Abstract
Purpose
Numerous studies have demonstrated that breast cancer in young women (BCY) has unfavorable prognostic features and more unfavorable subtypes. However, few studies have evaluated the effect of subtype disparities on breast cancer prognosis by age, especially for BCY. We analyzed breast cancer mortality stratified by tumor subtype according to age among patients younger than 50 years.
Methods
Data from the Korean Breast Cancer Society Registry for patients diagnosed with invasive breast cancer when aged less than 50 years between 2003 and 2010 were reviewed retrospectively.
Results
We identified 30,793 patients with breast cancer who were eligible for analysis. Of these, 793 (2.6%) were aged 20–29 and 8926 (28.8%) were aged 30–39. Median follow-up duration was 84 months. Mean age was 42.4 years. Patients in their 20s were more likely to have cancer of advanced stage and higher nuclear grade, present with lymphovascular invasion, and have unfavorable subtypes. Patients in the 20s group showed worse prognosis. In multivariate analysis for overall survival (OS), the hazard ratio (HR) for patients in the 20s group was higher than that for the 30s and 40s groups, and patients with triple-negative breast cancer (TNBC) showed higher HR than patients with HER-2 or luminal subtype (all p < 0.0001). When stratified by subtype, luminal subtype showed significantly worse prognosis in the 20s group than the 30s and 40s groups, whereas HER-2 and TNBC subtypes showed no significant difference.
Conclusion
Patients in their 20s with breast cancer had unfavorable characteristics and worse prognosis than patients in their 30s and 40s. When stratified by tumor subtype, patients in their 20s with luminal subtype of breast cancer showed worse prognosis than older patients, whereas HER-2 and TNBC subtypes showed no significant differences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is one of the most common cancers and a leading cause of death among women worldwide. Numerous studies have demonstrated that breast cancer in young women (BCY) has unfavorable prognostic features. These cancers are more likely to be high nuclear grade (NG), estrogen receptor (ER) negative, progesterone receptor (PR) negative, and human epidermal growth factor-2 (HER-2) positive, have a high proliferation fraction, present with lymphovascular invasion (LVI), and be diagnosed at more advanced stage [1,2,3,4]. Furthermore, some studies demonstrate that young age is an independent unfavorable prognostic factor [5, 6].
The molecular subtype of breast cancer is associated with prognosis and response to treatment such as chemotherapy and endocrine therapy [7]. BCY tends to have a higher proportion of intrinsic breast cancer subtypes associated with a poorer prognosis such as triple-negative, HER-2, and luminal B subtypes [3, 8, 9]. Few studies have evaluated the effect of subtype disparities on breast cancer prognosis by age, especially BCY [10,11,12].
In the United States, approximately 230,000 women are diagnosed with breast cancer annually; among them, 4.7–4.9%, or approximately 11,000 patients, are diagnosed when they are younger than 40 years [13, 14]. According to the 2014 annual report of the Korean Breast Cancer Society Registry (KBCSR), of 21,484 patients diagnosed with new breast cancer, more than 10.5% were younger than 40 years [15]. We analyzed breast cancer mortality stratified by tumor subtype according to age among patients younger than 50 years using the KBCSR database, which has a higher proportion of BCY than Western populations.
Materials and methods
We identified 37,865 patients who were diagnosed at 20–49 years of age. We excluded male patients and patients who underwent neoadjuvant chemotherapy, had distant metastasis or inflammatory breast cancer at presentation, or had histopathology other than invasive ductal or invasive lobular carcinoma. We also excluded patients who lacked immunohistochemistry data (ER, PR, HER-2) or had short follow-up duration (<12 months).
Data collection
Data from an online breast cancer registration program collected by the KBCSR on patients diagnosed with invasive breast cancer between January 2003 and December 2010 were retrospectively reviewed. The database collected information on more than 50 demographic and clinicopathological characteristics including sex, age at diagnosis, method of surgical treatment, stage according to American Joint Committee on Cancer (AJCC) classification [16], histopathological characteristics, adjuvant therapy (chemotherapy, radiotherapy, and endocrine therapy), and date of death from the Ministry of Health and Welfare, Republic of Korea. The KBCSR has been described in detail previously [17]. We collected data on age at diagnosis, family history of breast cancer, type of operation, pathologic stage, NG, LVI, ER/PR/HER-2 status, and type of adjuvant treatment. Patient tumors were classified into four subtypes: luminal A (positive for ER and/or PR and negative for HER-2); luminal B (positive for ER and/or PR and HER-2); HER-2-enriched (negative for ER/PR and positive for HER-2); and triple-negative breast cancer (TNBC) (negative for ER/PR and HER-2). ER, PR, and HER-2 status in surgical specimens were assessed at each center using routine immunohistochemistry protocols.
Statistical analysis
Patient characteristics were compared using independent t tests for continuous variables and the Chi-square or Fisher’s exact test for categorical variables. Values are reported as mean ± standard deviation (SD) or median with ranges. Kaplan–Meier curves with the corresponding results of log-rank tests were constructed for overall survival (OS). Univariate and multivariate analyses for OS were conducted with a Cox proportional hazards model to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Patients with any missing or unknown data were excluded from analysis by Cox models. OS was defined as the time between date of surgery and date of death from any cause. All tests were two sided, and p < 0.05 was considered significant. All statistical analyses used SAS version 9.4 (SAS Institute, Cary, NC, USA) and R3.2.1 (Vienna, Austria; http://www.R-project.org). This study adhered to the ethical tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Samsung Medical Center in Seoul, Korea (IRB number: 2017-04-021). The need for informed consent was waived because of the low risk posed by this investigation.
Results
Patient selection
A schematic diagram of patient selection is shown in Fig. 1. We identified 37,865 patients between 20 and 49 years of age with invasive breast cancer registered in the KBCSR database. Of these patients, we included only those eligible based on the following inclusion criteria: stage I–III breast cancer, no neoadjuvant chemotherapy, invasive ductal or invasive lobular carcinoma, follow-up longer than 12 months, and existing ER/PR/HER-2 data.
Baseline characteristics by age group
Among 30,793 patients eligible for analysis, 793 (2.6%) were aged 20–29 years, 8133 (26.4%) were 30–39, and 21,867 (71.0%) were 40–49. Among all eligible patients, 2471 (8.0%) died after undergoing an operation for breast cancer. Clinicopathological characteristics and adjuvant treatments according to age group are summarized in Table 1. Median follow-up duration was 84.8 (12.0–132.1) months. Mean age was 42.1 (±5.4) years. Patients in the 20s group were more likely to have cancers with advanced stage, higher NG, presence of LVI, and TNBC subtype than those in the 30s and 40s age groups (p < 0.0001 for all). In addition, patients in the 20s group were more likely to undergo chemotherapy than those in the 30s and 40s age groups (p < 0.0001).
Association between age group/tumor subtype and overall survival
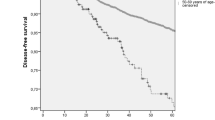
Patients in the 20s age group had worse OS than patients in the 30s and 40s age groups (p < 0.001; Fig. 2). Patients in the younger group had increased HR in univariate and multivariate analyses for OS (Table 2). In these analyses, patients with TNBC showed higher HR for OS than patients with HER-2, luminal B, or luminal A subtype: TNBC, 2.514 (2.075–3.045); HER-2 subtype, 2.262 (1.829–2.797), luminal B subtype, 1.437 (1.220, 1.692), and luminal A subtype (reference) (all p < 0.001) (Table 2).
Relationship of age group and overall survival stratified by tumor subtype
Patients with cancer of luminal A or B subtype showed significantly worse prognosis for the 20s age group than the 30s and 40s age groups (both p < 0.0001; Fig. 3). However, the prognosis of cancer of HER-2 subtype was not significantly different by age group (p = 0.440; Fig. 3), and the TNBC subtype also showed no significant difference between women in the age groups of 20s versus 30s, and 20s versus 40s (p = 0.445 and p = 0.592; Fig. 3). When stratified by tumor subtype, luminal A and B subtypes showed higher HRs in the 20s age group than in the 30s and 40s age groups, whereas HER-2 and TNBC subtypes were not significantly different among the age groups after additional adjustment for pathological stage, NG, LVI, tumor subtype, adjuvant chemotherapy, adjuvant radiotherapy, and adjuvant hormonal therapy (Table 3).
Discussion
We analyzed the relationship between mortality of breast cancer patients who were younger than 50 years and tumor subtype and age. We showed that patients diagnosed in their 20s tended to have unfavorable prognostic factors and worse prognosis than those in their 30s and 40s. However, when cancers were stratified by tumor subtype, no significant difference was observed for HER-2 and TNBC subtypes according to age group. Luminal subtypes showed worse prognosis in the 20s age group than in the 30s and 40s age groups.
Previous articles demonstrated that BCY is an independent risk factor for recurrence and mortality [5, 18, 19]. However, these studies did not consider the prognostic impact of age stratified by breast cancer subtype. A retrospective analysis of a randomized controlled trial of patients with early-stage HER-2-positive breast cancer who underwent chemotherapy followed by trastuzumab or no trastuzumab suggested that BCY is neither prognostic nor predictive of short-term oncological outcome [20]. For the TNBC subtype, Sheridan et al. [21] reported no differences according to age. Our study results were consistent with previous studies in which patients with HER-2 and TNBC subtype cancer showed no significant difference in mortality.
For luminal subtype, many studies report that BCY has a worse prognosis than breast cancer in older patients [21,22,23,24]. Partridge et al. [22] reported that age less than 40 years was associated with significant increases in risk of breast cancer-specific death from luminal A (HR 2.1, 95% CI 1.4–3.2) or luminal B (HR 1.4, 95% CI 1.1–1.9) subtype compared with older age groups. Of 17,575 patients with stage I–III breast cancer, 1916 were younger than 40 years and 1298 of them had luminal subtype cancer. BCY in western countries is relatively rare, and breast cancer in patients younger than 30 years is extremely rare, with an incidence lower than 1%. Furthermore, the proportion of luminal subtypes among BCY patients is lower than that of older patients with breast cancer. As precise analysis of this group is difficult, few studies show relationships between age and subtype for BCY. To the best of our knowledge, our study is the first to describe the characteristics of a large number of women in their 20s with breast cancer and the largest study on the relationships among breast cancer mortality, subtype, and age in BCY. More than 30,000 patients were included in our analysis, with approximately 9000 being younger than 40 years. We subgrouped the patients into 20s and 30s. Luminal A and B subtypes had higher risks of mortality in patients diagnosed at an earlier age (Fig. 3).
There are few potential hypotheses for why younger patients have worse prognosis than older patients with luminal breast cancer. In younger patients, the incidence of chemotherapy-induced amenorrhea is reduced, resulting in worse prognosis for hormone receptor-positive breast cancer [25,26,27]. Regan et al. [28] reported that weakly ER-positive and/or PR-positive tumors were less responsive to adjuvant endocrine therapy in the SOFT and TEXT randomized phase III trials. Viale et al. [29] also suggested that weakly ER-positive tumors are less responsive to adjuvant endocrine therapy in analyses using the Breast International Group 1-98 trial database. Sheffield et al. [30] reported that 90% of patients with weak ER positivity by immunohistochemistry (IHC) were classified as basal-like or HER-2-enriched subtypes by gene expression profiling using RT-qPCR. BCY was more likely to have weaker mRNA expression for ERα (p < 0.0001), ERβ (p = 0.02), and PR (p < 0.0001), but often had higher expression of HER-2 (p < 0.0001) and epidermal growth factor receptor (p < 0.0001), resulting in a lower response to adjuvant endocrine therapy [31]. Even though BRCA 1/2 mutations and other genetically related tumors are not clearly associated with breast cancer prognosis, BCY is more likely to have genetic mutations such as BRCA 1/2 than other genetic-related tumors [7, 32]. Finally, studies show that BCY is a risk factor for nonadherence and discontinuance of adjuvant endocrine therapy, resulting in worse oncological outcomes [33,34,35]. However, the issues of nonadherence and nonpersistence have many compound factors including age, increased out-of-pocket costs, social support, and treatment side effects [34].
A major strength of our study is the large number of BCY patients, at approximately 9000. This number allowed evaluation of the association of age and subtype for women in their 20s with breast cancer. We are about to use the eighth AJCC staging based on TNM anatomical factors and biological factors such as tumor grade, proliferation rate, and ER, PR, and HER-2 status in the staging system [36]. ER- and PR-positive breast cancer will be down-staged in the 8th AJCC staging. However, young patients with luminal subtype had worse prognosis so it should not be underestimated. Another change in the 8th AJCC staging system is that gene expression prognostic panels are incorporated into the staging system. Among the patients with luminal A subtype, node-negative cancers with tumor size less than or equal to 5 cm combined with low risk of multigene panels are expected to be categorized as stage I. However, gene expression prognostic panels are usually developed for postmenopausal patients and their applications in BCY patients are uncertain, especially for breast cancer patients in their 20s [37,38,39,40]. In the future, studies on gene expression prognostic panels validated for BCY are needed.
Our study had a few limitations. First, our study had a retrospective design based on analysis of the KBCSR database. We lacked information on detailed patient oncological outcome such as locoregional recurrence, distant metastasis, and contralateral recurrence. We also lacked information on proliferation markers such as Ki-67 and on administration of adjuvant treatment such as trastuzumab and goserelin. In addition, the database lacked information about adherence to adjuvant endocrine therapy, adjuvant chemotherapy, or goserelin. Adherence to adjuvant endocrine therapy is important for breast cancer of luminal subtype and could affect mortality from breast cancer. Second, the median follow-up of 84 months was relatively short, especially for luminal subtype cancer. Finally, we used IHC markers (ER, PR, and HER-2) as surrogates for gene expression. Although IHC profiles have been successfully used as surrogates, they can lead to misclassification. Despite these limitations, our study used a nationwide database linked to survival data officially confirmed by the KBCSR. All patients analyzed in the study were ethnically homogeneous (all patients were Korean), and the database had detailed clinicopathological characteristics including HER-2 status and treatment information. Our findings were consistent with results from the National Comprehensive Cancer Network Breast Cancer Outcomes Database Project [22].
In conclusion, breast cancer patients in their 20s had unfavorable characteristics and worse prognosis than patients in their 30s and 40s. When stratified by tumor subtype, women in their 20s with breast cancer of luminal subtype showed worse prognosis, while cancer of HER-2 and TNBC subtypes was not significantly different according to age.
References
Colleoni M, Rotmensz N, Peruzzotti G, Maisonneuve P, Orlando L, Ghisini R, Viale G, Pruneri G, Veronesi P, Luini A, Intra M, Cardillo A, Torrisi R, Rocca A, Goldhirsch A (2006) Role of endocrine responsiveness and adjuvant therapy in very young women (below 35 years) with operable breast cancer and node negative disease. Ann Oncol 17:1497–1503
Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H (2009) Breast cancer in young women: poor survival despite intensive treatment. PLoS ONE 4:e7695
Chollet-Hinton L, Anders CK, Tse CK, Bell MB, Yang YC, Carey LA, Olshan AF, Troester MA (2016) Breast cancer biologic and etiologic heterogeneity by young age and menopausal status in the Carolina Breast Cancer Study: a case-control study. Breast Cancer Res 18:79
Park YH, Lee SJ, Jung HA, Kim SM, Kim MJ, Kil WH, Lee JE, Nam SJ, Ahn JS, Im YH (2015) Prevalence and clinical outcomes of young breast cancer (YBC) patients according to intrinsic breast cancer subtypes: single institutional experience in Korea. Breast 24:213–217
Kataoka A, Iwamoto T, Tokunaga E, Tomotaki A, Kumamaru H, Miyata H, Niikura N, Kawai M, Anan K, Hayashi N, Masuda S, Tsugawa K, Aogi K, Ishida T, Masuoka H, Iijima K, Kinoshita T, Nakamura S, Tokuda Y (2016) Young adult breast cancer patients have a poor prognosis independent of prognostic clinicopathological factors: a study from the Japanese Breast Cancer Registry. Breast Cancer Res Treat 160:163–172
Fredholm H, Magnusson K, Lindstrom LS, Garmo H, Falt SE, Lindman H, Bergh J, Holmberg L, Ponten F, Frisell J, Fredriksson I (2016) Long-term outcome in young women with breast cancer: a population-based study. Breast Cancer Res Treat 160:131–143
Kao KJ, Chang KM, Hsu HC, Huang AT (2011) Correlation of microarray-based breast cancer molecular subtypes and clinical outcomes: implications for treatment optimization. BMC Cancer 11:143
Menen RS, Hunt KK (2016) Considerations for the treatment of young patients with breast cancer. Breast J 22:667–672
Sabiani L, Houvenaeghel G, Heinemann M, Reyal F, Classe JM, Cohen M, Garbay JR, Giard S, Charitansky H, Chopin N, Rouzier R, Darai E, Coutant C, Azuar P, Gimbergues P, Villet R, Tunon de Lara C, Lambaudie E (2016) Breast cancer in young women: pathologic features and molecular phenotype. Breast 29:109–116
De Camargo Cancela M, Comber H, Sharp L (2016) HR +/Her2- breast cancer in pre-menopausal women: the impact of younger age on clinical characteristics at diagnosis, disease management and survival. Cancer Epidemiol 45:162–168
Liedtke C, Rody A, Gluz O, Baumann K, Beyer D, Kohls EB, Lausen K, Hanker L, Holtrich U, Becker S, Karn T (2015) The prognostic impact of age in different molecular subtypes of breast cancer. Breast Cancer Res Treat 152:667–673
Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO, Blomqvist C, Heikkila P, Heikkinen T, Nevanlinna H, Akslen LA, Begin LR, Foulkes WD, Couch FJ, Wang X, Cafourek V, Olson JE, Baglietto L, Giles GG, Severi G, McLean CA, Southey MC, Rakha E, Green AR, Ellis IO, Sherman ME, Lissowska J, Anderson WF, Cox A, Cross SS, Reed MW, Provenzano E, Dawson SJ, Dunning AM, Humphreys M, Easton DF, Garcia-Closas M, Caldas C, Pharoah PD, Huntsman D (2010) Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med 7:e1000279
Samuel CA, Pinheiro LC, Reeder-Hayes KE, Walker JS, Corbie-Smith G, Fashaw SA, Woods-Giscombe C, Wheeler SB (2016) To be young, black, and living with breast cancer: a systematic review of health-related quality of life in young black breast cancer survivors. Breast Cancer Res Treat 160:1–15
Warner ET, Hu R, Collins LC, Beck AH, Schnitt S, Rosner B, Eliassen AH, Michels KB, Willett WC, Tamimi RM (2016) Height and body size in childhood, adolescence, and young adulthood and breast cancer risk according to molecular subtype in the nurses’ health studies. Cancer Prev Res (Phila) 9:732–738
Park EH, Min SY, Kim Z, Yoon CS, Jung KW, Nam SJ, Oh SJ, Lee S, Park BW, Lim W, Hur MH (2017) Basic facts of breast cancer in Korea in 2014: the 10-year overall survival progress. J Breast Cancer 20:1–11
Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A (2010) AJCC cancer staging manual, 7th edn. Springer, New York
Park B, Choi KS, Lee YY, Jun JK, Seo HG (2012) Trends in cancer screening rates among Korean men and women: results from the Korean national cancer screening survey (KNCSS), 2004–2011. Cancer Res Treat 44:113–120
Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS, Han W (2007) Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea–a report from the Korean Breast Cancer Society. J Clin Oncol 25:2360–2368
Adami HO, Malker B, Holmberg L, Persson I, Stone B (1986) The relation between survival and age at diagnosis in breast cancer. N Engl J Med 315:559–563
Partridge AH, Gelber S, Piccart-Gebhart MJ, Focant F, Scullion M, Holmes E, Winer EP, Gelber RD (2013) Effect of age on breast cancer outcomes in women with human epidermal growth factor receptor 2-positive breast cancer: results from a herceptin adjuvant trial. J Clin Oncol 31:2692–2698
Sheridan W, Scott T, Caroline S, Yvonne Z, Vanessa B, David V, Karen G, Stephen C (2014) Breast cancer in young women: have the prognostic implications of breast cancer subtypes changed over time? Breast Cancer Res Treat 147:617–629
Partridge AH, Hughes ME, Warner ET, Ottesen RA, Wong YN, Edge SB, Theriault RL, Blayney DW, Niland JC, Winer EP, Weeks JC, Tamimi RM (2016) Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol 34:3308–3314
Kim EK, Noh WC, Han W, Noh DY (2011) Prognostic significance of young age (<35 years) by subtype based on ER, PR, and HER2 status in breast cancer: a nationwide registry-based study. World J Surg 35:1244–1253
Han W, Kang SY (2010) Relationship between age at diagnosis and outcome of premenopausal breast cancer: age less than 35 years is a reasonable cut-off for defining young age-onset breast cancer. Breast Cancer Res Treat 119:193–200
Walshe JM, Denduluri N, Swain SM (2006) Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol 24:5769–5779
Howell A, Swindell R, Ribeire GC (1996) Ovarian ablation in early breast cancer: overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 348:1189–1196
Perez-Fidalgo JA, Rosello S, Garcia-Garre E, Jorda E, Martin-Martorell P, Bermejo B, Chirivella I, Guzman C, Lluch A (2010) Incidence of chemotherapy-induced amenorrhea in hormone-sensitive breast cancer patients: the impact of addition of taxanes to anthracycline-based regimens. Breast Cancer Res Treat 120:245–251
Regan MM, Pagani O, Francis PA, Fleming GF, Walley BA, Kammler R, Dell’Orto P, Russo L, Szoke J, Doimi F, Villani L, Pizzolitto S, Ohlschlegel C, Sessa F, Peg Camara V, Rodriguez Peralto JL, MacGrogan G, Colleoni M, Goldhirsch A, Price KN, Coates AS, Gelber RD, Viale G (2015) Predictive value and clinical utility of centrally assessed ER, PgR, and Ki-67 to select adjuvant endocrine therapy for premenopausal women with hormone receptor-positive, HER2-negative early breast cancer: TEXT and SOFT trials. Breast Cancer Res Treat 154:275–286
Viale G, Regan MM, Maiorano E, Mastropasqua MG, Dell’Orto P, Rasmussen BB, Raffoul J, Neven P, Orosz Z, Braye S, Ohlschlegel C, Thurlimann B, Gelber RD, Castiglione-Gertsch M, Price KN, Goldhirsch A, Gusterson BA, Coates AS (2007) Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1–98. J Clin Oncol 25:3846–3852
Sheffield BS, Kos Z, Asleh-Aburaya K, Wang XQ, Leung S, Gao D, Won J, Chow C, Rachamadugu R, Stijleman I, Wolber R, Gilks CB, Myles N, Thomson T, Hayes MM, Bernard PS, Nielsen TO, Chia SK (2016) Molecular subtype profiling of invasive breast cancers weakly positive for estrogen receptor. Breast Cancer Res Treat 155:483–490
Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG, Nevins JR, Potti A, Blackwell KL (2008) Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 26:3324–3330
Kang E, Park SK, Lee JW, Kim Z, Noh WC, Jung Y, Yang JH, Jung SH, Kim SW (2016) KOHBRA BRCA risk calculator (KOHCal): a model for predicting BRCA1 and BRCA2 mutations in Korean breast cancer patients. J Hum Genet 61:365–371
Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, Fehrenbacher L, Gomez SL, Miles S, Neugut AI (2010) Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8769 early-stage breast cancer patients. J Clin Oncol 28:4120–4128
Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW (2012) Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat 134:459–478
Rosenberg SM, Partridge AH (2015) New insights into nonadherence with adjuvant endocrine therapy among young women with breast cancer. J Natl Cancer Inst. doi:10.1093/jnci/djv245
Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, Weaver DL, Winchester DJ, Hortobagyi GN (2017) Breast cancer-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 67:290–303
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Perez EA, Olson JA Jr, Zujewski J, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin P, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Atkins JN, Berenberg JL, Sledge GW (2015) Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 373:2005–2014
Jasem J, Fisher CM, Amini A, Shagisultanova E, Rabinovitch R, Borges VF, Elias A, Kabos P (2017) The 21-gene recurrence score assay for node-positive, early-stage breast cancer and impact of RxPONDER trial on chemotherapy decision-making: have clinicians already decided? J Natl Compr Canc Netw 15:494–503
Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, Pierga JY, Brain E, Causeret S, DeLorenzi M, Glas AM, Golfinopoulos V, Goulioti T, Knox S, Matos E, Meulemans B, Neijenhuis PA, Nitz U, Passalacqua R, Ravdin P, Rubio IT, Saghatchian M, Smilde TJ, Sotiriou C, Stork L, Straehle C, Thomas G, Thompson AM, van der Hoeven JM, Vuylsteke P, Bernards R, Tryfonidis K, Rutgers E, Piccart M (2016) 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375:717–729
Wallden B, Storhoff J, Nielsen T, Dowidar N, Schaper C, Ferree S, Liu S, Leung S, Geiss G, Snider J, Vickery T, Davies SR, Mardis ER, Gnant M, Sestak I, Ellis MJ, Perou CM, Bernard PS, Parker JS (2015) Development and verification of the PAM50-based Prosigna breast cancer gene signature assay. BMC Med Genomics 8:54
Acknowledgements
This article was supported by the Korean Breast Cancer Society, by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2015R1D1A1A01057585) and by the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MSIP) (2016R1A5A2945889), and by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI17C1142).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Ryu, J.M., Yu, J., Kim, S.I. et al. Different prognosis of young breast cancer patients in their 20s and 30s depending on subtype: a nationwide study from the Korean Breast Cancer Society. Breast Cancer Res Treat 166, 833–842 (2017). https://doi.org/10.1007/s10549-017-4472-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4472-5