Abstract
The significant increase in the detection and treatment of ductal carcinoma in situ (DCIS) since the introduction of screening mammography has not been accompanied by the anticipated reduction in invasive breast cancer (IBC) incidence. The prevalence of DCIS requires a reexamination of the population level effects of detecting and treating DCIS. To further our understanding of the possible impact of DCIS diagnosis and treatment on IBC incidence in the U.S., we simulated breast cancer incidence over 25 years under various assumptions regarding the baseline incidence of IBC and the progression of DCIS to IBC. The simulations demonstrate a tradeoff between the expected increased incidence of IBC absent any DCIS detection and treatment and the rate of progression of DCIS to IBC. Our analyses indicate that a high progression of DCIS to IBC implies a significant increase in incidence of IBC over what is observed had we not detected and treated DCIS. Conversely, if we assume that there would not have been a significant increase over and above the observed incidence evident in SEER, then our model indicates that the rate of DCIS progression to clinically significant IBC is low. Given the tradeoff illustrated by our model, we must reevaluate the assumption that DCIS is a short-term obligate precursor of invasive cancer and instead focus on further exploration of the true natural history of DCIS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer screening programs aim to reduce population disease-specific morbidity and mortality through either early detection of disease or the identification and removal of pre-cancerous lesions. To date, several national screening initiatives have successfully achieved one or both of these objectives. Notably, the Pap smear detects high-grade cervical intraepithelial neoplasia (CIN), which may progress to cervical cancer if untreated [1, 2]. Similarly, colonoscopy detects and removes potentially precancerous polyps [3]. Figure 1 illustrates the U.S. trends for cervical and colorectal cancer incidence based on the Surveillance, Epidemiology and End Results Registries (SEER) data, showing a clear decrease over time in the overall incidence of cervical and colorectal cancers in the years after the introduction of these screening programs [4]. These screening programs are widely thought to be effective in significantly reducing cancer incidence [5, 6].
U.S. trends in incidence in cervical (a) and colon cancer rates (b) based on the National Cancer Institute’s Surveillance, Epidemiology and, End Results Registries (SEER) data (1975–2005). Screening for cervical cancer using the Pap smear was introduced in the early 1950s while colorectal screening accompanied by polypectomy began in the early 1960s
Screening mammography was introduced in the early 1980s with similar goals. Since the adoption of screening mammography, the diagnosis and treatment rates of non-invasive breast cancer, or ductal carcinoma in situ (DCIS), have increased greatly. After more than 25 years of screening, the rate of invasive breast cancer (IBC) has continued to increase despite widespread treatment of DCIS, except for a recent modest drop in incidence rates. It is possible that the recent drop seen in breast cancer incidence rates is due to effective screening and treatment programs. However, numerous studies have shown that this drop directly corresponds to the decreased use of hormone replacement therapy (HRT) in postmenopausal women [7–9]. While a decrease in cancer incidence is evident for cervical and colon cancer after the adoption of widespread screening (Fig. 1a, b), a similar trend is not clearly seen for breast cancer (Fig. 2). This is troubling, as one would expect that improved detection and eradication of preinvasive disease would lead to a reduction in the incidence of IBC.
Additionally, there is increasing evidence suggesting nationwide screening programs may lead to over-diagnosis, or the detection of indolent cancers that would not cause death or symptoms within a patient’s lifetime. Recent studies estimate the risk of indolent disease being detected to be as high as one in three to four cancers detected [11, 12]. In fact, due to a better understanding of the limitations and benefits of screening mammography as implemented in this country, there have been recent calls to rethink current mammography screening polices and practices [13, 14].
While there is increasing uncertainty regarding the impact of mammography screening on breast cancer morbidity and mortality in relation to the diagnosis and treatment of IBC, there is even more uncertainty about the benefits of diagnosing and treating DCIS. Clearly women with DCIS face an increased risk for developing IBC [15–18]. However, it remains difficult to predict which individuals with DCIS will develop IBC, and when invasion is likely to happen. Therefore, widespread treatment of DCIS has become the default, with 97% of patients with DCIS undergoing surgical excision, one-third of whom will have mastectomy [19, 20].
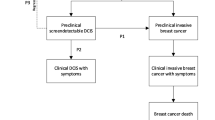
Considering that both the risks associated with DCIS are not well understood and that there is evidence suggesting over-diagnosis in IBC, it is reasonable to consider the possibility of over-diagnosis of DCIS [12, 21]. Over-diagnosis of DCIS could happen in two ways: DCIS lesions could be precursors of IBC that turn out to be indolent disease, or some cases of DCIS are indolent themselves and will not progress to IBC even in the absence of treatment. Inadvertent overestimation of the risk of progression of DCIS to IBC is illustrated by Fig. 3, where two screening scenarios are represented. Figure 3a represents a best-case scenario for a population screening program. Here, the introduction of screening results in cancer detection at an earlier stage. An increase in both DCIS and IBC incidence rates upon the introduction of screening stabilize after some time, followed by a decline in IBC after some time lag that depends on both the size of the reservoir of undetected disease, and the lead time between detection with screening versus clinical presentation. This represents the successful treatment and removal of DCIS with resultant reduction in IBC incidence. In this scenario, the overall incidence returns to the pre-screening rate. This stage-shift is what we would expect if DCIS is a true precursor and screening for it effectively reduces the burden of IBC. In contrast, the second scenario (Fig. 3b) represents a screening program that contributes to the overall incidence of cancer. Here, screening identifies DCIS lesions that ultimately do not progress to IBC and would not come to clinical attention in the absence of screening. In essence, this scenario increases the detection rate of DCIS, but has no effect on IBC incidence. In Fig. 3b, we see a long-term rise in DCIS and overall incidence, but no affect on IBC incidence. While these scenarios are quite different (one effectively reduces invasive cancer incidence, the other does not), as shown by the total incidence in DCIS and IBC (Fig. 3c), the relative percentage of DCIS as a fraction of IBC cases look very similar (Fig. 3d).
Two hypothetical screening scenarios are presented, a represents the scenario which assumes greatest potential impact from DCIS treatment (best-case scenario) and b represents the scenario which assumes no impact from DCIS treatment (worst case scenario). Each graph shows the impact a screening program might have on DCIS, invasive and the overall (invasive and DCIS) annual incidence rates. a Shows that the overall incidence returns to baseline after the introduction of widespread screening, with an accompanying stage shift (more DCIS, but fewer invasive cancers). Hypothetical best-case scenario: 100% of DCIS progresses to invasive cancer without treatment, 0% of DCIS progresses to invasive cancer with treatment. b Shows the worst case where the overall incidence is increased through additional detection of DCIS with no accompanying change in invasive cancer incidence. Hypothetical worst-case scenario: 0% of DCIS progresses to invasive cancer without treatment. c Compares the total annual distribution of DCIS and invasive cancer incidence for each scenario compared to pre-screening rates. (Total incidence). d Shows the relative percentage of DCIS versus invasive cancer incidence for the prescreening period and for each scenario. (Relative incidence). Note that the two scenarios appear very similar, and it is not possible to distinguish between Scenarios a and b
Observed SEER data shows a dramatic rise in the incidence of DCIS. However, there does not appear to be a corresponding drop in IBC rates that would account for successful detection and removal of DCIS. Instead, there is an overall increase in incidence across the 25-year period with only a small drop in recent years suggesting that the observed incidence trends (Fig. 2) do not match the ideal scenario in Fig. 3a and may be closer to the scenario in Fig. 3b. To better understand the observed trends, we explored the tradeoffs in assumptions related to the incidence rates of DCIS and IBC. The prevalence of DCIS in the U.S. and the potential morbidity and resource allocations resulting from its widespread treatment require that we critically examine the assumptions about DCIS and current treatment practices.
Methods
To better understand the possible impact that DCIS detection and treatment have had in the U.S., we simulated breast cancer incidence over 25 years (1978–2003) under multiple assumptions. In this study, we sought to gain insight into the assumed tradeoff between the background incidence rate of IBC as well as the potential for DCIS to progress to IBC, when replicating historical breast cancer incidence. Our simulation expands on a method previously presented by Welch [22], and models the baseline IBC incidence as the average IBC incidence observed in the mid 1970s, prior to the introduction of screening mammography [23]. Breast cancer incidence is then simulated using this underlying baseline incidence rate which is influenced by other factors related to the development of invasive cancer and the impact of diagnosing and treating DCIS as described below.
Variables
The simulated incidence rate of IBC was assumed to be impacted by the following variables: (1) the average annual baseline IBC incidence rate; (2) the percentage of DCIS that has the potential to progress into invasive cancer; and (3) the rate at which the progression from DCIS to IBC occurs. The baseline rate of IBC and DCIS are based on SEER data, beginning with the average, age-adjusted invasive cancer incidence observed prior to the introduction of screening mammography (100 per 100,000) [23]. The baseline rate of IBC incidence was assumed to be stable or increasing at a specified rate per year and is then influenced by the other variables to develop a simulated overall rate of IBC that is compared to the observed rate of IBC.
Although there are likely additional factors that contribute to baseline incidence of IBC, the intent of the model exercise was to determine the relationship between DCIS progression on IBC incidence; thus the baseline rate is assumed to be the cumulative result of all risk factors for IBC. For example, convincing evidence points to hormone replacement therapy (HRT) use as a factor influencing breast cancer rates [7–10]. Because the time interval examined in the simulation (1978–2003) largely preceded the observed decline in IBC incidence that has been attributed to reduction in HRT use, this factor is assumed to be captured in the underlying baseline rate. Similarly, the effects of specific treatments for DCIS were not modeled. Instead, we took a conservative approach and assumed that treatment of DCIS is 100% effective in preventing progression of DCIS to invasive cancer and model the percentage of DCIS that would have progressed to IBC in the absence of treatment. The resulting bias somewhat overestimates the benefit of treatment for DCIS.
Simulated scenarios
We simulate two scenarios (Scenario A and B) to illustrate the possible impact of diagnosing and treating DCIS. Scenario A determines the set of parameters that might explain the observed SEER invasive breast cancer data. This scenario assumes that the underlying baseline rate of invasive cancer, 100 per 100,000 women, starts at the rate observed prior to the introduction of mammography. This rate was simulated as either constant or increasing at a specified rate per year, ranging from 0% to 2% annually. From this baseline incidence rate, a percentage of the observed DCIS, as reported by SEER data, was removed in years prior. These lesions represent the DCIS that is detected and uniformly treated, and that are assumed to progress to IBC in absence of treatment for some women in later years. Through treatment, it is assumed that these DCIS lesions are removed from the pool of invasive cancers that would have otherwise developed and been diagnosed subsequently. The percentage of the DCIS lesions that are assumed to progress to IBC was allowed to vary between 0% and 100%. The simulation assumes that the time of DCIS progression to invasive cancer occurs between 1 and 15 years, and an average of 5 years was used for the baseline analyses. We simulated invasive cancer incidence over time and then compared the results to the observed incidence rates for IBC, as reported by SEER between 1978 and 2003. Using least square methodology, we solved for the parameter values that best fit the SEER data. These analyses illustrate the tradeoff between the proportion of detected DCIS that progress to invasive cancer and the increase in baseline incidence of IBC that would have to occur to explain the observed incidence rates.
In Scenario B, we project what the observed rates of IBC might have been in the hypothetical scenario where we had not detected and treated DCIS over the past 25 years. Currently, we treat DCIS under the assumption that we are preventing the development of invasive cancer. If this were true, then we would expect a higher rate of IBC in the absence of detection and treatment of DCIS. In Scenario B, we begin with observed SEER rates of IBC. We then assume that a percentage of detected DCIS would have progressed to invasive cancer within an average of 5 years. This percentage of DCIS, ranging from 0% to 100%, is added to the observed SEER rate of invasive cancer, representing the progression of DCIS to invasive cancer we might have seen in the absence of detection and treatment. In this scenario, we project the expected rates of invasive cancer for various DCIS progression rates had we not detected and treated DCIS for the past 25 years.
Results
Our results show that both the underlying baseline rate of IBC and the percentage of DCIS that progress to invasive cancer have a significant impact on the expected incidence. One parameter set for Scenario A is illustrated by Fig. 4a. Here it is assumed that 50% of DCIS would progress in the absence of detection or treatment, but are successfully treated and prevented from developing into invasive cancer. The simulation shows three assumptions regarding the baseline rate of IBC, assuming a 5-year rate of progression from DCIS to IBC. In Fig. 4a, the thick line represents observed SEER data for IBC rates between 1978 and 2003. The first simulation assumes the rate of IBC observed from 1978 to 2003 does not change (bottom line). The second assumes a 2% increase in the underlying IBC rate (top line), and the third is the best fit to the observed SEER data (thin solid line). An underlying baseline rate of increase of 1.39% is required to fit SEER data when 50% of the detected DCIS would have progressed to IBC in absence of treatment but are successfully treated and prevented from developing into invasive cancer. This translates into an underlying IBC incidence rate of 142 cases per 100,000 by 2003, increased from 100 per 100,000 in 1978. However, if we assume 50% of DCIS is successfully treated and prevented from becoming IBC and we maintain a constant underlying incidence rate of 100 per 100,000 as seen prior to mammography (no increase), the overall rate of IBC would decrease, as seen by the bottom line in Fig. 4a.
Model-simulated trends are compared to SEER, assuming a 5-year, 50% rate of progression from DCIS to invasive cancer (a). The thick solid line represents SEER data. The solid thin line and the dotted thick lines represent possible scenarios with differing assumptions for the baseline increase of invasive cancer incidence. The thin solid line represents the best fit to SEER data, assuming the 50% rate of progression. b Presents pairs of parameters that best fit the SEER trends for each assumed percentage of DCIS progression and increase in underlying baseline rate of IBC. Assumption of a longer time course of progression results in a lower estimation of baseline incidence rates for all scenarios (see text)
Figure 4b shows multiple parameter sets for scenarios like those shown in Fig. 4a for different DCIS progression rates. Here we see pairs of values for the assumed DCIS progression rate and rates of increase in IBC incidence over the baseline that are the best fit to observed SEER data in the presence of uniform successful treatment. For example, if we assume that 20% of DCIS would progress but are successfully removed with treatment, our model implies an annual increase of 1.22% in the baseline rate of breast cancer. Alternatively, if 90% of DCIS is detected and successfully removed from the population of IBC, the model implies a 1.61% annual increase. These results changed somewhat when the average time lag between DCIS detection and invasive cancer development was extended from 5 to 15 years; the required increase in the baseline incidence was lower when the time lag to progression was 15 years across all assumed scenarios for the percent of DCIS cases that progress.
The results of Scenario B are illustrated in Fig. 5a, where the expected IBC rates in the absence of DCIS detection and treatment are simulated. Here we see the impact of varying only the assumed proportion of DCIS that progresses to IBC, given the SEER DCIS incidence rates, and assuming that these lesions are not prevented from progressing. Figure 5a shows how the SEER incidence of IBC has continued to increase since the introduction of screening (bottom line). The thin lines represent hypothetical scenarios where the rate of IBC would have increased over time if a percentage of DCIS had not been detected and treated but instead had progressed to IBC. The top solid line represents what we would have expected for IBC incidence if we assume 100% detected DCIS would have progressed to IBC without treatment, and similarly if 60% of DCIS progressed (top dotted line), and 20% DCIS progressed (bottom dotted line). Figure 5b shows the corresponding rates of IBC that we would have expected and the equivalent increase in the observed rate of IBC over 25 years that we would have seen in each case.
SEER incidence rate is shown in a (bottom line) as are hypothetical invasive cancer incidence trends if we were to assume that 100% of DCIS had progressed to invasive cancer in absence of treatment (top solid line), 60% progressed (top dotted line), and 20% progressed (bottom dotted line). b Shows the increase in IBC incidence per 100,000 over 25 years and the annual increase in IBC incidence rate
The current SEER rates of IBC are in the range of 130 per 100,000 annually, which translates to an approximate 1% annual increase in IBC rate when assuming a DCIS progression rate of 20% (Fig. 5b). However, DCIS subtypes may have differing progression rates, and we can develop a plausible set of assumptions about these rates to invasive cancer based on DCIS size, grade, and age. Table 1 illustrates how the rates of progression might vary from as high as 60% for larger high grade DCIS in young women to as low as 5% for low grade DCIS in older women while still observing an overall progression rate of approximately 20% for all DCIS. Table 1 illustrates one possible scenario that could result in a 20% overall progression rate while capturing the known heterogeneity DCIS progression.
Discussion
For decades, DCIS detection, incidence, and treatment have been steadily rising. However, it is not evident that a significant reduction in invasive cancer incidence accompanies this increase. This observation raises questions about whether DCIS is an obligate precursor and would thus progress to invasive cancer if left untreated [22]. Our results show that different sets of assumptions can produce similar projected trends, illustrating the plausible tradeoff between the presumed progression of DCIS and the increase in the background rate of IBC. Based on our results, if the majority of DCIS were destined to progress to IBC, then it is reasonable to assume that there is a significant increase in the baseline rate of IBC over what we have seen. In contrast, if there is only a modest increase in the baseline rate of IBC after the introduction of screening, more in keeping with trends observed prior to the introduction of screening, it then follows that the majority of DCIS would not have progressed to IBC, even in the absence of treatment.
These results suggest that the observable impact of screening mammography on breast cancer incidence trends is likely to be closely connected to the assumptions regarding the underlying baseline rate of incidence. Data suggest it is possible that there has been an increase in the overall baseline rate of IBC. For example, data from the Connecticut Tumor Registry suggest that the rate of IBC incidence has been steadily rising since the 1940s at 1.0% annually [24]. However, other data suggest little change in incidence over this time period [25]. In addition, SEER data indicate no significant change in incidence rate during the 10 years prior to the introduction of mammography screening. Many argue that diet, lifestyle changes, and other environmental factors have caused an increase in baseline rates [26–29]. While this is likely, these are no data suggesting that the increase would be as high as it would need to be if we were to assume that the majority of DCIS has the potential to progress to IBC as shown by our analyses in Scenario B (Fig. 5).
These models compel us to ask if we have over-estimated the potential DCIS has to progress to IBC, and if so, are we over-diagnosing and therefore over-treating DCIS? Evidence in support of spontaneous regression for IBC has recently emerged, which may have even greater relevance for DCIS than for invasive cancer [21, 30, 31]. In other modeling efforts examining U.S. breast cancer trends, similar conclusions have been reached. For example, the model by Mandelblatt et al. [32] consistently underestimated rates of DCIS as compared to observed SEER rates, suggesting not all DCIS progresses to invasive cancer. In a similar model of breast cancer development, Fryback et al. [33] modeled a substantial proportion of tumors having limited malignant potential in order to account for the high observed DCIS rates.
While it is difficult to reach definitive conclusions from any single model, such analyses can shed light on potential inconsistencies in assumptions that inform treatment practices and can help clinicians examine current practices and consider alternative approaches. Current locoregional treatment for DCIS is often indistinguishable from that for early stage IBC. Is the treatment-related morbidity justified in light of the benefits gained? Our analyses suggest that it is possible that a modest increase in the baseline incidence can be paired with a high progression rate if we assume a long lag time between DCIS detection and progression to invasive cancer. This scenario would suggest that widespread treatment for DCIS is warranted, yet the long time interval for invasive suggests that many women may not be affected depending on her comorbidities at diagnosis and overall life expectancy. Although published data are limited, there are data that indicate that not all comedo DCIS treated by biopsy alone progress to IBC even with long-term follow up [15, 34]. Furthermore, autopsy data suggest that many patients with untreated DCIS may not develop IBC in a timeframe that will impact their lives [35]. Based on these studies, it is feasible that there is a potential reservoir of DCIS in the population that is never diagnosed, and never attains clinical relevance. It is likely that more DCIS in this reservoir will come to clinical attention, with increased use of digital mammography and screening MRI.
The possibility of low rates of DCIS progression are also supported by the ECOG trial data [36]. In this study, the rates of progression to IBC at 5-years for low and intermediate grade DCIS, after excision alone, were in the range of 3–5% for lesions less than 2.5 cm. For small high-grade lesions, in women over age 45, the rate of progression was 5%. While the rates of early progression were not affected by age for low and intermediate grade DCIS, young age makes an enormous difference on the rates of progression of high grade DCIS. Although the natural history of DCIS is unknown, there certainly appears to be a differential rate of disease progression of DCIS to IBC based on a number of studies looking at both biology and age and excision of DCIS [37]. It must also be considered that these subtypes are likely to have different impacts on survival. Given what little we know about DCIS, it is possible that treating DCIS has successfully eliminated more aggressive IBC, and is in fact partly responsible for the drop in breast cancer mortality. Alternately, it is possible that treating DCIS has had little effect on mortality rates.
It is likely that the degree of risk conferred by DCIS is less than that in BRCA1 mutation carriers for whom the lifetime risk of developing IBC may be up to 85%, yet the proportion of DCIS patients who receive a mastectomy are roughly equivalent to BRCA1 carriers [38]. In contrast, watchful waiting is the most commonly chosen management approach for women diagnosed with ADH, which confers a 4–7 fold risk of developing IBC over 30 years, or an approximate 35% lifetime risk [39, 40]. Although it is possible that DCIS confers an analogous risk to ADH, watchful waiting is almost never chosen as an option [41]. When comparing each of these diagnoses in context of their associated risks and clinical practices, it appears that our guidelines for treatment interventions are considerably inconsistent.
Elucidation of molecular subtypes has demonstrated the heterogeneity of IBC that probably also extend to DCIS [16]. Additionally, prognostic signatures such as NKI70 suggest that screening has increased the proportion of good prognosis cancers. It is possible that many of the in situ lesions, even if they do progress, are precursors of these more indolent cancers [42]. Going forward, we need to better understand the malignant potential of DCIS. Improved understanding of: (1) which prognostic biomarkers are associated with greatest risk of invasive progression, and (2) which predictive biomarkers are associated with best biologic response to nonsurgical treatment, will allow us to limit invasive interventions to women who are most likely to derive benefit.
Addressing the DCIS conundrum will require a cultural shift in the management of DCIS. Recognizing that DCIS does not require urgent treatment is the first step. This would allow patients and their clinicians to comfortably pursue strategies such as window trials (e.g. herceptin for HER2-positive DCIS), neoadjuvant trials (e.g. endocrine therapy for 3–6 months before surgery), or active surveillance with close clinical monitoring. Part of this cultural shift includes rethinking the terminology used to describe these in situ lesions. Many have suggested that “ductal intraepithelial neoplasia (DIN)” or the more general term “increased risk lesions” be used, and that the term “carcinoma” be removed from the lexicon that describes in situ lesions [43].
There remains much to understand about the forces influencing breast cancer incidence, as well as the role that DCIS treatment plays. However, there are clearly interdependencies among the variables involved. Our model highlights one set of tradeoffs and assumptions that is consistent with the observed trends in breast cancer incidence. In the presence of detection and successful treatment of DCIS, a high DCIS progression rate to IBC, implies that the observed rates of IBC are the result of a significant increase in the baseline rates of breast cancer. Conversely, only a modest increase in the baseline rate of breast cancer implies that the DCIS progression rates are low and that in some women, the morbidity of treatment is greater than the benefit. This possibility mandates a greater understanding of those biologic factors that impact DCIS progression which can in turn inform future clinical trial designs for less invasive treatment of DCIS.
References
Peto J et al (2004) The cervical cancer epidemic that screening has prevented in the UK. Lancet 364(9430):249–256
McCredie MR et al (2008) Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol 9(5):425–434
Winawer SJ et al (1993) Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 329(27):1977–1981
Ries L et al (2008) SEER cancer statistics review. National Cancer Institute, Bethesda
Pignone M et al (2002) Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 137(2):132–141
Janicek MF, Averette HE (2001) Cervical cancer: prevention diagnosis, and therapeutics. CA Cancer J Clin 51(2):92–114 (quiz 115–118)
Ravdin PM et al (2007) The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med 356(16):1670–1674
Kerlikowske K et al (2007) Declines in invasive breast cancer and use of postmenopausal hormone therapy in a screening mammography population. JNCI 99(17):1335–1339
Glass AG et al (2007) Breast cancer incidence, 1980–2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst 99(15):1152–1161
Simon JA et al (2010) The breast cancer “plunge” after initial publication of the WHI results: an alternative explanation. Maturitas 66(3):277–284
Welch HG, Black WC (2010) Overdiagnosis in cancer. J Natl Cancer Inst 102(9):605–613
Jorgensen KJ, Gotzsche PC (2009) Overdiagnosis in publicly organised mammography screening programmes: systematic review of incidence trends. BMJ 339:b2587
Esserman L, Shieh Y, Thompson I (2009) Rethinking screening for breast cancer and prostate cancer. JAMA 302(15):1685–1692
U.S. Preventive services task force (2009) Screening for breast cancer: U.S. Preventive services task force recommendation statement. Ann Intern Med 151(10): 716–26, W-236
Sanders ME et al (2005) The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer 103(12):2481–2484
Collins LC et al (2005) Outcome of patients with ductal carcinoma in situ untreated after diagnostic biopsy: results from the Nurses’ Health Study. Cancer 103(9):1778–1784
Warnberg F, Yuen J, Holmberg L (2000) Risk of subsequent invasive breast cancer after breast carcinoma in situ. Lancet 355(9205):724–725
Fisher ER et al (1999) Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: intraductal carcinoma. Cancer 86(3):429–438
Baxter NN et al (2004) Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst 96(6):443–448
Claus EB et al (2003) The risk of a contralateral breast cancer among women diagnosed with ductal and lobular breast carcinoma in situ: data from the Connecticut Tumor Registry. Breast 12(6):451–456
Morrell S et al (2010) Estimates of overdiagnosis of invasive breast cancer associated with screening mammography. Cancer Causes Control 21(2):275–282
Welch HG (2004) Should I be tested for cancer?: maybe not and here’s why. University of California Press, Berkeley, p 224
SEER, SEER*Stat Database (2009) Incidence - SEER 9 Regs Limited-Use, Nov 2008 Sub (1973–2006) <Katrina/Rita Population Adjustment> -Linked To County Attributes - Total U.S., 1969–2006 Counties, National Cancer Institute, DCCPS. Surveillance Research Program, Cancer Statistics
Miller BA, Feuer EJ, Hankey BF (1994) The significance of the rising incidence of breast cancer in the United States. Important Adv Oncol 193–207
Devesa SS, Silverman DT (1978) Cancer incidence and mortality trends in the United States: 1935–74. J Natl Cancer Inst 60(3):545–571
Zhang SM et al (2007) Alcohol consumption and breast cancer risk in the Women’s Health Study. Am J Epidemiol 165(6):667–676
Silvera SA et al (2006) Energy balance and breast cancer risk: a prospective cohort study. Breast Cancer Res Treat 97(1):97–106
Eliassen AH et al (2010) Physical activity and risk of breast cancer among postmenopausal women. Arch Intern Med 170(19):1758–1764
Peplonska B et al (2008) Adulthood lifetime physical activity and breast cancer. Epidemiology 19(2):226–236
Zahl PH, Maehlen J, Welch HG (2008) The natural history of invasive breast cancers detected by screening mammography. Arch Intern Med 168(21):2311–2316
Welch HG, Black WC (2010) Overdiagnosis in cancer. J Natl Cancer Inst 102(9):605–613
Mandelblatt J et al (2006) The SPECTRUM population model of the impact of screening and treatment on U.S. breast cancer trends from 1975 to 2000: principles and practice of the model methods. J Natl Cancer Inst Monogr 36:47–55
Fryback DG et al (2006) The Wisconsin breast cancer epidemiology simulation model. J Natl Cancer Inst Monogr 36:37–47
Lagios MD et al (1989) Mammographically detected duct carcinoma in situ: frequency of local recurrence following tylectomy and prognostic effect of nuclear grade on local recurrence. Cancer 63:616–624
Welch HG, Black WC (1997) Using autopsy series to estimate the disease “reservoir” for ductal carcinoma in situ of the breast: how much more breast cancer can we find? Ann Intern Med 127(11):1023–1028
Hughes LL et al (2009) Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 27(32):5319–5324
Solin LJ et al (2005) Long-term outcome after breast-conservation treatment with radiation for mammographically detected ductal carcinoma in situ of the breast. Cancer 103(6):1137–1146
Friebel TM et al (2007) Bilateral prophylactic oophorectomy and bilateral prophylactic mastectomy in a prospective cohort of unaffected BRCA1 and BRCA2 mutation carriers. Clin Breast Cancer 7(11):875–882
Hartmann LC et al (2005) Benign breast disease and the risk of breast cancer. N Engl J Med 353(3):229–237
Degnim AC et al (2007) Stratification of breast cancer risk in women with atypia: a Mayo cohort study. J Clin Oncol 25(19):2671–2677
Esserman L et al (2004) Applying the neoadjuvant paradigm to ductal carcinoma in situ. Ann Surg Oncol 11(1 Suppl):28S–36S
van’t Veer LJ et al (2009) Evaluation of the effect of screening on the detection of good and poor prognosis breast cancers. Am Soc Clin Oncol 27 (abstr 1525)
Jensen RA, P DL (2003) Ductal carcinoma in situ of the breast: impact of pathology on therapeutic decisions. Am J Surg Pathol 27(6):828–831
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozanne, E.M., Shieh, Y., Barnes, J. et al. Characterizing the impact of 25 years of DCIS treatment. Breast Cancer Res Treat 129, 165–173 (2011). https://doi.org/10.1007/s10549-011-1430-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1430-5