Abstract
Purpose
Previous studies suggest that coffee and caffeine intake may be associated with reduced breast cancer risk. To date, there is limited and inconsistent epidemiologic evidence for associations of adolescent diet with mammographic breast density, a strong and consistent predictor of breast cancer. We investigated the association of adolescent caffeine intake with mammographic density in premenopausal women.
Methods
This study included 751 cancer-free women within the Nurses’ Health Study II cohort. Percent breast density (PD), absolute dense (DA) and non-dense areas (NDA) were measured from digitized film mammograms using a computer-assisted thresholding technique; all measures were square root-transformed. Energy-adjusted adolescent caffeine intake was estimated using the data from a food frequency questionnaire. Information regarding breast cancer risk factors was obtained from questionnaires closest to the mammogram date. We used generalized linear regression to quantify associations of caffeine intake with breast density measures.
Results
In multivariable analyses, adolescent caffeine intake was not associated with any of the density phenotypes (caffeine 4th vs. 1st quartile: β = − 1.27, 95% CI − 4.62; 2.09, p-trend = 0.55 for percent density; β = − 0.21, 95% CI − 0.76, 0.34, p-trend = 0.65 for absolute dense area, and β = 0.23, 95% CI − 0.28, 0.74, p-trend = 0.50 for non-dense area). Additional adjustment of the models for body mass index at age 18 resulted in attenuation of the risk estimates.
Conclusions
Our findings do not support the hypothesis that adolescent caffeine intake is associated with premenopausal mammographic breast density.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammographic breast density is a well-established and strong predictor of breast cancer risk [1]. Appearance of the breast on the mammogram is a reflection of the amount of fat, connective tissue, and epithelial tissue in the breast [2]. Light (non-radiolucent) areas on the mammogram represent the fibrous and glandular tissues (mammographicallyi dense), whereas, the dark (radiolucent) areas are primarily fat. Women with breasts of ≥ 75% density (proportion of the total breast area that appears dense on the mammogram) are at four–sixfold greater risk of breast cancer compared to women with more fat tissues in the breasts [1].
Some previous studies have suggested that coffee and caffeine intake may be associated with reduced breast cancer risk [3,4,5,6]. A few mechanisms have been suggested to explain these associations including changes in bioavailable estrogen, antioxidative properties of coffee constituents (such as polyphenols), and changes in DNA methylation and cellular differentiation [7,8,9,10,11,12,13]. However, the epidemiologic evidence on the association of coffee and caffeine with breast density remains very limited. An earlier investigation within Minnesota Breast Cancer Family Study found no associations between caffeine intake and percent breast density [14]. Within Nurses’ Health Study and Nurses’ Health Study II cohorts, we found that decaffeinated coffee consumption was positively associated with percent density in premenopausal women (2 + cups/day: β = 0.23, p-trend = 0.03), but we observed no associations with caffeine intake (4th vs. 1st quartile: β = − 0.17, p-trend = 0.18). In postmenopausal women, decaffeinated and total coffee were inversely associated with percent density (decaffeinated 2 + cups/day: β = − 0.24, p-trend = 0.04; total 4 + cups/day: β = − 0.16, p-trend = 0.02) and among current hormone replacement therapy users, caffeine was inversely associated with percent density (4th vs. 1st quartile: β = − 0.32, p-trend = 0.01) [15].
Adolescence represents a critical window of susceptibility to breast cancer during which the breast undergoes rapid growth and development and emerging evidence suggests that exposures during this time period, including nutrition, may be particularly important for subsequent adult breast cancer risk [16,17,18,19,20,21]. These dietary influences could potentially be reflected in the degree of breast density. However, none of the previous studies have examined the associations of coffee and caffeine intake in adolescence with mammographic breast density. To address these knowledge gaps, we examined associations of caffeine intake with percent breast density in healthy pre- and postmenopausal women using prospective data from the Nurses’ Health Study and Nurses’ Health Study II cohorts.
Methods
Study population and design
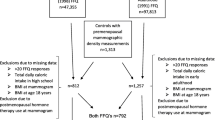
Women included in this study were selected from participants of a breast cancer case–control study nested within the Nurses’ Health Study II (NHSII) cohort. This prospective cohort was established in 1989 and followed 116, 430 female registered nurses in the United States who were 25–42 years old (NHSII) at enrollment. After administration of the initial questionnaire, information on breast cancer risk factors [body mass index (BMI), reproductive history, and alcohol use] and any diagnoses of cancer or other diseases was updated through biennial questionnaires [2, 22].
A nested case–control approach was originally used as an efficient sampling design to examine the association between selected biomarkers and breast cancer risk within the NHS II cohort [23]. Using incidence density sampling, women who did not have any type of cancer (other than non-melanoma skin cancer) at the time of the case’s cancer diagnosis (controls) were matched 1:2 with women diagnosed with in situ or invasive breast cancer (cases) on age at the time of blood collection, menopausal status and postmenopausal hormone use (current vs. not current) at blood draw, day/time of blood draw, race/ethnicity and day in the luteal phase [24]. Our analysis included controls from this nested case–control study as well as additional eligible women within this cohort (without a history of any cancer other than non-melanoma skin) who were not included in the original nested breast cancer case–control study. We attempted to obtain mammograms closest to the time of blood collection (or ~ 1997 for those who did not provide blood samples). From all eligible women, 1292 premenopausal women provided consent and had a usable mammogram for density estimation. Of these women, 751 had data on adolescent caffeine intake and covariates and were included in the analysis. This study was approved by the Institutional Review Board at the Brigham and Women’s Hospital. Consent was obtained or implied by return of questionnaires.
Dietary assessment
Usual dietary intake and coffee consumption during the past year were assessed with a semi-quantitative food frequency questionnaire (FFQ) with approximately 130 items which were included in the 1991, 1995, 1999, 2003, and 2007 questionnaires [25]. Responses were recorded in nine categories of intake frequency ranging from “never or less than once per month” to “six or more per day” for given portion sizes. Nutrient intakes were calculated by multiplying the frequency of consumption of each item by the nutrient content of the specified portions and then summing across all items, as previously described [25]. Nutrient values in foods were obtained from the US Department of Agriculture, food manufacturers, and independent academic sources [26,27,28]. Every 4 years, the food composition database was updated to account for changes in the food supply.
In 1997, participants were asked if they would be willing to complete a supplemental FFQ about diet during high school (HS-FFQ). From the entire cohort, 49% of women indicated willingness to complete the HS-FFQ, and of those 83% returned the HS-FFQ in 1998. There were minimal differences in baseline demographic characteristics between participants who completed the HS-FFQ and women who did not provide information on high school diet [20]. Food intake during adolescence was measured using a 124-item high school-FFQ, which was specifically designed to contain foods that were usually consumed during the periods from 1960 to 1980 when these women would have been in high school. Food items included in the FFQ for adolescents and response categories were similar to those in the FFQ for adults. Previous studies demonstrated high reproducibility of the nutrient intake estimates from these FFQs [29]. Caffeine intake was derived from self-reported intakes of coffee, soda, tea, and chocolate by multiplying the frequency of consumption of each item by their caffeine content per serving and then summing across all items, as previously described [7]. Energy-adjusted caffeine intake was defined as quartiles based on the distribution in the study sample (< 23.2, 23.2–< 52.6, 52.6–< 105.8, and ≥ 105.8 mg).
Assessment of mammographic density
Mammographic density was assessed in three batches approximately 2–3 years apart. To quantify mammographic density, the craniocaudal views of both breasts for first two batches of mammograms in the NHSII were digitized at 261 μm per pixel with a Lumisys 85 laser film scanner (Lumisys, Sunnyvale, California). The third batch of NHSII mammograms was digitized using a VIDAR CAD PRO Advantage scanner (VIDAR Systems Corporation; Herndon, VA, USA) and comparable resolution of 150 dots per inch and 12 bit depth. The Cumulus software (University of Toronto, Toronto, Canada) was used for computer-assisted determination of the absolute dense area, non-dense area, and percent mammographic density on all mammograms [2, 30]. All NHSII images were read by a single reader. Although within-batch reproducibility was high (intraclass correlation coefficient ≥ 0.90) [31], density measures varied across the NHSII batches. The density measures from the second and third batches of NHSII mammograms were adjusted to account for the batch effect (whether due to intra-reader variability or scanner), as previously described [32].
Percent breast density was measured as percentage of the total area occupied by epithelial/stromal tissue (absolute dense area) divided by the total breast area. Since breast densities of the right and left breast for an individual woman are strongly correlated [30], the average density of both breasts was used in this analysis.
Covariate information
Information on breast cancer risk factors was obtained from the biennial questionnaires closest to the date of the mammogram. For exclusion from this analysis, women were considered to be postmenopausal if reported: (1) no menstrual periods within the 12 months before blood collection with natural menopause, (2) bilateral oophorectomy, or (3) hysterectomy with one or both ovaries retained, and were 54 years or older for ever smokers or 56 years or older for never smokers [33, 34]. Height and weight at age 18 years was reported on the 1989 questionnaire.
Statistical analysis
We used generalized linear regression to examine the associations of adolescent caffeine intake with percent density, absolute dense and non-dense areas, while taking into account the correlation between matched controls [35]. Since absolute dense and non-dense area measures were non-normally distributed, we used the square root transformation to improve normality in all the regression analyses. Percent breast density did not require transformation. The lowest caffeine intake category was used as the reference. The regression estimates were adjusted for age (continuous), body mass index (continuous), age at menarche (< 12, 12–13, > 13 years), parity and age at first child’s birth (nulliparous, parous with age at first birth < 25, parous with age at first birth ≥ 25), a confirmed history of benign breast disease (yes, no), a family history of breast cancer (yes, no), alcohol consumption (0, 0–< 5, ≥ 5 g/day, unknown) and cumulative average caffeine consumption in adulthood (quartiles: < 71.0, 71.0 < 176.3, 176.3–< 329.4, and ≥ 329.4 mg) which was calculated as the average of the estimates from all available FFQs administered before the mammogram date. A two-sided test for trend was performed, modeling caffeine consumption as an ordinal variable and using the median consumption level in each category. In the additional analysis, we adjusted the risk estimates for all covariates described above and BMI at age 18. To assess the overall trend for caffeine intake, we used respective medians within each category. Statistical significance in all the analyses was assessed at 0.05 level. The analyses were performed using SAS software (version 9.2, SAS Institute, Cary, NC, USA).
Results
In this study of 751cancer-free women, the average age at the mammogram was 45 years (range 30–56). Women in the highest and lowest adolescent caffeine intake quartile had a mean percent density of 39.1% and 43.7%, respectively. As compared to women in the lowest quartile of caffeine intake, women in the highest quartile had smaller absolute dense area (91.3 vs. 98.2 cm2) and larger non-dense area (157.2 vs. 130.3 cm2). Distributions of breast cancer risk factors by the quartiles of adolescent caffeine intake are presented in Table 1. Women in the highest quartile of caffeine intake as compared to the lowest quartile consumed more alcohol, had greater BMI, were less likely to have benign breast disease, and were more likely to be nulliparous. Distributions of other risk factors were similar across the caffeine intake quartiles.
In multivariable analysis, adolescent caffeine intake was not associated with any of the density phenotypes (caffeine 4th vs. 1st quartile: β = − 1.27, 95% CI − 4.62; 2.09, p-trend = 0.55 for percent density; β = − 0.21, 95% CI − 0.76, 0.34, p-trend = 0.65 for absolute dense area, and β = 0.23, 95% CI − 0.28, 0.74, p-trend = 0.50 for non-dense area) (Table 2). The risk estimates remained similar when adult caffeine intake was excluded from the models. Additional adjustment of the models for BMI at age 18 resulted in attenuation of the risk estimates.
Discussion
In this study of cancer-free premenopausal women, we examined the associations of caffeine consumption in adolescence with mammographic breast density. We found no associations of adolescent caffeine intake with any of the three breast density phenotypes.
The evidence on the association of coffee and caffeine intake with mammographic breast density remains very limited. A study among 1508 cancer-free women participating in the Minnesota Breast Cancer Family Study (MBCFS) found no statistically significant association between caffeine intake and percent breast density overall and when stratified by menopausal status [14]. In contrast, our recent investigation found inverse associations of total and decaffeinated coffee intake with percent breast density among postmenopausal women and a positive association of decaffeinated coffee with percent density in premenopausal women [15]. None of the previous studies, however, explored the associations of adolescent caffeine intake with breast density.
Coffee and caffeine intake may affect breast cancer and breast density by several mechanisms, including an influence on circulating estrogen levels and bioavailable estrogen [7,8,9], antioxidative potential due to a high content of wide variety of phytochemicals [10], stimulating effects on variety of enzymes, including those involved in estrogen and xenobiotic metabolism [11], and inhibition of DNA methylation [12] all of which eventually can disrupt normal cell proliferation and differentiation. Even though our results do not support the hypothesis that adolescent caffeine intake may be important for adult breast density, it is important to note that majority of our study population (92%) reported consuming less than a cup of coffee per day. In contrast, in adulthood, more than 76% of women consumed ≥ 1 cups/day. Given this distribution, it is likely that the observed null findings for caffeine resulted from low prevalence of coffee consumption in our study population and were mainly driven by other sources of dietary caffeine, including soda, tea, and chocolate which have different antioxidant/antiradical activity and total polyphenolic content as compared to coffee, and thus, may have different effects on the breast tissue [36]. In addition, the levels of caffeine intake in adolescence (mean 84 mg/day and median of 53 mg/day) were lower than those in adulthood (mean of 223 mg and median of 176 mg/day), with only a weak correlation between these two intake measures (correlation coefficient = 0.35, p < 0.0001).
In the models with BMI at age 18, the risk estimates were attenuated. Adolescent obesity is positively associated with increased adiposity in adulthood [37, 38] and was inversely associated with breast density, independent of current BMI in premenopausal women [39]. However, evidence on association between adolescent obesity and coffee/caffeine intake is extremely limited. In a prospective cohort study using data from the National Heart, Lung, and Blood Institute Growth and Health Study, consumption of coffee or tea was not associated with BMI among 2371 girls who were followed from ages 9 or 10 years until age 19 years [40]. Similarly, another study among 319 pre-adolescent and adolescent girls (ages 10–17) found no association between caffeine consumption and BMI [41]. However, inclusion of BMI at age 18 in the full models with all breast cancer risk factors in our study resulted in moderate change in the risk estimates suggesting potential confounding. In addition, there was a strong correlation of adult BMI with BMI at age 18 (correlation coefficient = 0.61, p < 0.001) which may explain attenuation of the effects due to collinearity.
Our study is the first study to investigate the association of adolescent caffeine intake with mammographic density. The analysis used data from the NHSII, an established cohort with more than 25 years of follow-up, ascertainment of disease status, and comprehensive information on breast cancer risk factors and breast density. While we found no statistically significant associations with 751 women in our study we had sufficient statistical power (> 80%) to detect even modest effects (e.g., 5–10 percentage points in average percent density which translates into approximately 7% change in breast cancer risk [42, 43]) thus making our null result informative.
Our study has limitations. The examined associations are based on the density measures from a single mammogram rather than the woman’s life-long density pattern. However previous studies have suggested that a single breast density measure can predict breast cancer risk for up to 10 years in both pre- and postmenopausal women [42, 44] and that breast density measures of a woman over a long period of time are highly correlated [45] Despite the prospective nature of the cohort, potential errors in recall of coffee and caffeine intake, especially for adolescent diet, is possible, since women recalled their high school diet on average 20–23 years from before the questionnaire date. However, recall of adolescent diet is reasonably reproducible and sufficiently precise to examine associations of adolescent diet with health outcomes in epidemiologic studies [46]. Finally, as noted above, we could not examine separately associations of adolescent coffee intake with breast density.
In conclusion, we investigated the associations of adolescent caffeine intake with percent breast density, absolute dense and non-dense area in premenopausal women. Our findings suggest that adolescent caffeine intake is not associated with adult breast density phenotypes. However, further studies in populations with higher prevalence of coffee consumption in adolescence are needed to confirm our findings.
References
McCormack VA, dos Santos Silva I (2006) Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomark Prev 15(6):1159–1169
Tamimi RM, Byrne C, Colditz GA, Hankinson SE (2007) Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst 99(15):1178–1187
Baker JA, Beehler GP, Sawant AC, Jayaprakash V, McCann SE, Moysich KB (2006) Consumption of coffee, but not black tea, is associated with decreased risk of premenopausal breast cancer. J Nutr 136(1):166–171
Lowcock EC, Cotterchio M, Anderson LN, Boucher BA, El-Sohemy A (2013) High coffee intake, but not caffeine, is associated with reduced estrogen receptor negative and postmenopausal breast cancer risk with no effect modification by CYP1A2 genotype. Nutr Cancer 65(3):398–409
Oh JK, Sandin S, Strom P, Lof M, Adami HO, Weiderpass E (2015) Prospective study of breast cancer in relation to coffee, tea and caffeine in Sweden. Int J Cancer 137(8):1979–1989
Bhoo-Pathy N, Peeters PHM, Uiterwaal CSPM, Bueno-de-Mesquita HB, Bulgiba AM, Bech BH, Overvad K, Tjønneland A, Olsen A, Clavel-Chapelon F, Fagherazzi G, Perquier F, Teucher B, Kaaks R, Schütze M, Boeing H, Lagiou P, Orfanos P, Trichopoulou A, Agnoli C, Mattiello A, Palli D, Tumino R, Sacerdote C, van Duijnhoven FJB, Braaten T, Lund E, Skeie G, Redondo M-L, Buckland G, Pérez MJS, Chirlaque M-D, Ardanaz E, Amiano P, Wirfält E, Wallström P, Johansson I, Nilsson LM, Khaw K-T, Wareham N, Allen NE, Key TJ, Rinaldi S, Romieu I, Gallo V, Riboli E, van Gils CH (2015) Coffee and tea consumption and risk of pre- and postmenopausal breast cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Breast Cancer Res: BCR 17(1):15
Sisti JS, Hankinson SE, Caporaso NE, Gu F, Tamimi RM, Rosner B, Xu X, Ziegler R, Eliassen AH (2015) Caffeine, coffee, and tea intake and urinary estrogens and estrogen metabolites in premenopausal women. Cancer Epidemiol Biomark Prev 24(8):1174–1183
Kotsopoulos J, Eliassen AH, Missmer SA, Hankinson SE, Tworoger SS (2009) Relationship between caffeine intake and plasma sex hormone concentrations in premenopausal and postmenopausal women. Cancer 115(12):2765–2774
Kotsopoulos J, Eliassen AH, Missmer SA, Hankinson SE, Tworoger SS (2009) Relationship between caffeine intake and plasma sex hormone concentrations in premenopausal and postmenopausal women. Cancer 115(12):2765–2774
Halvorsen BL, Carlsen MH, Phillips KM, Bøhn SK, Holte K, Jacobs DR, Blomhoff R (2006) Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am J Clin Nutr 84(1):95–135
Rahmioglu N, Heaton J, Clement G, Gill R, Surdulescu G, Zlobecka K, Hodgkiss D, Ma Y, Hider RC, Smith NW, Ahmadi KR (2011) Genetic epidemiology of induced CYP3A4 activity. Pharmacogenet Genom 21(10):642–651
Lee WJ, Zhu BT (2006) Inhibition of DNA methylation by caffeic acid and chlorogenic acid, two common catechol-containing coffee polyphenols. Carcinogenesis 27(2):269–277
Pozner J, Papatestas AE, Fagerstrom R, Schwartz I, Saevitz J, Feinberg M, Aufses AH Jr (1986) Association of tumor differentiation with caffeine and coffee intake in women with breast cancer. Surgery 100(3):482–488
Vachon CM, Kushi LH, Cerhan JR, Kuni CC, Sellers TA (2000) Association of diet and mammographic breast density in the Minnesota breast cancer family cohort. Cancer Epidem Biomar 9(2):151–160
Yaghjyan L, Colditz G, Rosner B, Gasparova A, Tamimi RM (2018) Associations of coffee consumption and caffeine intake with mammographic breast density. Breast Cancer Res Treat 169(1):115–123
Mahabir S (2013) Association between diet during preadolescence and adolescence and risk for breast cancer during adulthood. J Adolesc Health 52(5 Suppl):S30–S35
Berkey CS, Frazier AL, Gardner JD, Colditz GA (1999) Adolescence and breast carcinoma risk. Cancer 85(11):2400–2409
Frazier AL, Ryan CT, Rockett H, Willett WC, Colditz GA (2003) Adolescent diet and risk of breast cancer. Breast Cancer Res 5(3):R59–R64
Zhao Y, Tan YS, Aupperlee MD, Langohr IM, Kirk EL, Troester MA, Schwartz RC, Haslam SZ (2013) Pubertal high fat diet: effects on mammary cancer development. Breast Cancer Res: BCR 15(5):R100
Linos E, Willett WC, Cho E, Frazier L (2010) Adolescent diet in relation to breast cancer risk among premenopausal women. Cancer Epidemiol, Biomark Prev: Publ Am Assoc Cancer Res, Cosponsored Am Soc Prev Oncol 19(3):689–696
Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC (2015) Adolescent meat intake and breast cancer risk. Int J Cancer 136(8):1909–1920
Colditz GA, Hankinson SE (2005) The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer 5(5):388–396
Tworoger SS, Sluss P, Hankinson SE (2006) Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Cancer Res 66(4):2476–2482
Bertrand KA, Rosner B, Eliassen AH, Hankinson SE, Rexrode KM, Willett W, Tamimi RM (2015) Premenopausal plasma 25-hydroxyvitamin D, mammographic density, and risk of breast cancer. Breast Cancer Res Treat 149(2):479–487
Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC (2014) Dietary protein sources in early adulthood and breast cancer incidence: prospective cohort study. BMJ 348:g3437
Nutrient Database for Standard Reference, Release 14. Department of Agriculture ARS, 2001. http://www.ars.usda.gov/Services/docs.htm?docid=21215. Accessed March 20, 2019
Holland GWA, Unwin ID, Buss DH, Paul AA, Dat S (1991) The composition of foods. Cambridge, UK: The Royal Society of Chemistry and Ministry of Agriculture, Fisheries and Food
Dial S, Eitenmiller RR (1995) Tocopherols and tocotrienols in key foods in the US diet. In: Ong ASH, Niki E, Packer L (eds) Nutrition, lipids, health, and disease. AOCS Press, Champaign, pp 327–342
Maruti SS, Feskanich D, Colditz GA, Frazier AL, Sampson LA, Michels KB, Hunter DJ, Spiegelman D, Willett WC (2005) Adult recall of adolescent diet: reproducibility and comparison with maternal reporting. Am J Epidemiol 161(1):89–97
Byng JW, Boyd NF, Little L, Lockwood G, Fishell E, Jong RA, Yaffe MJ (1996) Symmetry of projection in the quantitative analysis of mammographic images. Eur J Cancer Prev 5(5):319–327
Pettersson A, Hankinson SE, Willett WC, Lagiou P, Trichopoulos D, Tamimi RM (2011) Nondense mammographic area and risk of breast cancer. Breast Cancer Res 13(5):R100
Bertrand K, Eliassen AH, Hankinson S, Gierach G, Xu X, Rosner B, Ziegler R, Tamimi R (2012) Urinary estrogens and estrogen metabolites and mammographic density in premenopausal women. Breast Cancer Res Treat 136(1):277–287
Willett W, Stampfer MJ, Bain C, Lipnick R, Speizer FE, Rosner B, Cramer D, Hennekens CH (1983) Cigarette smoking, relative weight, and menopause. Am J Epidemiol 117(6):651–658
Stampfer MJ, Willett WC, Colditz GA, Rosner B, Speizer FE, Hennekens CH (1985) A prospective study of postmenopausal estrogen therapy and coronary heart disease. N Engl J Med 313(17):1044–1049
Zeger SL, Liang KY (1986) Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42(1):121–130
Yashin A, Yashin Y, Wang JY, Nemzer B (2013) Antioxidant and antiradical activity of coffee. Antioxidants 2(4):230–245
Wang LY, Chyen D, Lee S, Lowry R (2008) The association between body mass index in adolescence and obesity in adulthood. J Adolesc Health 42(5):512–518
Simmonds M, Llewellyn A, Owen CG, Woolacott N (2016) Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev 17(2):95–107
Bertrand KA, Baer HJ, Orav EJ, Klifa C, Shepherd JA, Van Horn L, Snetselaar L, Stevens VJ, Hylton NM, Dorgan JF (2015) Body fatness during childhood and adolescence and breast density in young women: a prospective analysis. Breast Cancer Res: BCR 17(1):95
Striegel-Moore RH, Thompson D, Affenito SG, Franko DL, Obarzanek E, Barton BA, Schreiber GB, Daniels SR, Schmidt M, Crawford PB (2006) Correlates of beverage intake in adolescent girls: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr 148(2):183–187
Drescher AA, Goodwin JL, Silva GE, Quan SF (2011) Caffeine and screen time in adolescence: associations with short sleep and obesity. J Clin Sleep Med: JCSM 7(4):337–342
Byrne C, Schairer C, Wolfe J, Parekh N, Salane M, Brinton LA, Hoover R, Haile R (1995) Mammographic features and breast cancer risk: effects with time, age, and menopause status. J Natl Cancer Inst 87(21):1622–1629
Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, Forbes JF, Warren RM (2011) Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst 103(9):744–752
Wijayabahu AT, Zhou Z, Cook RL, Brumback B, Ennis N, Yaghjyan L (2019) Healthy behavioral choices and cancer screening in persons living with HIV/AIDS are different by sex and years since HIV diagnosis. Cancer Causes & Control
Krishnan K, Baglietto L, Stone J, Simpson JA, Severi G, Evans CF, MacInnis RJ, Giles GG, Apicella C, Hopper JL (2017) Longitudinal study of mammographic density measures that predict breast cancer risk. Cancer Epidemiol, Biomark Prev 26(4):651–660
Frazier AL, Willett WC, Colditz GA (1995) Reproducibility of recall of adolescent diet: nurses’ Health Study (United States). Cancer Causes Control: CCC 6(6):499–506
Acknowledgements
This work was supported by the National Cancer Institute at the National Institutes of Health [CA131332, CA175080 to R.M.T., UM1 CA186107 and P01 CA087969, to M.S., UM1 CA176726 to W.W], Avon Foundation for Women, Susan G. Komen for the Cure®, and Breast Cancer Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yaghjyan, L., Colditz, G., Rosner, B. et al. Adolescent caffeine consumption and mammographic breast density in premenopausal women. Eur J Nutr 59, 1633–1639 (2020). https://doi.org/10.1007/s00394-019-02018-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-02018-0