Abstract
Adolescence is hypothesized to be a time period of particular susceptibility to breast cancer risk factors. Red meat and fat intake during high school was positively associated with risk of breast cancer among premenopausal women in the Nurses’ Health Study II (NHSII). High mammographic density is a strong predictor of breast cancer risk but there is limited research on dietary factors associated with breast density. To test the hypothesis that high intake of animal fat or red meat during adolescence is associated with mammographic density, we analyzed data from premenopausal women in the NHSII. Participants recalled adolescent diet on a high school food frequency questionnaire. We assessed absolute and percent mammographic density on digitized analog film mammograms for 687 premenopausal women with no history of cancer. We used generalized linear regression to quantify associations of adolescent animal fat and red meat intake with mammographic density, adjusting for age, body mass index, and other predictors of mammographic density. Adolescent animal fat intake was significantly positively associated with premenopausal mammographic density, with a mean percent density of 39.2 % in the lowest quartile of adolescent animal fat intake versus 43.1 % in the highest quartile (p trend: 0.03). A non-significant positive association was also observed for adolescent red meat intake (p trend: 0.14). These findings suggest that higher adolescent animal fat intake is weakly associated with percent mammographic density in premenopausal women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adolescence is a time period of rapid growth and development. In the investigation of diseases with a long latency period like cancer, puberty has become a focus of research for early life exposures [1]. For determining future breast cancer risk, exposures between menarche and first pregnancy might be particularly relevant as the breast tissue undergoes increased proliferation during this time and only finally differentiates with the first pregnancy. This may render adolescence a time period of particular susceptibility to carcinogenic influences [2, 3].
Nutrition during adolescence, a potentially modifiable risk factor, may be important in the development of breast cancer later in life [4–6]. Animal studies have demonstrated effects of a high fat diet during adolescence on breast cancer development [7]. In recent analyses in the Nurses’ Health Study II (NHSII), adolescent total fat and red meat intakes were positively associated with risk of breast cancer in premenopausal women, independent of adult diet. Specifically, for total fat intake during high school, the hazard ratio (HR) comparing the top versus bottom quintiles of intake was 1.47 (95 % confidence interval (CI): 1.08, 2.01; p trend: 0.02) [8] and for red meat intake during high school, the corresponding HR was 1.42 (95 % CI: 1.04, 1.95; p trend: 0.009) [9].
High mammographic density is one of the strongest predictors of breast cancer risk [10–12] but little research has focused on early life dietary factors in relation to mammographic density [13], which may be an intermediate marker for breast cancer risk. Only three small studies (sample sizes from 91 to 451) have evaluated adolescent dietary fat and red meat intake in relation to premenopausal mammographic density; no associations were found [14–16]. Our analysis aims to add to the knowledge on adolescent diet—specifically, animal fat and red meat intake—and its possible influence on breast tissue composition as assessed by mammographic density.
Methods
Study population
The Nurses’ Health Study II (NHSII) is an on-going prospective cohort study that began in 1989. Then 116,430 female registered nurses, aged 25–42 years, from 14 US states were enrolled in the study and completed the baseline questionnaire on demographic and lifestyle factors, anthropometric measures, and medical history. Since its inception, the cohort has been followed biennially via a mailed self-administered questionnaire updating the information on incident diseases and potential risk factors like reproductive and family history, medication, smoking, and alcohol consumption [17]. Since 1991, every 4 years a supplementary semi-quantitative food frequency questionnaire (FFQ) gathers information on the nurses’ average diet during the past year; information on diet during adolescent years was also collected (see below). The response rate to questionnaires remains high (~90 % each cycle).
Mammograms were collected on a subset of participants who were part of a previously established breast cancer nested case–control study that included biospecimen samples. Each breast cancer case was matched with two controls on age; menopausal status; postmenopausal hormone use (current vs. not current) at time of blood draw and breast cancer diagnosis; and the equivalent time for controls; race/ethnicity; and time of day, month and fasting status at blood draw [18]. The screening mammogram collection was targeted close to the years of the blood collection (1996–1999) and mammograms were received from approximately 80 % of eligible women in the nested case–control study. Further, we collected additional mammograms conducted around 1997 from eligible women (breast cancer cases and non-cases) in NHSII who were not in the original nested breast cancer case–control study. Women for whom mammograms could not be obtained did not differ from those with available mammograms with regard to breast cancer risk factors, including BMI, parity, and family history of breast cancer [19].
Adolescent diet assessment
About half of the entire cohort (56,928 = 49 %) indicated willingness to complete a separate high school FFQ (HS-FFQ) and of those, 83 % (n = 47,355) returned the completed HS-FFQ in 1998. The HS-FFQ, which was a self-administered semi-quantitative questionnaire tailored to include food items commonly consumed during the period from 1960 to 1980 when participants would have been between 13 and 18 years old and attending high school. Respondents were asked to recall their dietary habits during high school (approximately ages 13–18) and provided information on 124 food items grouped under the following eight headings: beverages (including alcohol); dairy; fruits; vegetables; bread/cereal/grain; condiments; snack foods/desserts; and main dishes. The section on main dishes included questions on eggs, chicken, fish, and on the following eight red meat food items: beef or lamb as a main dish; pork as a main dish; beef, pork, or lamb as a sandwich or mixed dish; hamburger; bacon; hotdogs; and other processed meats. Commonly used serving sizes were used where possible (e.g., two slices of bacon or one hamburger equaling one serving). Additionally, for each food item the participants could specify the frequencies of consumption by marking one out of nine categories that ranged from ‘never, or less than once a month’ to ‘six and more times per day.’ There were no major differences between participants who provided information on their high school diet and those who did not with regard to their breast cancer risk factors (e.g., body mass index (BMI), age at menarche, age at first birth, parity, oral contraceptive use, or their adult red meat intake [20]).
Participants recalled their adolescent diet 15–34 years after high school (at ages 33–52). Reproducibility was assessed by administering the HS-FFQ to a random sample of 333 NHSII participants twice at a 4-year interval: their recalled adolescent animal fat and red meat and total calorie intakes correlated moderately (animal fat: r = 0.62, red meat dishes: r = 0.52, total calorie intake: r = 0.69) [21] while the adolescent and adult intakes correlated only weakly (animal fat: r = 0.21, total calorie intake r = 0.43). Validity was evaluated in another population by comparing the information collected using the NHSII HS-FFQ with the information gathered using another FFQ from the same eighty individuals 10 years earlier during high school: the mean correlation between the two questionnaires for animal fat was 0.40 (range 0.18–0.59) and for total calorie intake 0.62 (range 0.40–0.78) [22]. In addition, information from the mothers of 272 NHSII participants on their nurse-daughters’ high school diet was collected and compared with the results from the respective HS-FFQs: the correlation between the nurse-daughters’ own information on their high school diet and their mothers’ recall of it was moderate (animal fat: r = 0.51, total calorie intake: 0.13) [23]. Therefore, the HS-FFQ appears to be an acceptable means to capture past adolescent diet.
The three main food groups contributing to animal fat and accounting for about 70 % of its intake among the women who had provided information on their adolescent diet were red meat (~37 %), dairy products (milk ~13 %, ice cream ~5 %), and chicken (~7 %). Intake of animal fat was derived by multiplying the frequency of consumption of each food item by the animal fat content in the specified portions. Then the results from all the food items contributing to animal fat were summed. The nutrient content for the food items in the HS-FFQ for the respective time period were obtained primarily from the U.S. Department of Agriculture [24]. Animal fat was energy-adjusted using the residuals from the regression of nutrient intake on participants’ total caloric intake/day [25].
Total red meat intake as a food group was calculated by converting responses to the individual red meat food items from their original nine categories of consumption into servings/day and then taking their overall sum. In this analysis of red meat intake, we summed the eight red meat food items on the HS-FFQ described above.
Mammographic density assessment
To assess mammographic density, the cranio-caudal views of screening film mammograms were digitized at 261 µm/pixel with a Lumysis 85 laser film scanner (Lumysis, Sunnyvale, CA) or a VIDAR CAD PRO Advantage scanner (VIDAR Systems Corporation; Herndon, VA) using comparable resolution of 150 dots per inch and 12 bit depth. Density measurements were performed using the Cumulus software, a thresholding computer-assisted method (University of Toronto, Toronto, Canada). The observer selects two thresholds according to the intensity of the pixels: one to delineate the breast edge and the other to distinguish dense tissue from non-dense tissue. The software then calculates the number of pixels of the entire breast and those of the area identified as dense. From these measurements, the following three density measures are calculated: absolute dense area (in cm2), absolute non-dense area (in cm2) (total breast area minus dense area), and percent mammographic density (dense area divided by the total breast area). All images were read by a single reader who was blinded to case–control status. The density measurements for this study were highly reproducible, with a within-person intra-class correlation coefficient of >0.90. As the images had been read in three different batches about 3 years apart each, we adjusted for batch-to-batch variability in density measurements, as previously described [19]. Mammographic density measures of the right and left breast of a woman are strongly correlated (r = 0.92–0.96 [26]) and therefore the density measurements of both breasts were averaged for this analysis.
Covariate data
Information on breast cancer risk factors was collected from the biennial questionnaires. The baseline questionnaire in 1989 provided information on the nurses’ height, BMI at age 18, and age at menarche. The biennial questionnaire directly preceding the mammogram date was used to obtain information on the following covariates at the time of mammogram: age at first birth, parity, family history of breast cancer, history of benign breast disease, current body mass index (BMI, kg/m2), physical activity (METs/wk), oral contraceptive use, current smoking, and alcohol consumption.
The long-term average adult diet up to the time of mammogram was calculated using the diet information from all FFQs preceding the mammogram date. As in the HS-FFQ, the nurses could choose between the same nine categories for frequency of consumption and similar individual red meat food items. The mean correlations in our analysis between adult and adolescent intake were weak for animal fat (r = 0.19) and moderate for red meat (r = 0.37).
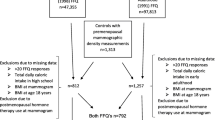
The current analysis was restricted to premenopausal controls (n = 809) for whom both adolescent diet and mammographic density was available. Women with missing data on adolescent red meat intake (n = 4), total calorie intake (n = 21), adult BMI (n = 31), or BMI at age 18 (n = 4) were excluded. Another 62 women were excluded because they reported using postmenopausal hormones near the time of mammogram. The final analytic sample included 687 premenopausal women (Fig. 1).
This study was approved by the institutional review board of Brigham and Women’s Hospital.
Statistical analysis
We assessed age-adjusted distributions of breast cancer risk factors according to quartiles of adolescent animal fat and a prior categories of red meat intake. We used generalized linear models to evaluate the association between adolescent exposures and the three different mammographic density phenotypes. Because two controls were matched to each case, they cannot be assumed to be independent observations. Therefore, generalized estimating equations [27] were used to take into account the correlation between paired controls matched to the same case. For each category of adolescent animal fat and red meat the least square mean percent mammographic density, dense area, and non-dense area were estimated. Linear trends across quartiles of dietary exposures were tested using Wald tests by entering the median of the quartiles into the models as continuous variables. Similar results were observed when outcomes were square-root transformed in sensitivity analyses (data not shown).
In three successive models, we adjusted for known and potential predictors of mammographic density and breast cancer risk factors. The baseline model (Model 1) adjusted for adolescent calorie intake, age at time of mammogram, and adult BMI. Model 2 additionally adjusted for adult diet-related variables: either adult animal fat or red meat intake and adult total calorie intake. This was done to assess whether any observed associations between adolescent intake and mammographic density were confounded by adult diet. The third and final model additionally included further adolescent and reproductive factors known or suspected to be associated with breast cancer risk: BMI at age 18, adolescent physical activity (METs/wk, four categories), adolescent alcohol intake (drinkers vs. non-drinkers), adult alcohol intake (0 g/day, 0.1 to <5 g/day, 5+ g/day), age at menarche, age at first birth and parity combined, biopsy confirmed benign breast disease, first degree family history of breast cancer (see footnotes to Tables 2, 3). Oral contraceptive use near the time of mammogram did not appreciably change effect estimates and was not included in the final multivariable models.
Analyses were conducted using SAS version 9.3 for UNIX (SAS Institute, Cary, NC). All p values were based on two-sided tests and considered statistically significant if ≤0.05.
Results
The distribution of breast cancer risk factors according to quartiles of adolescent animal fat and adolescent red meat intake is shown in Table 1. During high school women consumed a mean of 1.5 servings/day of red meat (range 0–3.79 servings/day) and a mean of 81 grams of animal fat per day (range: 13.6–130.8 g/day). The mean percent mammographic density was 41 %. The median age of the nurses was 45 years when they had their mammogram (range 34–55 years).
Women with higher intake of animal fat during high school were slightly older at the time of mammogram, had a lower energy intake but higher red meat intake during high school, and were more likely to be smokers, to be parous and younger at first birth and to consume more animal fat as adults. Women with higher intake of red meat during high school were more likely to have a higher total energy and animal fat intake and higher BMI during adulthood, to be smokers, and to consume more red meat as adults (Table 1). The correlation between adolescent animal fat and red meat intake was 0.39.
We observed a positive association between adolescent animal fat intake and percent mammographic density, after adjusting for age and BMI (Model 1; Table 2). There was virtually no change in mean estimates of percent mammographic density after further adjustment for adult dietary factors (Model 2; Table 2) or other predictors of mammographic density (Model 3; Table 2). The association was most apparent for women in the top quartile of intake (multivariable-adjusted mean percent density: 43.1 %), whereas mean percent mammography density was similar for women in the first, second, and third quartiles of intake (39.2, 40.8, and 41.0 %, respectively), although there was significant evidence of a linear trend (p trend 0.03). The observed association of animal fat intake with percent mammographic density reflected a weak positive association with dense area as well as a weak inverse association for non-dense area, although neither of these associations were statistically significant (Table 2).
We also observed a weak non-significant positive association between adolescent red meat intake and percent mammographic density (Table 3). In the fully adjusted model, mean percent mammographic density was 40.4 % for women who consumed 2 or more servings of red meat per day during high school versus 37.8 % for those who consumed <1 serving/day (p trend 0.14).
The association between adolescent animal fat intake and percent mammographic density was similar in magnitude after mutual adjustment for adolescent red meat intake (difference between extreme quartiles: 3.4 % points), although the linear trend was no longer statistically significant (p for trend 0.07). There was no association between adolescent intake of processed meat only and mammographic density (data not shown). Adolescent intakes of chicken, milk, or ice cream, the next main contributors to adolescent animal fat following red meat, were not significantly associated with percent or absolute mammographic density; however, adolescent intake of milk was inversely associated with absolute non-dense area (p for trend 0.03) while ice cream was positively associated with absolute non-dense area (p for trend 0.04; Supplementary Table). Finally, there were no associations of current adult intake of animal fat or red meat with percent mammographic density in multivariable models that also included the adolescent dietary factors (data not shown).
Discussion
In this analysis of adolescent diet and premenopausal mammographic density, animal fat intake was positively associated with percent mammographic density. Women in the highest quartile of adolescent animal fat intake had moderately higher percent mammographic density (difference: 3.9 % points) when compared to women in the lowest quartile of intake. The association remained statistically significant after adjusting for adult intake of animal fat and known predictors of mammographic density. We also observed a weak, but non-significant, positive association between adolescent red meat intake and percent mammographic density.
A difference in percent density of around five percentage points is biologically of interest with respect to future breast cancer risk: in postmenopausal women the use of combined postmenopausal hormones [28–30] has been shown to lead to a mean increase in density of about 5–6 %, and in premenopausal women tamoxifen has been shown to decrease mean mammographic density by around 5 % [31]. Whether the slightly smaller magnitude of association we observed for adolescent animal fat intake and mammographic density (i.e., 3.9 % points) is relevant for breast cancer risk prevention efforts warrants further study.
Three other studies, two observational studies and one clinical trial, evaluated associations between specific adolescent dietary factors and premenopausal mammographic density [14–16]. The Minnesota Breast Cancer Family Study Cohort reported no associations between adolescent intake of animal fat, high fat meats, dairy products, and fish-and-chicken measured in number of servings/month and percent mammographic density in 451 premenopausal women [14]. A smaller analysis among 91 premenopausal Chinese immigrant women also did not support an association between red meat intake during teenage years and adult premenopausal mammographic density. In that study, red meat intake was categorized into tertiles of intake frequency (1×/month, 2×/week, 1×/day), and the participants in the highest tertile consumed less red meat than the nurses in the highest quartile (1×/day vs. 2+ servings/day) [15]; associations with adolescent fat intake were not analyzed. Finally, results from the Dietary Intervention Study in Children (DISC) Follow-up Study suggested no association between a low-fat dietary intervention among 182 girls aged 8–10 and breast density assessed at ages 25–29; however, there was no evidence of a difference in total fat intake between the intervention and usual care groups at the end of the 7-year trial, which could explain the null result [16].
The underlying mechanisms for associations between adolescent red meat and fat intake and breast cancer, if causal, are not clear. It has been hypothesized that breast density may represent increased cellular proliferation and cumulative exposure to estrogens. Some dietary factors, including fat intake, may influence circulating levels of plasma sex steroid hormones and insulin-like growth factor [32–37]. Therefore, it is biologically plausible that associations between diet and both breast density and cancer could be mediated by sex hormones and/or growth factors.
Whereas our analysis provides suggestive evidence for a weak positive association between adolescent animal fat and premenopausal mammographic density, these findings are not consistent with the only two other studies that have evaluated this association [14, 16]. Further, though statistically significant, the magnitude of associations we observed were relatively weak and results should be interpreted with caution. However, if confirmed in larger studies and if the mechanism by which animal fat influences breast cancer risk operates through mammographic density, then this finding supports the results from a previous NHSII analysis that showed an association of adolescent total fat intake with an increased risk for breast cancer among premenopausal women (RR for top vs. bottom quintile of intake: 1.47; 95 % CI 1.08, 2.01; p trend 0.02) [8]. In the present analysis, adolescent animal fat was the principal source of and highly correlated with adolescent total fat intake (r = 0.72). Because adolescent red meat and animal fat intake were highly correlated, it was difficult to disentangle the effects of animal fat and red meat on mammographic density. None of the other major food sources of animal fat, however, explained the association we observed between animal fat and percent mammographic density.
The main limitation in this study is the retrospective assessment of high school diet. Measurement error is innate when adolescent diet is recalled 15–34 years after high school. In the NHS and NHSII, the average correlation between two HS-FFQs administered 4–8 years apart ranged from 0.57 to 0.65, suggesting reasonable reproducibility of recalled diet for a distinct time period such as high school [21, 38]. A recent review concluded that a HS-FFQ can be useful for assessing adolescent diet when recall time does not exceed 35 years [39], as in this study. Demonstrated associations between high school diet and breast cancer [8, 9, 20], diabetes [40], and colorectal adenomas [6] further support the validity of this exposure assessment. Most importantly, high school diet was assessed before the mammograms were obtained and read, thus eliminating potential sources of bias. An important strength of this analysis is the use of screening mammograms from women with no history of breast or other cancer. Mammograms came from different centers, which may contribute to measurement error. However, mammograms were digitized and assessed by a single reader who maintained a high intra-person correlation coefficient (>0.90). Furthermore, the associations of density measurements with established predictors such as BMI and age are in the expected directions [41–43]. Additional strengths include the assessment of the outcome measures as continuous measurements thus increasing the power of the analysis and the ability to adjust for breast cancer risk factors, including factors during adolescence and adulthood.
In conclusion, these findings from the largest study to date suggest that high intake of animal fat during adolescence may be associated with a small increase in premenopausal mammographic density. Future studies are needed to corroborate these findings and estimate the joint impact of adolescent diet and mammographic density on breast cancer risk.
References
Biro FM, Deardorff J (2013) Identifying opportunities for cancer prevention during preadolescence and adolescence: puberty as a window of susceptibility. J Adolesc Health 52(5 Suppl):019
Colditz GA, Frazier AL (1995) Models of breast cancer show that risk is set by events of early life: prevention efforts must shift focus. Cancer Epidemiol Biomark Prev 4(5):567–571
Berkey CS, Frazier AL, Gardner JD, Colditz GA (1999) Adolescence and breast carcinoma risk. Cancer 85(11):2400–2409
Mahabir S (2012) Association between diet during preadolescence and adolescence and risk for Breast Cancer During Adulthood. J Adolesc Health 8(12):00352–00357
Frazier AL, Ryan C, Rockett H, Willett W, Colditz G (2003) Adolescent diet and risk of breast cancer. Breast Cancer Res 5(3):R59–R64
Nimptsch K, Bernstein AM, Giovannucci E, Fuchs CS, Willett WC, Wu K (2013) Dietary intakes of red meat, poultry, and fish during high school and risk of colorectal adenomas in women. Am J Epidemiol 178(2):172–183
Zhao Y, Tan Y, Aupperlee M, Langohr I, Kirk E, Troester M, Schwartz R, Haslam S (2013) Pubertal high fat diet: effects on mammary cancer development. Breast Cancer Res 15(5):R100
Linos E, Willett WC, Cho E, Frazier L (2010) Adolescent diet in relation to breast cancer risk among premenopausal women. Cancer Epidemiol Biomark Prev 19(3):689–696
Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC (2015) Adolescent meat intake and breast cancer risk. Int J Cancer 136(8):1909–1920
McCormack VA, Silva IDS (2006) Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomark Prev 15(6):1159–1169
Boyd NF, Martin LJ, Yaffe MJ, Minkin S (2011) Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res 13(6):223
Vachon CM, Brandt KR, Ghosh K, Scott CG, Maloney SD, Carston MJ, Pankratz VS, Sellers TA (2007) Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiol Biomark Prev 16(1):43–49
Lindgren J, Dorgan J, Savage-Williams J, Coffman D, Hartman T (2013) Diet across the lifespan and the association with breast density in adulthood. Int J Breast Cancer 2013:808317
Sellers TA, Vachon CM, Pankratz VS, Janney CA, Fredericksen Z, Brandt KR, Huang Y, Couch FJ, Kushi LH, Cerhan JR (2007) Association of childhood and adolescent anthropometric factors, physical activity, and diet with adult mammographic breast density. Am J Epidemiol 166(4):456–464
Tseng M, Olufade TO, Evers KA, Byrne C (2011) Adolescent lifestyle factors and adult breast density in U.S. Chinese immigrant women. Nutr Cancer 63(3):342–349
Dorgan JF, Liu L, Klifa C, Hylton N, Shepherd JA, Stanczyk FZ, Snetselaar LG, Van Horn L, Stevens VJ, Robson A et al (2010) Adolescent diet and subsequent serum hormones, breast density, and bone mineral density in young women: results of the Dietary Intervention Study in Children follow-up study. Cancer Epidemiol Biomark Prev 19(6):1545–1556
Colditz GA, Hankinson SE (2005) The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer 5(5):388–396
Eliassen AH, Spiegelman D, Xu X, Keefer LK, Veenstra TD, Barbieri RL, Willett WC, Hankinson SE, Ziegler RG (2012) Urinary estrogens and estrogen metabolites and subsequent risk of breast cancer among premenopausal women. Cancer Res 72(3):696–706
Bertrand KA, Eliassen AH, Hankinson SE, Gierach GL, Xu X, Rosner B, Ziegler RG, Tamimi RM (2012) Urinary estrogens and estrogen metabolites and mammographic density in premenopausal women. Breast Cancer Res Treat 136(1):277–287
Linos E, Willett WC, Cho E, Colditz G, Frazier LA (2008) Red meat consumption during adolescence among premenopausal women and risk of breast cancer. Cancer Epidemiol Biomark Prev 17(8):2146–2151
Maruti SS, Feskanich D, Colditz GA, Frazier AL, Sampson LA, Michels KB, Hunter DJ, Spiegelman D, Willett WC (2005) Adult recall of adolescent diet: reproducibility and comparison with maternal reporting. Am J Epidemiol 161(1):89–97
Maruti SS, Feskanich D, Rockett HR, Colditz GA, Sampson LA, Willett WC (2006) Validation of adolescent diet recalled by adults. Epidemiology 17(2):226–229
Maruti SS, Feskanich D, Colditz GA, Frazier AL, Sampson LA, Michels KB, Hunter DJ, Spiegelman D, Willett WC (2005) Adult recall of adolescent diet: reproducibility and comparison with maternal reporting. Am J Epidemiol 161(1):89–97
U.S. Department of Agriculture, Agricultural Research Service (2001) USDA National Nutrient Database for Standard Reference, Release 14. Nutrient Data Laboratory Home Page. http://www.ars.usda.gov/nea/bhnrc/ndl
Willett WC, Howe GR, Kushi LH (1997) Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65(4 Suppl):1220S–1228S
Linos E, Willett W (2009) Meat, dairy, and breast cancer: do we have an answer? Am J Clin Nutr 90(3):455–456
Zeger SL, Liang KY (1986) Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42(1):121–130
Greendale GA, Reboussin BA, Slone S, Wasilauskas C, Pike MC, Ursin G (2003) Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst 95(1):30–37
Chlebowski RT, Hendrix SL, Langer RD et al (2003) Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the women’s health initiative randomized trial. JAMA 289(24):3243–3253
Lundström E, Christow A, Kersemaekers W, Svane G, Azavedo E, Söderqvist G, Mol-Arts M, Barkfeldt J, von Schoultz B (2002) Effects of tibolone and continuous combined hormone replacement therapy on mammographic breast density. Am J Obstet Gynecol 186(4):717–722
Cuzick J, Warwick J, Pinney E, Warren RML, Duffy SW (2004) Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst 96(8):621–628
Boyd NF, Lockwood GA, Greenberg CV, Martin LJ, Tritchler DL (1997) Effects of a low-fat high-carbohydrate diet on plasma sex hormones in premenopausal women: results from a randomized controlled trial. Canadian Diet and Breast Cancer Prevention Study Group. Br J Cancer 76(1):127–135
Crowe FL, Key TJ, Allen NE, Appleby PN, Roddam A, Overvad K, Gronbaek H, Tjonneland A, Halkjaer J, Dossus L et al (2009) The association between diet and serum concentrations of IGF-I, IGFBP-1, IGFBP-2, and IGFBP-3 in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomark Prev 18(5):1333–1340
Holmes MD, Pollak MN, Willett WC, Hankinson SE (2002) Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomark Prev 11(9):852–861
Kerver JM, Gardiner JC, Dorgan JF, Rosen CJ, Velie EM (2010) Dietary predictors of the insulin-like growth factor system in adolescent females: results from the Dietary Intervention Study in Children (DISC). Am J Clin Nutr 91(3):643–650
Vrieling A, Voskuil DW, Bueno de Mesquita HB, Kaaks R, van Noord PA, Keinan-Boker L, van Gils CH, Peeters PH (2004) Dietary determinants of circulating insulin-like growth factor (IGF)-I and IGF binding proteins 1, -2 and -3 in women in the Netherlands. Cancer Causes Control 15(8):787–796
Wu AH, Pike MC, Stram DO (1999) Meta-analysis: dietary fat intake, serum estrogen levels, and the risk of breast cancer. J Natl Cancer Inst 91(6):529–534
Frazier AL, Willett WC, Colditz GA (1995) Reproducibility of recall of adolescent diet: nurses’ Health Study (United States). Cancer Causes Control 6(6):499–506
Chavarro JE, Rosner BA, Sampson L, Willey C, Tocco P, Willett WC, Chumlea WC, Michels KB (2009) Validity of adolescent diet recall 48 years later. Am J Epidemiol 170(12):1563–1570
Malik VS, Fung TT, van Dam RM, Rimm EB, Rosner B, Hu FB (2012) Dietary patterns during adolescence and risk of type 2 diabetes in middle-aged women. Diabetes Care 35(1):12–18
Li T, Sun LM, Miller N, Nicklee T, Woo J, Hulse-Smith L, Tsao MS, Khokha R, Martin L, Boyd N (2005) The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomark Prev 14(2):343–349
Vachon CM, Kuni CC, Anderson K, Anderson VE, Sellers TA (2000) Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States). Cancer Causes Control 11(7):653–662
Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Paterson AD (2005) Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol 6(10):798–808
Acknowledgments
We thank Barbara DeSouza, Divya Prithviraj, and Ellen Hertzmark for their assistance with data collection and analysis. This work was supported by the Breast Cancer Research Foundation and the National Cancer Institute (UM1 CA176726, R01 CA124865, R01 CA67262, and R01 CA50385). KAB was supported by the Simeon J. Fortin Charitable Foundation Fellowship, Bank of America, N.A., Co-Trustee.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Kimberly A. Bertrand and Rosemarie A. Burian have contributed equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bertrand, K.A., Burian, R.A., Eliassen, A.H. et al. Adolescent intake of animal fat and red meat in relation to premenopausal mammographic density. Breast Cancer Res Treat 155, 385–393 (2016). https://doi.org/10.1007/s10549-016-3679-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3679-1