Abstract
Purpose
We investigated the usefulness of abbreviated breast MRI (AB-MRI), including fat-suppressed T2-weighted imaging, pre- and postcontrast image acquisition, and subtracted maximum-intensity projection imaging, for the screening of women with a history of breast cancer surgery.
Methods
Between October 2014 and March 2016, a total of 799 AB-MRI examinations were performed for 725 women with a history of breast cancer surgery. The image acquisition time was 8.5 min. Screening mammography, ultrasound, and AB-MRI were generally performed around the same time. The cancer detection rate, positive predictive values for recall and biopsy, sensitivity and specificity of screening MRI, and rate of malignancy belonging to each breast imaging reporting and data system (BI-RADS) category were assessed.
Results
AB-MRI detected 12 malignancies in 12 women (15.0 cancers per 1000 cases). Seven of these 12 malignancies were initially invisible on ultrasound and mammography, although subsequent targeted ultrasound revealed lesions corresponding to the MRI-detected lesions. The positive predictive values for recall and biopsy and sensitivity and specificity values for screening MRI were 12.4, 61.5, 100, and 89.2%, respectively. The rates of malignancies belonging to categories 1, 2, 3, and 4 of the BI-RADS were 0, 0, 4.8, and 57.1%, respectively.
Conclusions
The diagnostic performance of screening AB-MRI for women with a history of breast cancer surgery is acceptable, with the advantages of short examination and interpretation times and low costs. Thus, it could be used as a main screening modality that may replace conventional imaging in breast cancer survivors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Women with a personal history of breast cancer exhibit an increased risk of ipsilateral breast relapse (IBR) and contralateral breast cancer (CBC) [1,2,3,4]. The detection of secondary breast cancer in the asymptomatic phase can improve survival by 27–47% compared with their detection in the symptomatic phase [5]. Currently, mammography (MG) is the standard screening modality for this subset of patients. However, the detection of IBR may be compromised by post-treatment changes; furthermore, the low sensitivity of this modality for women with dense breasts is a major drawback [6]. In previous studies, MG detected 50–72% IBR lesions and 37–80% CBC lesions [7,8,9,10,11,12,13,14,15]. The higher-than-average risk in breast cancer survivors, improvement in survival with detection in the asymptomatic phase, and previously reported detection rates highlight the need for a supplemental modality for the detection of mammographically occult cancers.
Several studies have evaluated the use of screening MRI for women with a personal history of breast cancer and reported that this modality could detect mammographically or ultrasonographically occult breast cancers. Their findings suggested that breast MRI is a useful supplemental screening tool for women with a personal history of breast cancer. Brennan et al. reported that screening breast MRI alone accurately detected 10 malignancies in 144 women with a personal history of breast cancer, while Gweon et al. reported that 18.1 cancers per 1000 patients were accurately detected by screening breast MRI after exhibiting negative findings on ultrasound (US) and MG [16, 17]. However, the use of MRI in the screening setting is restricted because of high costs, limited availability of scanners, a longer scan time, and a relatively long interpretation time.
In a recent study, Kuhl et al. introduced abbreviated breast MRI (AB-MRI) with short acquisition and interpretation times and demonstrated a diagnostic accuracy comparable with that of the full diagnostic protocol [18]. From the findings of their study, it appeared that AB-MRI can detect all occult cancers. Furthermore, a great potential for cost savings, which was associated with the shorter scan time, was observed. However, the feasibility of AB-MRI as a screening tool is still under investigation and has not been established. Therefore, we conducted the present study to assess the usefulness of AB-MRI for the screening of women with a personal history of breast cancer surgery using our own imaging protocol.
Methods
Study population
The protocol for this research project, which involved a retrospective analysis of prospectively acquired data, was approved by the institutional review board and conforms to the provisions of the Declaration of Helsinki. The review board waived the requirement for informed consent. Between October 2014 and March 2016, 760 women underwent screening AB-MRI at our institution. Twenty of these 760 women were excluded because of unclear data regarding their cancer history (n = 5), performance of AB-MRI for diagnostic purposes (n = 8), or the absence of a history of breast cancer (n = 7). The remaining 740 women with a personal history of breast cancer and detailed information regarding their cancer history were initially enrolled in our study. Fifteen of these were lost to follow-up and excluded. Eventually, 725 women (median age 51 years; age range 26–84 years) with a personal history of breast cancer who underwent screening AB-MRI comprised our final study population.
At our institution, after breast cancer surgery, women routinely undergo follow-up with MG and US every 6 months for the first 2 years and annually thereafter. Screening AB-MRI is performed at the request of patients or clinicians. For our study population, mammography, US, and AB-MRI were performed on the same day or around the same time. The median interval between the initial surgery for breast cancer and the first AB-MRI examination was 26 months (range 3–295 months). At the time of AB-MRI screening, there was no radiological evidence of malignancy on previously performed MG and US.
AB-MRI technique
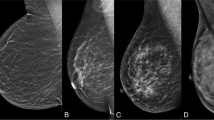
AB-MRI was performed using a 1.5T or 3.0T MRI system (Signa HDxt or Discovery 750; General Electric Medical Systems, Milwaukee, WI, USA). All patients were imaged in the prone position using a dedicated eight-channel bilateral breast coil (General Electric Medical Systems). Following the acquisition of transverse localizer images, sagittal fat-suppressed T2-weighted fast spin-echo images were acquired with a minimum repetition time and echo time (1.5T, 4000–4200/100 ms; 3.0T, 3400–3600/100 ms), a 20-cm field of view, 4-mm sections with no gap, and a 256 × 224 matrix. Before and immediately after contrast injection, three-dimensional sagittal T1-weighted fat-suppressed fast spoiled gradient-echo sequences with parallel imaging were acquired with a minimum repetition time and echo time (1.5T, 6.9/3.4 ms; 3.0T, 6.3/2.4 ms), a 10° flip angle, an 18-cm field of view, 1-mm sections with no gap, and a 288 × 150 matrix at 1.5T or a 288 × 192 matrix at 3.0T MRI system (Fig. 1). The total acquisition time was only 8.5 min. Gadoterate meglumine (Dotarem; Guerbet, Aulnay-Sous-Bois, France) was injected into the antecubital vein at a dose of 0.1 mmol/kg body weight and a rate of 3 ml/s using an automated injector (Spectris Solaris, Medrad Europe, Maastricht, The Netherlands), followed by a 20-ml saline flush. The precontrast T1 images were subtracted from the corresponding postcontrast images to achieve subtraction images. Subtraction was automatically achieved on a pixel-by-pixel basis using the software subtraction function available on the console. Reformatted images with a maximum-intensity projection (MIP) in the craniocaudal and mediolateral directions were created from the subtraction images.
Image interpretation
AB-MRI was prospectively interpreted by one of two radiologists with 7 and 12 years of experience in breast MRI interpretation. High-resolution picture archiving and communication system monitors were used for image interpretation in conjunction with the patient’s clinical history, preoperative MRI findings, and other breast imaging examinations, including MG or US when available.
The reader initially reviewed the subtracted images to determine the presence or absence of significant enhancement. If there was significant enhancement, its enhancement and morphology were assessed on the subtraction images and fat-suppressed T2-weighted images (Fig. 2). MIP images were used to estimate the lesion size and distance from the nipple and assess the distribution of nonmass enhancements.
Order of interpretation of abbreviated breast MRI (AB-MRI) sequences acquired for the screening of women with a history of breast cancer surgery. Subtracted T1-weighted images are viewed first (a), followed by fat-suppressed T2-weighted images (b) and subtracted maximum-intensity projection (MIP) images (c)
Of the 725 women, 92.8% (n = 673) had undergone preoperative MRI examinations at a median of 26 months (range 4–195 months) before the first AB-MRI examination, and all previous images were available for comparison. The breast lesions were prospectively described and assessed on the basis of their morphology and kinetic features in the initial phase using the breast imaging reporting and data system (BI-RADS) [19, 20]. Oval or round masses with smooth margins, focal or regional homogeneous nonmass enhancements, and foci without T2 hyperintensity exhibiting a fatty hilum were assigned to BI-RADS category 3. Bilateral multiple foci that were widely separated by normal fibroglandular tissue or fat, with a pattern of background parenchymal enhancement, were assigned to BI-RADS category 2 [21, 22]. If a category 3 lesion on AB-MRI appeared stable for more than 6 months when compared with the preoperative MRI findings, it was downgraded to BI-RADS category 2. Moreover, oval or round masses with circumscribed margins and dark internal septations or T2 hyperintensity or foci with T2 hyperintensity or a fatty hilum were assigned to BI-RADS category 2.
Validation of diagnoses
Women with BI-RADS category 1 or 2 lesions on AB-MRI were monitored with clinical breast examinations or imaging modalities such as MG and US or AB-MRI for at least 12 months (median 13 months; range 12–30 months). Women with BI-RADS category 3 lesions on AB-MRI underwent follow-up AB-MRI after 6 months. If the lesion was stable, it was downgraded to BI-RADS category 2, and the routine screening cycle with or without AB-MRI was resumed. Women with suspicious findings (BI-RADS category 4 or 5) on AB-MRI and lesions on US or MG that corresponded with those on MRI underwent US-guided core needle biopsy or surgical biopsy after US or MG-guided needle localization. MRI-guided biopsy was recommended for mammographically and ultrasonographically occult lesions, although no case in the present study was diagnosed by this modality.
Statistical analysis
Lesions with a final BI-RADS category of 1 or 2 were considered negative AB-MRI findings, while those with a final BI-RADS category of 3, 4, or 5 were considered positive AB-MRI findings. For patients with positive AB-MRI findings, pathology results after core needle biopsies and surgical excisions or follow-up imaging findings for at least 6 months were recorded. For AB-MRI screening, the cancer detection rate, positive predictive value (PPV) for recall (PPV1), PPV for biopsy (PPV3), sensitivity, specificity, and the rates of malignancies belonging to each BI-RADS category were calculated. This analysis was performed using SPSS, version 24.0 (SPSS Inc., Chicago, IL, USA).
Results
A total of 799 AB-MRI examinations were performed for the 725 women; 651 (89.8%) underwent one screening round and 74 (10.2%) underwent two screening rounds. Table 1 lists the clinicopathological findings for the 725 women. The distribution of the final BI-RADS categories was as follows: category 1 in 548 examinations (68.6%), category 2 in 154 examinations (19.3%), category 3 in 83 examinations (10.4%), and category 4 in 14 examinations (1.7%). For the women with negative AB-MRI findings, no malignancy was diagnosed for at least 1 year after screening.
Of the 83 category 3 lesions, 76 (91.6%) were followed up with AB-MRI, of which 71 were downgraded to category 1 or 2 and five were upgraded to category 4. Four of the five upgraded lesions were confirmed to be malignancies. These four malignancies (ductal carcinoma in situ (DCIS), n = 2; invasive ductal carcinoma, n = 1; mucinous carcinoma, n = 1) initially appeared as enhancing foci or nonmass enhancements on AB-MRI, with negative findings on MG or US, and exhibited interval growth during the follow-up period. The remaining one upgraded lesion was not biopsied, but it remained stable for 18 months on US and was finally categorized as a benign lesion. The seven lesions that were not followed up with AB-MRI were monitored using US or MG and exhibited no suspicious malignant findings for at least 1 year after the first AB-MRI screen.
Of the 14 category 4 lesions, 13 underwent MG-guided (n = 3) or US-guided (n = 10) biopsy, which revealed eight malignancies (invasive ductal carcinoma, n = 5; DCIS, n = 3) and five benign lesions (stromal fibrosis, n = 2; fibrocystic change, n = 1; ductal ectasia, n = 1; fibroadipose tissue, n = 1). One AB-MRI examination revealed a 2.9-cm linear nonmass enhancement that showed no corresponding abnormality on targeted US and MG. This lesion was not biopsied, and follow-up US and MG for 25 months showed negative findings.
The overall cancer detection rate with AB-MRI was 15.0 per 1000 cases [12 of 799 examinations (95% confidence interval (CI) 7.8, 26.1)], with the prevalence of invasive ductal carcinoma (median tumor size, 1.5 cm; range 0.6–2.0 cm), DCIS, and mucinous carcinoma being 50.0% (6/12), 41.7% (5/12), and 8.3% (1/12), respectively (Table 2). Seven of the 12 AB-MRI-detected lesions were mammographically and ultrasonographically occult lesions. All AB-MRI-detected cancers except one were node-negative T1 invasive cancers or DCIS. PPV1 was 12.4% (12/97) and PPV3 was 61.5% (8/13). The imaging and clinical features of the 12 malignancies detected by AB-MRI are listed in Table 2.
There were no false-negative AB-MRI findings; therefore, the sensitivity was 100% [12 of 12 (95% CI 73.5, 100%)] and specificity was 89.2% [702 of 787 (95% CI 86.8, 91.3%)]. The rates of malignancies according to the final BI-RADS category were as follows: category 1, 0% (0 of 548); category 2, 0% (0 of 154); category 3, 4.8% (4 of 83); and category 4, 57.1% (8 of 14).
Conclusions
In the present study, 799 screening AB-MRI examinations detected 12 malignancies (15.0 cancers per 1000 women), including seven mammographically and ultrasonographically occult lesions, with 100% sensitivity and 89.2% specificity in women with a personal history of breast cancer surgery. All AB-MRI-detected cancers except one were node-negative T1 invasive cancers or DCIS; this finding is comparable with those of several previous studies on MRI screening for women with a personal history of breast cancer [16, 17, 23,24,25,26,27,28]. In addition, no interval cancer was found in our study. These findings suggest that screening AB-MRI for women with a personal history of breast cancer is a useful imaging modality for the detection of early in-breast recurrence and/or contralateral cancer that may otherwise go undetected at such an early stage.
Currently, the National Comprehensive Cancer Network and the American Society for Clinical Oncology recommend MG and clinical breast examinations for the screening of women with a history of breast cancer surgery [29, 30]. However, in this patient population, the contribution of MG to the overall cancer yield has been limited [6, 12]. In clinical practice, supplemental US is occasionally used for screening. The cancer detection rate and sensitivity of combined MG and US are reportedly higher than those for MG only, although US has several limitations such as high operator dependency, a small field of view, and the inability to detect microcalcifications [23, 24]. Screening MRI can overcome these drawbacks by offering a large field of view, high sensitivity for the detection of DCIS, which presents as a microcalcified lesion, and low operator dependency with regard to image acquisition [31]. A previous study reported that the overall cancer detection rate (8.2 vs. 6.8 per 1000) and sensitivity (100% vs. 82.4%) were higher when MG was combined with MRI than when it was combined with US [24]. In our study, AB-MRI detected all 12 malignancies, five of which were also visible on US (n = 2) or MG (n = 3) and seven of which were not (Table 2). These findings demonstrate the superiority of AB-MRI as a screening tool for women with a history of breast cancer.
Several researchers have investigated the use of screening breast MRI for women with a personal history of breast cancer [16, 17, 24,25,26,27,28]. Table 3 lists the diagnostic performance of this tool in all these studies, which demonstrated a high sensitivity of 80.0–100% and a high specificity of 82.2–95.3% in the screening setting. The cancer detection rate fell into a relatively wide range (7.3–118.1 per 1000 women). The cancer detection rates reported by Brennan et al., Schacht et al., and Weinstock et al. were relatively high, although this can be explained by selection bias due to the small sample sizes [17, 25, 27]. Most previous reports about screening breast MRI for women with a personal history of breast cancer applied the full diagnostic protocol. The use of standard breast MRI in the screening setting has been restricted by high costs, limited availability of MRI scanners, a longer scan time, and a relatively long interpretation time. However, as mentioned above, the recent study by Kuhl et al. broke these barriers by introducing AB-MRI and demonstrating a diagnostic accuracy similar to that of the full diagnostic protocol [18]. To our knowledge, the present study is the first to evaluate the usefulness of AB-MRI screening for women with a history of breast cancer surgery. The cancer detection rate (15.0 per 1000 cases) and sensitivity (100%) observed in our study are comparable with those for MRI performed with the full diagnostic protocol (Table 3).
In the present study, the total image acquisition time during AB-MRI was 8.5 min, which is greater than the time reported by Kuhl et al. because we also acquired fat-suppressed T2-weighted sequences [18]. Several previous studies on breast MRI have shown that T2-weighted sequences are useful for the evaluation of breast lesions [32, 33]. While Mann et al. found these sequences to be helpful in lesion characterization, Heacock et al. showed that they increased lesion visibility despite failing to improve the detection of cancer by the observer [34, 35]. The lack of T2-weighted sequences may be problematic because, in some cases, T2 hyperintensity may result in a benign diagnosis, limit the identification of features characteristic of intramammary nodes, and result in unnecessary recalls. We did not evaluate the effects of T2-weighted images on specificity. In future, it would be worthwhile to evaluate an AB-MRI protocol with T2-weighted images in a screening setting where researchers would want to exclude false-positive findings, particularly for incidental lesions.
We retrospectively reviewed four malignancies detected on follow-up MRI after the initial screening AB-MRI, which categorized them as BI-RADS category 3. Explanations for the delayed diagnosis are as follows. The first lesion initially presented as a 5-mm enhancing mass that was too small for the accurate analysis of morphology, such as margin, shape, and the internal enhancement pattern in the early phase. The second lesion presented as a 4-mm enhancing focus with a subtle increase in the maximal dimension compared with that on preoperative MRI performed 15 months ago. After 10 months, follow-up MRI revealed a 6-mm growing, enhancing mass that was pathologically confirmed to be a low-grade DCIS, which may have been indolent and also stable because of adjuvant chemotherapy for 6 months after surgery [36, 37]. The other two lesions presented as nonmass enhancements that exhibited a slightly higher degree of contrast enhancement compared with normal breast tissue. These lesions were misinterpreted as heterogenous benign background enhancements. Finally, our institution does not have the equipment for MRI-guided biopsy. Therefore, we may have tended to diagnose small enhancing lesions with no correlates on MG, US, and even second-look US as category 3 lesions.
It should be noted that our AB-MRI protocol only included the early postcontrast phase, so we could not assess the kinetic curve that shows signal enhancement in relation to time after contrast injection. Recently, accelerated scan techniques were introduced, which provide a very high temporal resolution with a very early postcontrast phase and kinetic assessment [38, 39]. Abe et al. reported that analysis of the early enhancement rate and kinetic area under the curve using ultrafast dynamic contrast-enhanced MRI is useful for discriminating benign lesions from malignant ones [38]. Furthermore, a method known as differential subsampling with Cartesian ordering using pseudorandom k-space sampling provides a 6 times faster and effective temporal resolution without sacrificing the spatial resolution [39]. The combination of AB-MRI with these new scan techniques may be an important topic for future studies on breast MRI screening.
Our study has several limitations. First, it was limited by its retrospective, single-center design and the relatively small number of patients with malignancies. We did not include all women with a history of breast cancer surgery encountered during the study period, and this may have led to selection bias. Second, the number and timing of previous screening MG and US examinations varied and could have affected the outcomes of AB-MRI. Third, because the median follow-up duration (13 months; range 12–30 months) for our study was relatively short, there may have been a verification bias. Fourth, AB-MRI was not evaluated with regard to the survival outcomes, cost effectiveness, and patient tolerability. Despite these limitations, this study provides preliminary information on the usefulness of screening AB-MRI for women with a history of breast cancer surgery. Prospective, randomized multicenter studies with a large patient population are required for further validation of the applicability of our findings to a broader clinical setting.
In conclusion, the findings of our study suggest that the diagnostic performance of screening AB-MRI for women with a history of breast cancer surgery is comparable to that of the full diagnostic MRI protocol, with the advantages of short examination and interpretation times and low costs. Using our abbreviated protocol, we were able to detect all cases of secondary or recurrent breast cancer in the included patients, some of which were mammographically and ultrasonographically occult breast cancers. Thus, AB-MRI after breast cancer surgery may be used as a standard modality, which can improve the sensitivity and specificity of breast cancer screening. Furthermore, AB-MRI could be used not only as a supplemental modality but also as a main screening modality that may replace conventional imaging procedures such as MG or US in breast cancer survivors.
Change history
14 November 2017
In the original publication of the article, the acknowledgment section was missed out inadvertently. The acknowledgement section is below
References
Fisher B, Anderson S, Bryant J et al (2002) Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347:1233–1241
Veronesi U, Cascinelli N, Mariani L et al (2002) Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 347:1227–1232
Anderson SJ, Wapnir I, Dignam JJ et al (2009) Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five national surgical adjuvant breast and bowel project protocols of node-negative breast cancer. J Clin Oncol 27:2466–2473
Gao X, Fisher SG, Emami B (2003) Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: a population-based study. Int J Radiat Oncol Biol Phys 56:1038–1045
Houssami N, Ciatto S, Martinelli F et al (2009) Early detection of second breast cancers improves prognosis in breast cancer survivors. Ann Oncol 20:1505–1510
Dershaw DD, Shank B, Reisinger S (1987) Mammographic findings after breast cancer treatment with local excision and definitive irradiation. Radiology 164:455–461
De la Rochefordière A, Mouret-Fourme E, Asselain B et al (1996) Metachronous contralateral breast cancer as first event of relapse. Int J Radiat Oncol Biol Phys 36:615–621
Kollias J, Evans AJ, Wilson ARM et al (2000) Value of contralateral surveillance mammography for primary breast cancer follow-up. World J Surg 24:983–989
Hill-Kayser CE, Harris EE, Hwang W et al (2006) Twenty-year incidence and patterns of contralateral breast cancer after breast conservation treatment with radiation. Int J Radiat Oncol Biol Phys 66:1313–1319
Robinson A, Speers C, Olivotto I et al (2007) Method of detection of new contralateral primary breast cancer in younger versus older women. Clin Breast Cancer 7:705–709
Weinstock C, Bigenwald R, Hochman T et al (2012) Outcomes of surveillance for contralateral breast cancer in patients less than age 60 at the time of initial diagnosis. Curr Oncol 19:e160–e164
Orel SG, Troupin RH, Patterson EA et al (1992) Breast cancer recurrence after lumpectomy and irradiation: role of mammography in detection. Radiology 183:201–206
Grosse A, Schreer I, Frischbier H et al (1997) Results of breast conserving therapy for early breast cancer and the role of mammographic follow-up. Int J Radiat Oncol Biol Phys 38:761–767
Ashkanani F, Sarkar T, Needham G et al (2001) What is achieved by mammographic surveillance after breast conservation treatment for breast cancer? Am J Surg 182:207–210
Joseph E, Hyacinthe M, Lyman GH et al (1998) Evaluation of an intensive strategy for follow-up and surveillance of primary breast cancer. Ann Surg Oncol 5:522–528
Gweon HM, Cho N, Han W et al (2014) Breast MR imaging screening in women with a history of breast conservation therapy. Radiology 272:366–373
Brennan S, Liberman L, Dershaw DD et al (2010) Breast MRI screening of women with a personal history of breast cancer. Am J Roentgenol 195:510–516
Kuhl CK, Schrading S, Strobel K et al (2014) Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol 32:2304–2310
D’Orsi CJ (2013) ACR BI-RADS atlas: breast imaging reporting and data system
American College of Radiology (2003) Breast imaging reporting and data system atlas (BI-RADS atlas). American College of Radiology 98, Reston
Eby PR, DeMartini WB, Gutierrez RL et al (2009) Characteristics of probably benign breast MRI lesions. Am J Roentgenol 193:861–867
Eby PR, DeMartini WB, Gutierrez RL et al (2010) Probably benign lesions detected on breast MR imaging. Magn Reson Imaging Clin N Am 18:309–321
Berg WA, Zhang Z, Lehrer D et al (2012) Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 307:1394–1404
Cho N, Han W, Han BK et al (2017) Breast cancer screening with mammography plus ultrasonography or magnetic resonance imaging in women 50 years or younger at diagnosis and treated with breast conservation therapy. JAMA Oncol. doi:10.1001/jamaoncol.2017.1256
Schacht DV, Yamaguchi K, Lai J et al (2014) Importance of a personal history of breast cancer as a risk factor for the development of subsequent breast cancer: results from screening breast MRI. Am J Roentgenol 202:289–292
Giess CS, Poole PS, Chikarmane SA et al (2015) Screening breast MRI in patients previously treated for breast cancer: diagnostic yield for cancer and abnormal interpretation rate. Acad Radiol 22:1331–1337
Weinstock C, Campassi C, Goloubeva O et al (2015) Breast magnetic resonance imaging (MRI) surveillance in breast cancer survivors. SpringerPlus 4:459
Lehman CD, Lee JM, DeMartini WB et al (2016) Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst 108:djv349
Bevers TB, Anderson BO, Bonaccio E et al (2009) NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw 7:1060–1096
Khatcheressian JL, Wolff AC, Smith TJ et al (2006) American society of clinical oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol 24:5091–5097
Bennani-Baiti B, Baltzer PA (2017) MR imaging for diagnosis of malignancy in mammographic microcalcifications: a systematic review and meta-analysis. Radiology 283:692–701
Ballesio L, Savelli S, Angeletti M et al (2010) Breast MRI: are T2 IR sequences useful in the evaluation of breast lesions? Clin Imaging 34:77
Kuhl CK, Klaschik S, Mielcarek P et al (1999) Do T2-weighted pulse sequences help with the differential diagnosis of enhancing lesions in dynamic breast MRI? J Magn Reson Imaging 9:187–196
Mann RM, Kuhl CK, Kinkel K et al (2008) Breast MRI: guidelines from the European society of breast imaging. Eur Radiol 18:1307–1318
Heacock L, Melsaether AN, Heller SL et al (2016) Evaluation of a known breast cancer using an abbreviated breast MRI protocol: correlation of imaging characteristics and pathology with lesion detection and conspicuity. Eur J Radiol 85:815–823
Erbas B, Provenzano E, Armes J et al (2006) The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res Treat 97:135–144
Sanders ME, Schuyler PA, Dupont WD et al (2005) The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer 103:2481–2484
Abe H, Mori N, Tsuchiya K et al (2016) Kinetic analysis of benign and malignant breast lesions with ultrafast dynamic contrast-enhanced MRI: comparison with standard kinetic assessment. Am J Roentgenol 207:1159–1166
Morrison CK, Henze Bancroft LC, DeMartini WB et al (2017) Novel high spatiotemporal resolution versus standard-of-care dynamic contrast-enhanced breast MRI: comparison of image quality. Invest Radiol 52:198–205
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
A correction to this article is available online at https://doi.org/10.1007/s10549-017-4568-y.
Rights and permissions
About this article
Cite this article
Choi, B.H., Choi, N., Kim, M.Y. et al. Usefulness of abbreviated breast MRI screening for women with a history of breast cancer surgery. Breast Cancer Res Treat 167, 495–502 (2018). https://doi.org/10.1007/s10549-017-4530-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4530-z