Abstract
Purpose
Due to limitations in the ability to identify non-progressive disease, ductal carcinoma in situ (DCIS) is usually managed similarly to localized invasive breast cancer. We used simulation modeling to evaluate the potential impact of a hypothetical test that identifies non-progressive DCIS.
Methods
A discrete-event model simulated a cohort of U.S. women undergoing digital screening mammography. All women diagnosed with DCIS underwent the hypothetical DCIS prognostic test. Women with test results indicating progressive DCIS received standard breast cancer treatment and a decrement to quality of life corresponding to the treatment. If the DCIS test indicated non-progressive DCIS, no treatment was received and women continued routine annual surveillance mammography. A range of test performance characteristics and prevalence of non-progressive disease were simulated. Analysis compared discounted quality-adjusted life years (QALYs) and costs for test scenarios to base-case scenarios without the test.
Results
Compared to the base case, a perfect prognostic test resulted in a 40% decrease in treatment costs, from $13,321 to $8005 USD per DCIS case. A perfect test produced 0.04 additional QALYs (16 days) for women diagnosed with DCIS, added to the base case of 5.88 QALYs per DCIS case. The results were sensitive to the performance characteristics of the prognostic test, the proportion of DCIS cases that were non-progressive in the model, and the frequency of mammography screening in the population.
Conclusion
A prognostic test that identifies non-progressive DCIS would substantially reduce treatment costs but result in only modest improvements in quality of life when averaged over all DCIS cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Approximately 20% of U.S. women diagnosed with breast cancer have ductal carcinoma in situ (DCIS) [1]. This percentage is even greater (over 27%) among women who regularly participate in mammography screening [2]. Approximately 85% of DCIS is diagnosed asymptomatically through screening mammography [3]. As of 2016, over 1 million women are estimated to have a personal history of a DCIS diagnosis [4]. Several lines of research suggest that a substantial fraction of DCIS—perhaps more than half—would never have caused the women harm if the DCIS had remained undetected [5,6,7,8]. Because a significant proportion will progress to invasive disease, treatment for DCIS typically is similar to approaches for locally limited invasive breast cancer including excision, radiation, and adjuvant endocrine therapy. Increasingly, women are choosing ipsilateral mastectomy, contralateral risk reduction mastectomy, and breast reconstruction which have substantial impacts relating to quality of life and financial costs [9].
In 2011, a new multigene assay for estimating the 10-year risk of local recurrence, the Oncotype DX® DCIS Score™, became available for women with DCIS treated by local excision [10]. Although use of the DCIS score assay is limited due to concerns of patient eligibility and lack of consideration of non-molecular predictive factors such as margin width and nuclear grade [11], independent studies confirm that this 12-gene assay predicts local recurrence after DCIS [12] and influences decisions regarding radiotherapy after breast-conserving surgery [13]. Additional approaches using features to identify the malignant potential of a DCIS lesion are actively under investigation, most often by combining different sources of information regarding histological and molecular markers as well as method of detection [14,15,16].
In anticipation of the development of more accurate methods of identifying non-progressive DCIS, we used the University of Wisconsin Breast Cancer Simulation Model to evaluate the impact of a hypothetical prognostic test for DCIS. Simulation modeling is a valuable tool to estimate the population impact of a new or hypothetical intervention as it allows for using multiple sources of input data without risk to human subjects. We started by assuming a perfect prognostic test to estimate, under ideal circumstances, the maximum impact on costs and quality of life if non-progressive DCIS lesions were not treated beyond diagnostic biopsy, sparing women further treatment. We then explored the impact of alternative assumptions of test performance, DCIS tumor growth, and screening mammography utilization.
Methods
University of Wisconsin Breast Cancer Simulation Model
The University of Wisconsin Breast Cancer Simulation Model is a discrete-event simulation model that uses multiple sources of U.S. cancer data to generate registry-like datasets of breast cancer incidence and mortality statistics according to patient demographics [17]. The model was developed as part of the Cancer Intervention and Surveillance Modeling Network (CISNET), a consortium funded by the National Cancer Institute [18, 19]. The simulation model is used to consider the benefits and harms of alternate screening and treatment programs for breast cancer [20,21,22,23,24]. The University of Wisconsin Health Sciences Human Subjects Committee determined that this study was exempt from review.
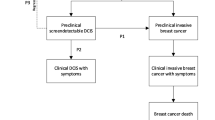
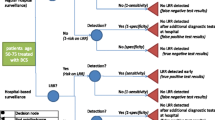
Briefly, the simulation model has four main components—natural history, detection, treatment, and non-breast cancer mortality; [25] additional information regarding the model is included in Fig. 1 and Online Appendix Table 1 [18, 23, 26,27,28,29,30,31,32,33,34,35,36,37,38,39]. The natural history component assumes that the probability of breast cancer onset is a function of risk based on trends in breast cancer according to single years of age at diagnosis, years of diagnosis, and years of birth [26, 27]. In the model, all invasive breast cancer tumors first progress through DCIS, so that a “tumor” can be either invasive or DCIS. Tumor growth is a function of a random initial growth parameter, with a fraction of tumors (initially set through calibration) having limited malignant potential, i.e., non-progressive growth. Thus, in the primary analysis, we did not assume a priori a specific fraction of non-progressive disease; rather, the fraction of non-progressive disease (30–50% with a “best” estimate of 42%) was determined by testing different fractions for the best fit to the observed historical patterns in stage-specific breast cancer incidence. In other words, not all DCIS or small localized invasive cancers progress to become a lethal event. After reaching a maximum size, also set through calibration, non-progressive tumors stop growing. If undetected, all non-progressive tumors become undetectable or disappear from the model after reaching their maximum size for a specified time set through calibration (2 years).
Schema representing breast cancer natural history, detection, and treatment as represented in the simulation model: a in the absence of a DCIS prognostic test and b in the presence of a perfect DCIS prognostic test. If the DCIS prognostic test is positive, indicating that the DCIS is non-progressive, then the woman receives no treatment and eventually dies from a cause other than breast cancer. If the DCIS prognostic test is negative, the DCIS is progressive, the woman receives breast cancer treatment, and survival depends on whether the cancer is cured (determined stochastically) and on the efficacy of treatment. See Table 1 for the description of the implementation of the DCIS prognostic test and cancer detection in the simulation model
The detection component assumes that breast cancer can be detected either by screening mammography or through clinical surfacing by clinical examination or self-detection [27]. The probability that any undetected tumor will be symptomatically detected is a function of tumor diameter, so that larger tumors are more likely to be clinically detected at any time relative to smaller tumors. Screen detection of a tumor during the preclinical detectable period could result in the identification and treatment of earlier stage or smaller tumors and lead to breast cancer mortality reduction. Sensitivity of digital mammography depends on a woman’s age (< 50, 50–69, and ≥ 70), breast density, and also on tumor diameter and calendar year [25]. In addition, the probability that a woman has a digital mammogram is a function of calendar year and age, so that it is more likely that a woman will have a mammogram with increasing age and time [30]. Screening mammography rates are based on observed dissemination patterns in the U.S. estimated from the Breast Cancer Surveillance Consortium [30, 40].
Breast cancer treatment is modeled using a “cured”/“not cured” approach. Uncured tumors continue to grow until they reach the distant disease stage and, eventually, cause death from breast cancer unless the woman dies from other causes or reaches age 100 (the end of the simulation). The probability of cure depends on the treatment given, tumor stage at detection, and age [25]. Surgical treatment assignment depends on the year when the tumor was detected based on patterns of care observed in the SEER Program and the National Cancer Data Base [9, 23]; adjuvant therapy is assigned based on stage of disease at detection and ER/HER2 status [41]. Survival rates for specific treatments including surgery, radiation, chemotherapy, endocrine therapy, and targeted therapy (e.g., trastuzumab) are based on recent clinical trial results [23, 32]. The non-breast cancer mortality component assumes that death from other causes will occur independently of a woman’s breast cancer status, and the probability of death from other causes depends on age [38, 42].
The simulated population included women born in 1970. This birth cohort was chosen since these women experience modern conditions (e.g., digital mammography performance, treatment effectiveness, competing mortality, etc.) and for consistency with recent collaborative modeling reports [18, 22]. Current digital mammography performance and treatment effectiveness were used for future calendar years. In each simulation, subgroups of women were followed from age 20 until death or age 100, whichever occurs first.
Outcomes
In all simulated scenarios, primary outcomes were quality-adjusted life years (QALYs) and costs. We used age-specific quality of life weights for general health based on utilities from noninstitutionalized female civilians in the U.S. (Online Appendix Table 1) [33]. Adjustments were made for stage-specific breast cancer treatment. Costs were divided into annual surveillance mammography costs among diagnosed DCIS cases who were not treated beyond biopsy, stage-specific treatment costs (including subsequent surveillance mammography among treated cases) [19, 36], and prognostic test costs, reported in 2015 U.S. dollars (Table 1). Cost of the hypothetical DCIS prognostic test was assumed to be $3419.42, based on Medicare reimbursement for a currently available diagnostic test (Oncotype DX® Breast DCIS Score™, HCPSC 81519). Life years, QALYs, and costs were discounted at 3% per year.
Secondary outcomes included breast cancer deaths, life years, the numbers of prognostic tests, and the number of DCIS cases treated (or not treated).
Comparison scenario: current practice for DCIS management
As our primary comparison scenario, we assumed current mammography dissemination and modern breast cancer treatment. The hypothetical DCIS prognostic test was not implemented. All women diagnosed with breast cancer were given treatment (100% adherence) according to guidelines.
Alternate scenarios: DCIS prognostic test
One primary alternate scenario was modeled by modifying the simulation model to include application of a perfect DCIS prognostic test at the time of diagnosis (Fig. 1, panel B). If the test was “positive,” indicating non-progressive disease, then the case was not treated beyond diagnostic biopsy, thus also avoiding a decrement to quality of life associated with treatment. DCIS cases with positive prognostic tests were assigned annual mammography (“surveillance” mammography after diagnosis) until death or a diagnosis with invasive breast cancer. (Under this scenario with the perfect test, no “non-progressive” DCIS progressed to invasive breast cancer; this only occurred with false-positive prognostic tests in the sensitivity analysis, described below.) If the prognostic test was “negative,” indicating that the tumor was progressive, then the case received standard treatment.
Analysis
Two million women were simulated for each scenario. Outcomes were compared between the scenarios with and without a perfect hypothetical DCIS prognostic test (100% sensitivity, 100% specificity). We compared additional scenarios to examine the effects of variation in prognostic test performance characteristics, quality of life decrements, and model parameters governing disease progression. Prognostic test performance was varied such that sensitivity of the prognostic test to identify non-progressive disease ranged from 50 to 95%, while specificity of the test to identify progressive disease ranged from 70 to 95%. We varied decrements in quality of life associated with breast cancer treatment; specifically, we increased the disutility of treatment for DCIS and localized invasive breast cancer (from 0.1 to 0.2) since stage-specific treatment disutilities were initially small for early-stage disease (Table 1) [19, 35]. We varied model parameters regarding non-progressive tumors by setting the percent of DCIS tumors designated as non-progressive as 30 or 50%, instead of the “best” value set through calibration (42%), to reflect the range generally reported in the literature. Finally, we examined alternative schedules for screening mammography (for women without a personal history of breast cancer) including those recommended by the U.S. Preventive Services Task Force (biennial ages 50–74 year), the American Cancer Society (annual 45–54 year, biennial 55–79 year), and the American College of Radiology (annual 40–74 year).
Results
In the absence of a DCIS prognostic test, the model predicted that 13.9% of women were diagnosed with breast cancer in their lifetimes. Slightly over 20% of breast cancers were DCIS (20.6%); overall, 8% of all breast cancer cases and 3% of DCIS cases died of breast cancer. Costs were estimated to equal $13,321 per DCIS case.
In the perfect test scenario, the DCIS prognostic test was applied to 2.8% of all women (2844 per 100,000), with approximately 60% of tests being positive for a non-progressive lesion (1696 per 100,000) (Table 2). Although 42% of all breast cancer tumors were non-progressive at onset, the percent of non-progressive tumors decreases with increasing stage at diagnosis. Thus, 60% of DCIS cases were spared treatment. Among women diagnosed with DCIS, the gain in QALYs was modest (0.044 per case, or 16 days of perfect health) and mortality was unchanged (3.0%); however, the reduction in costs was substantial. All DCIS cases experienced the cost of the prognostic test with an estimated total expected discounted cost per DCIS case of $1598 (assuming a 3% annual discounting factor for future costs). DCIS cases with positive prognostic tests incurred surveillance mammography costs ($705 per case), whereas DCIS cases with negative tests incurred both surveillance mammography and treatment costs. Total lifetime costs were reduced by 40% averaged across all DCIS cases.
In scenarios where specificity remained perfect (no progressive disease was misidentified as non-progressive) but sensitivity was decreased as low as 20% (as little as 20% of non-progressive disease tested positive), breast cancer mortality among cases diagnosed with DCIS remained unchanged and the effect on QALYs was small, but costs increased from $8334 to $13,521 per DCIS case (Table 3). In scenarios with perfect sensitivity but reduced specificity (some progressive disease was misidentified as non-progressive), the effect on QALYs and costs was modest and breast cancer deaths among women diagnosed with DCIS increased by 0.1–0.4% (2–11 per 100,000 women). Changes in sensitivity had a greater effect on the number of DCIS cases that avoided treatment than changes in specificity (Fig. 2).
Changing the decrement in quality of life associated with DCIS and localized invasive treatment from 0.1 to 0.2 for 2 years doubled the gain in QALYs for DCIS cases in the presence of a perfect test from 16 to 32 days (Table 2). When we varied assumptions by increasing the prevalence of non-progressive breast tumors, the benefits of a DCIS prognostic test were greater, with a savings of almost $1000 per DCIS case if 50% were non-progressive instead of 42%.
Varying screening schedules did not substantially impact outcomes (Online Appendix Table 2). Screening strategies with more frequent mammography generally lead to greater numbers of detected DCIS with slightly fewer cases avoiding treatment and slightly greater costs in the presence of a DCIS prognostic test, but these differences were small.
Discussion
This modeling study demonstrates the maximum potential impact of a hypothetical prognostic test for non-progressive DCIS. Results showed that, under optimal conditions, treatment cost savings would be substantial in the presence of an accurate prognostic test. Given that about 63,000 women are diagnosed with DCIS in the U.S. each year [43], this corresponds to an annual savings of over $334 million. The availability of a perfect DCIS prognostic test would improve quality of life, on average, about 2–4 weeks of perfect health across all women diagnosed with DCIS. The estimated utility of the perfect prognostic (0.044) is slightly greater than the quality of life impact of a screening mammogram (0.01–0.03) but smaller than the impact of breast-conserving surgery (0.07–0.09) [44,45,46].
Our primary analysis assumed that the hypothetical prognostic test was perfectly accurate. While this assumption is unrealistic, these results demonstrate the optimal impact of a prognostic test that can be anticipated. Our scenario analyses demonstrate how the projected costs and the number of DCIS cases that avoided treatment are dependent on test sensitivity; conversely, breast cancer mortality depends on test specificity. The cost of the prognostic test was based on current Medicare reimbursement for the Oncotype DX test which is greater than the costs of surveillance mammography or breast MRI but less than the costs of breast cancer treatment. We estimated costs of surveillance mammography in women with a positive diagnostic test, but it is likely that a variety of surveillance approaches would be utilized in the setting of a prognostic test (e.g., imaging with MRI or more frequent mammography), so that our analysis did not fully estimate the variety or magnitude of surveillance costs.
The breast cancer simulation model has been calibrated to match breast cancer incidence and mortality as observed in the US, and incorporates inputs on modern screening performance and treatment benefit. Other model inputs are unobservable or difficult to quantify, so that the model requires certain assumptions. We explored the extent that assumptions regarding tumor growth, quality of life utilities, and the frequency of mammography screening had on the results. In particular, the percent of tumors designated at biological onset as non-progressive (42%) was initially set through model calibration [17]. We conducted a series of secondary analyses to explore the impact of this parameter and found that the results were sensitive to the fraction of tumors that were non-progressive; the value of a prognostic test increases along with the fraction of tumors that are non-progressive. The proportion of DCIS lesions that are non-progressive is debated, with studies finding a very wide range of potential values, from 10 to 80% [5,6,7,8]. We also assumed that positive results of the hypothetical prognostic test meant that women avoided all treatments including excision, whereas potentially more likely clinical scenarios for such a test may include breast-conserving surgery or endocrine therapy and avoidance of some but not all treatment approaches. Thus, the cost savings resulting from our modeling experiments describe the most optimistic scenario in terms of cost savings. Treatment costs used in this study arise from studies that provided values for the phase of care (initial, interim, end of life) rather than for the type of treatment (surgery, radiation, endocrine therapy) [36], thus preventing the estimation of cost savings when specific treatments are omitted. Our model was also limited since women were at risk of only a single breast cancer diagnosis; future studies should consider risk of multiple primaries since over 10% of breast cancer survivors experience a second primary diagnosis [47]. In addition, future analysis should account for up-staging that occurs among approximately 24% of women who are diagnosed with DCIS at biopsy but have invasive breast cancer detected at the time of surgery [48]. Finally, we did not estimate emotional costs such as anxiety that might be associated with DCIS testing and forgoing treatment. Randomized trials investigating these issues will shed light on these qualitative components of the clinical cascade, which will then be available for modeling in the future.
Efforts to identify indolent DCIS and improve decision-making surrounding treatment for early-stage breast cancer are ongoing, and recent developments in molecular-based assays will likely play an important role in future DCIS management. Alternative approaches to immediate surgery, including active surveillance using advanced imaging, are also under evaluation [49]. While randomized trials will ultimately provide evidence regarding the benefits of actual prognostic tests in the future, simulation modeling is a valuable tool to explore unobservable phenomenon like tumor growth in asymptomatic women. Modeling also allows incorporation of multiple data sources to inform the analysis based on modern breast cancer screening and treatment. This study provides estimates of the potential impact of implementing a tailored approach to DCIS therapy, where women with greater risk of recurrence or a second breast cancer diagnosis receive more extensive treatment. Our findings suggest that a DCIS prognostic test that could identify non-progressive DCIS would substantially reduce costs associated with DCIS management.
References
Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016) SEER Cancer Statistics Review, 1975–2013
Miglioretti DL, Zhu W, Kerlikowske K, Sprague BL, Onega T, Buist DS, Henderson LM, Smith RA, Breast Cancer Surveillance C (2015) Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status. JAMA Oncol 1:1069–1077. https://doi.org/10.1001/jamaoncol.2015.3084
Sprague BL, McLaughlin V, Hampton JM, Newcomb PA, Trentham-Dietz A (2013) Disease-free survival by treatment after a DCIS diagnosis in a population-based cohort study. Breast Cancer Res Treat 141:145–154. https://doi.org/10.1007/s10549-013-2670-3
Sprague BL, Trentham-Dietz A (2009) Prevalence of breast carcinoma in situ in the United States. JAMA 302:846–848
Ozanne EM, Shieh Y, Barnes J, Bouzan C, Hwang ES, Esserman LJ (2011) Characterizing the impact of 25 years of DCIS treatment. Breast Cancer Res Treat 129:165–173. https://doi.org/10.1007/s10549-011-1430-5
Yen MF, Tabar L, Vitak B, Smith RA, Chen HH, Duffy SW (2003) Quantifying the potential problem of overdiagnosis of ductal carcinoma in situ in breast cancer screening. Eur J Cancer 39:1746–1754
Independent U. K. Panel on Breast Cancer Screening (2012) The benefits and harms of breast cancer screening: an independent review. Lancet 380:1778–1786. https://doi.org/10.1016/S0140-6736(12)61611-0
Etzioni R, Gulati R, Mallinger L, Mandelblatt J (2013) Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Intern Med 158:831–838. https://doi.org/10.7326/0003-4819-158-11-201306040-00008
Shiyanbola OO, Sprague BL, Hampton JM, Dittus K, James TA, Herschorn S, Gangnon RE, Weaver DL, Trentham-Dietz A (2016) Emerging trends in surgical and adjuvant radiation therapies among women diagnosed with ductal carcinoma in situ. Cancer 122:2810–2818. https://doi.org/10.1002/cncr.30105
Solin LJ, Gray R, Baehner FL, Butler SM, Hughes LL, Yoshizawa C, Cherbavaz DB, Shak S, Page DL, Sledge GW Jr, Davidson NE, Ingle JN, Perez EA, Wood WC, Sparano JA, Badve S (2013) A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst 105:701–710. https://doi.org/10.1093/jnci/djt067
Lagios MD, Silverstein MJ (2014) Risk of recurrence of ductal carcinoma in situ by oncotype Dx technology: some concerns. Cancer 120:1085. https://doi.org/10.1002/cncr.28523
Rakovitch E, Nofech-Mozes S, Hanna W, Baehner FL, Saskin R, Butler SM, Tuck A, Sengupta S, Elavathil L, Jani PA, Bonin M, Chang MC, Robertson SJ, Slodkowska E, Fong C, Anderson JM, Jamshidian F, Miller DP, Cherbavaz DB, Shak S, Paszat L (2015) A population-based validation study of the DCIS Score predicting recurrence risk in individuals treated by breast-conserving surgery alone. Breast Cancer Res Treat 152:389–398. https://doi.org/10.1007/s10549-015-3464-6
Alvarado M, Carter DL, Guenther JM, Hagans J, Lei RY, Leonard CE, Manders J, Sing AP, Broder MS, Cherepanov D, Chang E, Eagan M, Hsiao W, Schultz MJ (2015) The impact of genomic testing on the recommendation for radiation therapy in patients with ductal carcinoma in situ: a prospective clinical utility assessment of the 12-gene DCIS score result. J Surg Oncol 111:935–940. https://doi.org/10.1002/jso.23933
Molinaro AM, Sison JD, Ljung BM, Tlsty TD, Kerlikowske K (2016) Risk prediction for local versus regional/metastatic tumors after initial ductal carcinoma in situ diagnosis treated by lumpectomy. Breast Cancer Res Treat 157:351–361. https://doi.org/10.1007/s10549-016-3814-z
Benson JR, Wishart GC (2013) Predictors of recurrence for ductal carcinoma in situ after breast-conserving surgery. Lancet Oncol 14:e348–e357. https://doi.org/10.1016/S1470-2045(13)70135-9
Yi M, Meric-Bernstam F, Kuerer HM, Mittendorf EA, Bedrosian I, Lucci A, Hwang RF, Crow JR, Luo S, Hunt KK (2012) Evaluation of a breast cancer nomogram for predicting risk of ipsilateral breast tumor recurrences in patients with ductal carcinoma in situ after local excision. J Clin Oncol 30:600–607. https://doi.org/10.1200/JCO.2011.36.4976
Alagoz O, Ergun MA, Cevik M, Sprague BL, Fryback DG, Gangnon RE, Hampton JM, Stout NK, Trentham-Dietz A (in press) The University of Wisconsin breast cancer epidemiology simulation model: an update. Medical decision making
Trentham-Dietz A, Kerlikowske K, Stout NK, Miglioretti DL, Schechter CB, Ergun MA, van den Broek JJ, Alagoz O, Sprague BL, van Ravesteyn NT, Near AM, Gangnon RE, Hampton JM, Chandler Y, de Koning HJ, Mandelblatt JS, Tosteson AN, Breast Cancer Surveillance Consortium, the Cancer Intervention Surveillance Modeling Network (2016) Tailoring breast cancer screening intervals by breast density and risk for women aged 50 years or older: collaborative modeling of screening outcomes. Ann Intern Med 165:700–712. https://doi.org/10.7326/M16-0476
Stout NK, Lee SJ, Schechter CB, Kerlikowske K, Alagoz O, Berry D, Buist DS, Cevik M, Chisholm G, de Koning HJ, Huang H, Hubbard RA, Miglioretti DL, Munsell MF, Trentham-Dietz A, van Ravesteyn NT, Tosteson AN, Mandelblatt JS (2014) Benefits, harms, and costs for breast cancer screening after U.S. implementation of digital mammography. J Natl Cancer Inst. https://doi.org/10.1093/jnci/dju092
Lee CI, Cevik M, Alagoz O, Sprague BL, Tosteson AN, Miglioretti DL, Kerlikowske K, Stout NK, Jarvik JG, Ramsey SD, Lehman CD (2015) Comparative effectiveness of combined digital mammography and tomosynthesis screening for women with dense breasts. Radiology 274:772–780. https://doi.org/10.1148/radiol.14141237
Sprague BL, Stout NK, Schechter C, van Ravesteyn NT, Cevik M, Alagoz O, Lee CI, van den Broek JJ, Miglioretti DL, Mandelblatt JS, de Koning HJ, Kerlikowske K, Lehman CD, Tosteson AN (2015) Benefits, harms, and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med 162:157–166. https://doi.org/10.7326/M14-0692
Mandelblatt JS, Stout NK, Schechter CB, van den Broek JJ, Miglioretti DL, Krapcho M, Trentham-Dietz A, Munoz D, Lee SJ, Berry DA, van Ravesteyn NT, Alagoz O, Kerlikowske K, Tosteson AN, Near AM, Hoeffken A, Chang Y, Heijnsdijk EA, Chisholm G, Huang X, Huang H, Ergun MA, Gangnon R, Sprague BL, Plevritis S, Feuer E, de Koning HJ, Cronin KA (2016) collaborative modeling of the benefits and harms associated with different U.S. breast cancer screening strategies. Ann Intern Med 164:215–225. https://doi.org/10.7326/M15-1536
Munoz D, Near AM, van Ravesteyn NT, Lee SJ, Schechter CB, Alagoz O, Berry DA, Burnside ES, Chang Y, Chisholm G, de Koning HJ, Ali Ergun M, Heijnsdijk EA, Huang H, Stout NK, Sprague BL, Trentham-Dietz A, Mandelblatt JS, Plevritis SK (2014) Effects of screening and systemic adjuvant therapy on ER-specific U.S. breast cancer mortality. J Natl Cancer Inst. https://doi.org/10.1093/jnci/dju289
Batina NG, Trentham-Dietz A, Gangnon RE, Sprague BL, Rosenberg MA, Stout NK, Fryback DG, Alagoz O (2013) Variation in tumor natural history contributes to racial disparities in breast cancer stage at diagnosis. Breast Cancer Res Treat 138:519–528. https://doi.org/10.1007/s10549-013-2435-z
Mandelblatt J, Cronin KA, De Koning H, Miglioretti DL, Schechter C, Stout N, Breast Working Group of the Cancer Intevention and Surveillance Modeling Network (CISNET) and the Breast Cancer Surveillance Consortium (BCSC) (AHRQ Publication No. 14-05201-EF-4, December 2015. Collaborative Modeling of U.S. Breast Cancer Screening Strategies. In: Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services, Rockville, MD. http://www.uspreventiveservicestaskforce.org/Home/GetFile/1/16255/collabmodelingbc/pdf. Accessed May 2016
Gangnon RE, Sprague BL, Stout NK, Alagoz O, Weedon-Fekjaer H, Holford TR, Trentham-Dietz A (2015) The contribution of mammography screening to breast cancer incidence trends in the United States: an updated age-period-cohort model. Cancer Epidemiol Biomark Prev 24:905–912. https://doi.org/10.1158/1055-9965.EPI-14-1286
Fryback DG, Stout NK, Rosenberg MA, Trentham-Dietz A, Kuruchittham V, Remington PL (2006) The Wisconsin breast cancer epidemiology simulation model. J Natl Cancer Inst Monogr 36:37–47
Shwartz M (1978) A mathematical model used to analyze breast cancer screening strategies. Oper Res 26:937–955
Shwartz M (1981) Validation and use of a mathematical model to estimate the benefits of screening younger women for breast cancer. Cancer Detect Prev 4:595–601
Cronin KA, Mariotto AB, Clarke LD, Feuer EJ (2006) Additional common inputs for analyzing impact of adjuvant therapy and mammography on U.S. mortality. J Natl Cancer Inst Monogr 36:26–29
Cronin KA, Yu B, Krapcho M, Miglioretti DL, Fay MP, Izmirlian G, Ballard-Barbash R, Geller BM, Feuer EJ (2005) Modeling the dissemination of mammography in the United States. Cancer Causes Control 16:701–712
Early Breast Cancer Trialists’ Collaborative Group, Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P, Taylor C, Wang YC, Bergh J, Di Leo A, Albain K, Swain S, Piccart M, Pritchard K (2012) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379:432–444. https://doi.org/10.1016/S0140-6736(11)61625-5
Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG (2006) Report of nationally representative values for the noninstitutionalized U.S. adult population for 7 health-related quality-of-life scores. Med Decis Making 26:391–400. https://doi.org/10.1177/0272989X06290497
Mariotto AB, Feuer EJ, Harlan LC, Abrams J (2006) Dissemination of adjuvant multiagent chemotherapy and tamoxifen for breast cancer in the United States using estrogen receptor information: 1975-1999. J Natl Cancer Inst Monogr 36:7–15
Stout NK, Rosenberg MA, Trentham-Dietz A, Smith MA, Robinson SM, Fryback DG (2006) Retrospective cost-effectiveness analysis of screening mammography. J Natl Cancer Inst 98:774–782
Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, Brown ML (2008) Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst 100:630–641. https://doi.org/10.1093/jnci/djn103
Mandelblatt J, Near A, Miglioretti DL, Munoz D, Sprague B, Trentham Dietz A, Gangnon R, Kurian A, Weedon-Fekjaer H, Cronin K, Plevritis SK (in press) Common model inputs in collaborative breast cancer modeling. Med Decis Making
Gangnon RE, Stout NK, Alagoz O, Hampton JM, Sprague BL, Trentham Dietz A (in press) Contribution of breast cancer to overall mortality for U. S. women. Med Decis Making
Munoz D, Plevritis S (in press) Estimating breast cancer progression features and survival by molecular subtype in the absence of screening and treatment. Med Decis Making
Cronin KA, Feuer EJ, Clarke LD, Plevritis SK (2006) Impact of adjuvant therapy and mammography on U.S. mortality from 1975 to 2000: comparison of mortality results from the CISNET breast cancer base case analysis. J Natl Cancer Inst Monogr 36:112–121
National Comprehensive Cancer Network (2015) NCCN clinical practice guidelines in oncology: breast cancer
Rosenberg MA (2006) The impact of mammography and adjuvant therapy on U.S. breast cancer mortality (1975–2000): collective results from the cancer intervention and surveillance modeling network. Competing risks to breast cancer mortality. J Natl Cancer Inst Monogr 36:15–19
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30. https://doi.org/10.3322/caac.21387
de Koning HJ, van Ineveld BM, van Oortmarssen GJ, de Haes JC, Collette HJ, Hendriks JH, van der Maas PJ (1991) Breast cancer screening and cost-effectiveness; policy alternatives, quality of life considerations and the possible impact of uncertain factors. Int J Cancer 49:531–537
Grann VR, Patel PR, Jacobson JS, Warner E, Heitjan DF, Ashby-Thompson M, Hershman DL, Neugut AI (2011) Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat 125:837–847. https://doi.org/10.1007/s10549-010-1043-4
Roberts A, Habibi M, Frick KD (2014) Cost-effectiveness of contralateral prophylactic mastectomy for prevention of contralateral breast cancer. Ann Surg Oncol 21:2209–2217. https://doi.org/10.1245/s10434-014-3588-7
Trentham-Dietz A, Newcomb PA, Nichols HB, Hampton JM (2007) Breast cancer risk factors and second primary malignancies among women with breast cancer. Breast Cancer Res Treat 105:195–207
Bruening W, Fontanarosa J, Tipton K, Treadwell JR, Launders J, Schoelles K (2010) Systematic review: comparative effectiveness of core-needle and open surgical biopsy to diagnose breast lesions. Ann Intern Med 152:238–246. https://doi.org/10.7326/0003-4819-152-1-201001050-00190
Francis A, Thomas J, Fallowfield L, Wallis M, Bartlett JM, Brookes C, Roberts T, Pirrie S, Gaunt C, Young J, Billingham L, Dodwell D, Hanby A, Pinder SE, Evans A, Reed M, Jenkins V, Matthews L, Wilcox M, Fairbrother P, Bowden S, Rea D (2015) Addressing overtreatment of screen detected DCIS; the LORIS trial. Eur J Cancer 51:2296–2303. https://doi.org/10.1016/j.ejca.2015.07.017
Acknowledgements
We thank Berta Geller, Julie McGregor, Kathy Peck, Oyewale Shiyanbola, Dawn Pelkey, and Kathleen Howe for their advice, project management, and assistance with data for this project.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health for the Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) Program (Grant No. U54 CA163303) and other projects (Grant Nos. U01 CA199218, P30 CA014520, and U54 CA163307). Data collection for model inputs from the Breast Cancer Surveillance Consortium (BCSC) was supported by the National Cancer Institute Grant P01 CA154292, contract HSN261201100031C, and Grant U54 CA163303. The collection of BCSC cancer and vital status data used in this study was supported in part by several state public health departments and cancer registries throughout the US. For a full description of these sources, please see: http://breastscreening.cancer.gov/work/acknowledgement.html.
Author information
Authors and Affiliations
Contributions
The authors are solely responsible for the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Corresponding authors
Ethics declarations
Conflicts of interest
Dr. Herschorn reports previously owning stock and currently serving as an unpaid advisor for Hologic. All other authors have no conflicts to disclose.
Ethical approval
The University of Wisconsin Health Sciences Human Subjects Committee determined that this study was exempt from review.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Trentham-Dietz, A., Ergun, M.A., Alagoz, O. et al. Comparative effectiveness of incorporating a hypothetical DCIS prognostic marker into breast cancer screening. Breast Cancer Res Treat 168, 229–239 (2018). https://doi.org/10.1007/s10549-017-4582-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4582-0