-years matter in cost-effectiveness analysis? Health Econ. https://doi.org/10.1002/hec.853
Laupacis A, Feeny D, Detsky AS, Tugwell PX (1992) How attrative does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clincal and economic evaluations. Can Med Assoc J. https://doi.org/10.1016/S0140-6736(98)07019-6
Cleemput I, Neyt M, Thiry N, De Laet C, Leys M (2011) Using threshold values for cost per quality-adjusted life-year gained in healthcare decisions. Int J Technol Assess Health Care. https://doi.org/10.1017/S0266462310001194
Beauchemin C, Letarte N, Mathurin K, Yelle L, Lachaine J (2016) A global economic model to assess the cost-effectiveness of new treatments for advanced breast cancer in Canada. J Med Econ 19(6):619–629. https://doi.org/10.3111/13696998.2016.1151431
Diaby V, Adunlin G, Ali AA et al (2016) Cost-effectiveness analysis of 1st through 3rd line sequential targeted therapy in HER2-positive metastatic breast cancer in the United States. Breast Cancer Res Treat 160(1):187–196. https://doi.org/10.1007/s10549-016-3978-6
Diaby V, Ali AA, Williams KJ et al. Economic evaluation of sequencing strategies in HER2-positive metastatic breast cancer in Mexico: a contrast between public and private payer perspectives. Breast Cancer Res Treat 2017:1–13
Ding H, Fang L, Xin W, Tong Y, Zhou Q, Huang P (2017) Cost-effectiveness analysis of fulvestrant versus anastrozole as first-line treatment for hormone receptor-positive advanced breast cancer. Eur J Cancer Care 26(6):e12733. https://doi.org/10.1111/ecc.12733
Dranitsaris G, King J, Kaura S, Yu B, Zhang A (2015) Nab-paclitaxel, docetaxel, or solvent-based paclitaxel in metastatic breast cancer: a cost-utility analysis from a Chinese health care perspective. Clin Outcomes Res. https://doi.org/10.2147/CEOR.S82194
Durkee BY, Qian Y, Pollom EL et al (2016) Cost-effectiveness of pertuzumab in human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 34(9):902–909. https://doi.org/10.1200/JCO.2015.62.9105
Greenhalgh J, Bagust A, Boland A et al (2015) Eribulin for the treatment of advanced or metastatic breast cancer: a NICE single technology appraisal. Pharmacoeconomics 33(2):137–148. https://doi.org/10.1007/s40273-014-0214-2
Le QA, Bae YH, Kang JH (2016) Cost-effectiveness analysis of trastuzumab emtansine (T-DM1) in human epidermal growth factor receptor 2 (HER2): positive advanced breast cancer. Breast Cancer Res Treat 159(3):565–573. https://doi.org/10.1007/s10549-016-3958-x
Leung HWC, Chan ALF, Muo C-H, Leung JH. Cost-effectiveness of pertuzumab combined with trastuzumab and docetaxel as a first-line treatment for HER-2 positive metastatic breast cancer. Expert Rev Pharmacoecon Outcomes Res 2017:1–7. https://doi.org/10.1080/14737167.2018.1386559
Leung HW, Chan AL, Wang S-Y (2018) Cost-utility analysis of trastuzumab combined with Docetaxel for patients with HER-2 positive metastatic breast cancer—real world claim data. J Oncol Pharm Pract. https://doi.org/10.1177/1078155218755548
Mamiya H, Tahara RK, Tolaney SM, Choudhry NK, Najafzadeh M (2017) Cost-effectiveness of palbociclib in hormone receptor-positive advanced breast cancer. Ann Oncol 28(8):1825–1831. https://doi.org/10.1093/annonc/mdx201
Matter-Walstra K, Ruhstaller T, Klingbiel D, Schwenkglenks M, Dedes KJ (2016) Palbociclib as a first-line treatment in oestrogen receptor-positive, HER2-negative, advanced breast cancer not cost-effective with current pricing: a health economic analysis of the Swiss Group for Clinical Cancer Research (SAKK). Breast Cancer Res Treat 158(1):51–57. https://doi.org/10.1007/s10549-016-3822-z
Raphael J, Helou J, Pritchard KI, Naimark DM (2017) Palbociclib in hormone receptor positive advanced breast cancer: a cost-utility analysis. Eur J Cancer 85:146–154. https://doi.org/10.1016/j.ejca.2017.08.018
Sabale U, Ekman M, Thunstrom D, Telford C, Livings C (2017) Economic evaluation of fulvestrant 500 mg compared to generic aromatase inhibitors in patients with advanced breast cancer in Sweden. PharmacoEconomics Open 1(4):279–290. https://doi.org/10.1007/s41669-017-0031-6
Squires H, Stevenson M, Simpson E, Harvey R, Stevens J (2016) Trastuzumab emtansine for treating HER2-positive, unresectable, locally advanced or metastatic breast cancer after treatment with trastuzumab and a taxane: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics 34(7):673–680. https://doi.org/10.1007/s40273-016-0386-z
Tremblay G, Majethia U, Breeze JL, Kontoudis I, Park J (2016) Economic evaluation of eribulin as second-line treatment for metastatic breast cancer in South Korea. Clin Outcomes Res 8:485–493. https://doi.org/10.2147/CEOR.S110553
Xie J, Hao Y, Zhou Z-Y, Qi CZ, De G, Glück S (2015) Economic evaluations of everolimus versus other hormonal therapies in the treatment of HR+/HER2− advanced breast cancer from a US payer perspective. Clin Breast Cancer 15(5):e263–e276. https://doi.org/10.1016/j.clbc.2015.04.001
Husereau D, Drummond M, Petrou S et al (2013) Consolidated health economic evaluation reporting standards (CHEERS)-explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Heal. https://doi.org/10.1016/j.jval.2013.02.002
Delea TE, Amdahl J, Chit A, Amonkar MM (2013) Cost-effectiveness of lapatinib plus letrozole in her2-positive, hormone receptor–positive metastatic breast cancer in Canada. Curr Oncol 20(5):371. https://doi.org/10.3747/co.20.1394
Sanders GD, Neumann PJ, Basu A et al (2016) Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. https://doi.org/10.1001/jama.2016.12195
Gold MR, Siegel JE, Russell LB, Weinstein MC (1996) Cost-effectiveness in health and medicine. Oxford University Press, Oxford
Higgins MJ, Baselga J, Sorlie T et al (2011) Targeted therapies for breast cancer. J Clin Investig. https://doi.org/10.1172/JCI57152
Zeichner SB, Terawaki H, Gogineni K (2016) A review of systemic treatment in metastatic triple-negative breast cancer. Breast Cancer 10:25–36. https://doi.org/10.4137/BCBCR.S32783
Naidoo S, Friedman ML, Paly VF, Hansen R, Sidhu MK, Smith I (2017) Targeted literature review of advanced/metastatic triple-negative breast cancer burden of illness. In: ISPOR 22nd annual international meeting, Boston
Acknowledgements
This work was supported, in part, by the Centers for Disease Control and Prevention and National Cancer Institute Special Interest Project entitled “Economic Burden of Metastatic Breast Cancer across the Life Course” (3-U48-DP005017-04S4, PIs: Trogdon and Wheeler) and by the Cancer Information and Population Health Resource, UNC Lineberger Comprehensive Cancer Center, with funding provided by the University Cancer Research Fund via the state of North Carolina.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AG held an internship position with Janssen Pharmaceuticals, Inc. for work external to this study. JT received research funding to his institution from Merck, Inc. for another project. SW receives grant funding to their institution from Pfizer. JR, CB, KRH, and KM do not have any conflicts of interest to disclose.
Informed consent
For this type of study formal consent is not required.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Gogate, A., Rotter, J.S., Trogdon, J.G. et al. An updated systematic review of the cost-effectiveness of therapies for metastatic breast cancer. Breast Cancer Res Treat 174, 343–355 (2019). https://doi.org/10.1007/s10549-018-05099-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-05099-3