Abstract
To examine the relationship between local recurrence and breast cancer mortality in women with early-stage breast cancer. We studied 1675 women with stage 0 (DCIS), stage I or stage II breast cancer who were treated with breast-conserving surgery at Women’s College Hospital between 1987 and 2009. For each patient, we obtained information on age at diagnosis, tumour size, lymph node status, tumour grade, lymphovascular invasion, oestrogen receptor status, progesterone receptor status, HER2 status and treatments received (radiotherapy, chemotherapy and tamoxifen). Patients were followed from the date of diagnosis until local recurrence, death from breast cancer or the date of last follow-up. We used the Kaplan–Meier method to estimate 15-year local recurrence-free and breast cancer-specific survival rates for each stage at diagnosis. For each stage, the two rates were compared. After a mean follow-up of 13.1 years, 243 women (14.5 %) experienced a local recurrence and 281 women (16.8 %) died of breast cancer. The 15-year actuarial rate of local recurrence was 16 % for women with DCIS, 15 % for women with stage I cancer and 16 % for women with stage II cancer. The 15-year breast cancer-specific mortality rate was 3 % for women with DCIS, 10 % for women with stage I breast cancer and 30 % for women with stage II breast cancer. After experiencing a local recurrence, the 15-year breast cancer mortality rate was 16 % for women with DCIS, 32 % for women with stage I breast cancer and 59 % for women with stage II breast cancer. Across the spectrum of the early stages of breast cancer, the risk of local recurrence does not correlate with the risk of death from breast cancer. After local recurrence, the risk of death from breast cancer depends on the initial stage at diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Screening mammography detects a large number of early-stage breast cancers for which the optimal treatment is not always clear. Many breast cancers present with no evidence of invasion within the breast (DCIS) or beyond the breast (node-negative breast cancer). It is difficult to distinguish patients with indolent breast cancers that are unlikely to affect survival from those with subclinical metastases who might benefit from systemic therapy. It is proposed that the development of new markers that predict which patients are at high risk for tumour recurrence will be helpful in guiding treatment decisions (e.g. lumpectomy versus mastectomy, radiotherapy versus no radiotherapy, tamoxifen versus no tamoxifen, chemotherapy versus no chemotherapy) [1].
Some patients who die from early-stage breast cancer experience a local (in-breast) recurrence prior to the development of clinically detectable distant metastases, and some patients experience a distant recurrence as the first event. The risk of death from breast cancer increases after a local recurrence, but not all patients who experience a local recurrence develop distant metastases or die [2–4]. It has been proposed that by predicting the risk of local recurrence, we can minimize the under-treatment and over-treatment of small screen-detected breast cancers [5, 6]. For women with DCIS or with invasive breast cancer, preventing local recurrence (with mastectomy or with radiation therapy) does not prevent death from breast cancer [7–11]; in order to impact on breast cancer mortality, a predictive test must help guide decisions about adjuvant chemotherapy. To justify chemotherapy, the reduction in mortality from breast cancer must be sufficiently large to outweigh the side effects. Much research has been conducted on risk factors for local recurrence; however, it is not clear to what extent the risk of local recurrence correlates with the risk of death across the early stages of breast cancer or if biomarkers of local recurrence will predict breast cancer mortality or will help guide treatment.
Here, we explore the relationship between rates of local recurrence and breast cancer mortality in groups of women with DCIS (stage 0), stage I and stage II breast cancers. We compare the rates of local recurrence and of breast cancer mortality across the three stages and within each stage. We compare the impacts of various factors on the risks of local recurrence and of breast cancer death for all women with early-stage breast cancer. If factors which predict local recurrence also predict mortality, then we should be optimistic that new markers of local recurrence will have the potential to guide chemotherapy. If the factors are distinct, then future studies should focus on mortality itself and not on local recurrence.
Methods
We studied a cohort of 1675 women diagnosed with primary DCIS (stage 0), stage I breast cancer or stage II breast cancer who were treated with breast-conserving surgery at the Henrietta Banting Breast Center at Women’s College Hospital between 1987 and 2009. For each patient, we abstracted information on age at diagnosis, tumour size, lymph node status, tumour grade, lymphovascular invasion (LVI), oestrogen receptor (ER) status, progesterone receptor (PR) status, HER2 status, all treatments received (surgery, radiotherapy, chemotherapy, hormone therapy), all recurrences (local, regional, distant) and dates and causes of death. ‘DCIS’ refers to patients with ductal carcinoma, primary tumour in situ. ‘Stage I breast cancer’ refers to all patients with invasive primary tumours of 2.0 cm or less in size with negative lymph nodes. ‘Stage II breast cancer’ refers to patients with invasive primary tumours of 2.0 cm or less in size with positive lymph nodes, 2.1–5.0 cm in size with negative or positive lymph nodes or more than 5.0 cm in size with negative lymph nodes.
We stratified the patients into three groups according to the type of first recurrence. ‘Local recurrence’ includes all patients who experienced a local invasive recurrence (defined as in-breast or chest wall) as the first event. Patients with a non-invasive local recurrence were not included in this group. ‘Distant recurrence’ includes all patients who had a distant recurrence as the first event (alone or at the same time as a local or regional recurrence). ‘No recurrence’ includes all patients with no documented recurrence as of the date of last follow-up. Patients who experienced a regional (axillary) or loco-regional recurrence as the first event were excluded from this study.
We used the Kaplan–Meier method to estimate 15-year local recurrence-free survival rates and breast cancer-specific survival rates. Patients were followed for local recurrence from the date of diagnosis to local recurrence, death or date of last follow-up. Patients were followed for breast cancer death from the date of diagnosis to death from breast cancer, death from another cause or date of last follow-up. To estimate breast cancer-specific survival after local recurrence, we followed patients from the date of local recurrence to death from breast cancer, death from another cause or date of last follow-up.
The Cox proportional hazards model was used to estimate the unadjusted (univariate) and adjusted (multivariate) hazard ratios for local recurrence and for death from breast cancer. Covariates included age at diagnosis, grade, LVI, ER status, PR status, stage at diagnosis, radiotherapy (no/yes), chemotherapy (no/yes) and tamoxifen (no/yes). A time-dependent Cox proportional hazards model was used to estimate the impact of experiencing a local recurrence on the risk of death from breast cancer at 15 years for patients in each stage group.
The ratio of distant metastasis to local recurrence can be thought of as an index of metastatic potential. The ratio was estimated by dividing the number of patients who either died of breast cancer or experienced a distant recurrence in the study period by the number of patients who experienced a local recurrence as the first event in the study period.
The (crude) probability of death from breast cancer (P death) can be expressed as a function of the probability of local recurrence (P LR), the probability of death given local recurrence (\(P_{\text{death}}{\mid}{\text{LR}}\)), the probability of distant recurrence (P DR), the probability of death given distant recurrence (\(P_{\text{death}}{\mid}{\text{DR}}\)), the probability of no recurrence (P NR) and the probability of death given no recurrence (\(P_{\text{death}}{\mid}{\text{NR}}\)), using the following equation:
We estimated these values for the members of the study cohort according to stage at diagnosis. These results are presented and discussed in the supplement.
Results
We followed 1675 women with DCIS (n = 254), stage I (n = 697) or stage II (n = 724) breast cancer who were treated with breast-conserving surgery for a mean of 13.1 years (range 1–27) (Table 1). By the end of the study period, 243 (14.5 %) of the 1689 women had experienced a local recurrence as the first event; 217 women (13.0 %) had experienced a distant recurrence as the first event. 281 women (16.8 %) died of breast cancer.
The actuarial rate of local recurrence at 15 years was 15.6 % for women with DCIS, 15.3 % for women with stage I breast cancer and 15.9 % for women with stage II breast cancer (Table 2; Fig. 1a). Among women who did not receive radiotherapy, the risk of local recurrence at 15 years was 16.9 % for women with DCIS, 22.9 % for women with stage I breast cancer and 33.3 % for women with stage II breast cancer (Fig. 1b). After adjusting for all adjuvant treatments received (radiotherapy, chemotherapy, tamoxifen) and other factors (age, grade, LVI, hormone-receptor status), the risk of local recurrence at 15 years was significantly higher for patients with stage I cancer compared to that for patients with DCIS (adjusted HR 2.47, 95 % CI 1.32–4.61; p = 0.005) and for patients with stage II cancer compared to that for patients with DCIS (adjusted HR 3.05; 95 % CI 1.54–6.03; p = 0.001) (Table 3).
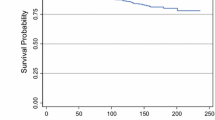
The 15-year breast cancer-specific mortality rate was 2.7 % for patients with DCIS, 9.6 % for patients with stage I cancer and 29.6 % for patients with stage II cancer (Fig. 2). The gradient in mortality was similar among patients who did and who did not receive radiotherapy (Table 2). The adjusted hazard ratio of breast cancer death at 15 years was 5.8 (95 % CI 2.1–16.7; p = 0.001) for patients with stage I cancer compared to that for patients with DCIS and was 14.2 (95 % CI 5.0–40.5; p < 0.0001) for patients with stage II cancer compared to that for patients with DCIS (Table 3).
For each stage group, we compared the risks of local recurrence and the risks of breast cancer mortality at 15 years (Fig. 3). Among the 254 women with DCIS, 35 women (13.8 %) experienced a local recurrence in the follow-up period and six women (2.4 %) died of breast cancer. Among the 679 women with stage I breast cancer, 101 women (14.9 %) experienced a local recurrence in the follow-up period and 73 women (10.8 %) died of breast cancer. Among the 727 women with stage II breast cancer, 107 women (14.7 %) experienced a local recurrence in the follow-up period and 202 women (27.8 %) died of breast cancer. In women with DCIS, the risk of local recurrence is much higher than the risk of breast cancer death (Fig. 3a). In women with stage I breast cancer, the risk of local recurrence is similar to the risk of breast cancer death (Fig. 3b). In women with stage II breast cancer, the risk of breast cancer death is higher than the risk of local recurrence (Fig. 3c).
a Local recurrence-free survival and breast cancer-specific survival at 15 years in patients with DCIS treated with lumpectomy. b Local recurrence-free survival and breast cancer-specific survival at 15 years in patients with stage I breast cancer treated with lumpectomy. c Local recurrence-free survival and breast cancer-specific survival at 15 years in patients with stage II breast cancer treated with lumpectomy
Among all patients in the cohort, local recurrence was associated with an increased risk of subsequent breast cancer mortality (adjusted HR 4.2; 95 % CI 3.1–5.7; p < 0.0001). The adjusted hazard ratio for death associated with a local recurrence was 18.6 for patients with DCIS (95 % CI 1.9–183.5; p = 0.01), 3.8 for patients with stage I breast cancer (95 % CI 2.0–7.2; p < 0.0001) and 3.9 for patients with stage II breast cancer (95 % CI 2.7–5.7; p < 0.0001). The actuarial 15-year breast cancer mortality rate after local recurrence was 16.0 % for patients with DCIS, 31.9 % for patients with stage I breast cancer and 58.9 % for patients with stage II breast cancer (Fig. 4).
We compared the impact of various factors on both the risk of local recurrence at 15 years and the risk of death from breast cancer at 15 years for all patients in the cohort (Table 3). In a multivariable analysis, positive PR status did not predict local recurrence (adjusted HR 0.90; 95 % CI 0.62–1.31; p = 0.58) but was a strong predictor of death from breast cancer (adjusted HR 0.49; 95 % CI 0.36–0.67; p < 0.0001). PR expression was associated with a reduced risk of death both for women with ER-positive breast cancers (adjusted HR 0.43; 95 % CI 0.30–0.60; p < 0.0001) and ER-negative breast cancers (adjusted HR 0.40; 95 % CI 0.19–0.83; p = 0.01). ER status was associated with good survival in a crude analysis but not after adjusting for PR status. Positive ER status was not associated with a reduced risk of death in women with PR-positive breast cancers (adjusted HR 0.88; 95 % CI 0.46–1.69; p = 0.71) or with PR-negative breast cancers (adjusted HR 1.05; 95 % CI 0.70–1.59; p = 0.81). Among patients with stage I and stage II breast cancers, high tumour grade (vs. low tumour grade) was a moderate predictor of local recurrence (adjusted HR 1.80; 95 % CI 1.07–3.03; p = 0.03) but was a strong predictor of death from breast cancer (adjusted HR 3.49; 95 % CI 1.91–6.38; p < 0.0001). Lympho-vascular invasion (stage I and stage II patients only) was an independent predictor both of local recurrence (adjusted HR 1.73; 95 % CI 1.22–2.46; p = 0.002) and of breast cancer mortality (adjusted HR 1.94; 95 % CI 1.47–2.56; p < 0.0001).
The risk of local recurrence at 15 years was significantly reduced with radiotherapy (adjusted HR 0.40; 95 % CI 0.29–0.55; p < 0.0001), chemotherapy (adjusted HR 0.61; 95 % CI 0.41–0.91; p = 0.002) and tamoxifen (adjusted HR 0.56; 95 % CI 0.40–0.79; p = 0.001); however, none of the three therapies predicted breast cancer death at 15 years (Table 3).
We compared the number of patients who either died of breast cancer or experienced a distant recurrence in the follow-up period and the number of patients who experienced a local recurrence as the first event (distant metastasis to local recurrence ratio) for 1666 women with early-stage (non-metastatic) breast cancer, according to tumour size (Table S1). The ratio of distant metastasis to local recurrence was 0.2 for patients with DCIS (6 distant recurrences/deaths and 35 local recurrences), 0.8 for patients with tumours 2.0 cm or less in diameter (39 distant recurrences/deaths and 47 local recurrences), 1.2 for patients with tumours 2.1–5.0 cm in diameter (103 distant recurrences/deaths and 86 local recurrences) and 2.2 for patients with tumours 5.0 cm or more in diameter (157 distant recurrences/deaths and 71 local recurrences) (Figure S1).
For each stage group, we compared the relative contributions of local recurrences, distant recurrences and no recurrence to the overall probability of death from breast cancer (see Results section in the supplement). To determine what factors account for the difference in mortality rates for women with DCIS, stage I cancer and stage II cancer, we examined the probability of death from breast cancer according to type of first recurrence (Tables S2 and S3).
Discussion
Based on the results of a recent study, we concluded that in the absence of invasion, DCIS has the potential to metastasize and to cause death [9]. This prompted us to consider DCIS to be an early stage of breast cancer, which differs from stage I and II breast cancer only by a matter of degree. Under this paradigm, we examined the relationship between local recurrence and distant recurrence/death in women with stage 0 to stage II breast cancer. The actuarial rate of local recurrence at 15 years was similar for women with DCIS (16 %), stage I breast cancer (15 %) and stage II breast cancer (16 %). In contrast, the actuarial rate of breast cancer-specific mortality at 15 years was much lower for women with DCIS (3 %) than that for women with stage I breast cancer (10 %) or stage II breast cancer (30 %). The rates of local recurrence and of breast cancer death reported here are comparable to the rates reported in randomized trials of DCIS [2, 12] and stage I–II breast cancers [3, 4, 7]. The patients in our study were followed closely from diagnosis, and all recurrences have been confirmed by a pathologist. Variation in local recurrence rates between studies is likely due to underlying differences in the patient cohorts (e.g. differences in age, stage at diagnosis or treatments received). The extent of discordance between local recurrence rates and breast cancer mortality rates calls into question the relevance of using the risk of local recurrence to predict cancer-related death in women with early-stage breast cancer or the goal of identifying new markers of local recurrence in order to guide future treatment decisions.
After adjusting for treatments received, the risk of local recurrence at 15 years was higher for stage I cancer compared to that for DCIS (HR 2.47; 95 % CI 1.32–4.61; p = 0.005) and for stage II cancer compared to that for DCIS (HR 3.05; 95 % CI 1.54–6.03; p = 0.001). These modest differences observed in the local recurrence rate cannot account for the much larger differences in breast cancer mortality observed with stage; for example, compared to DCIS, the adjusted hazard ratio for death from breast cancer at 15 years was 14.2 for stage II breast cancer (95 % CI 4.96–40.5; p < 0.0001).
The risk of breast cancer-specific death following local recurrence ranged from 16 % for patients diagnosed with DCIS to 59 % for patients diagnosed with stage II breast cancer (Table 2). After controlling for initial treatments received (and other factors), the risk of death following local recurrence was 2.4 times higher for patients with stage I cancer compared to that for DCIS (95 % CI 0.51–11.0; p = 0.27) and was 6.4 times higher for patients with stage II cancer compared to that for DCIS (95 % CI 1.34–30.3; p = 0.02). The hazard ratios for death after local recurrence between stage groups are comparable to the hazard ratios for death after primary diagnosis between stage groups (Table 3). This suggests that among patients with early-stage breast cancer, local recurrence is a marker for the presence of distant metastases but does not influence the probability of metastases in itself [13]. Others have also reported that survival after local recurrence depends on the initial stage at diagnosis [3, 4, 14–18].
Differences in the risks of local recurrence as the first event and the risks of death given local recurrence account for some (but not all) of the difference observed in breast cancer mortality between DCIS, stage I and stage II breast cancers (Supplementary Appendix). Breast cancer mortality is also influenced by the risk of experiencing a distant recurrence or of dying from breast cancer in the absence of an intervening local recurrence. In the current study, the (crude) probability of death from breast cancer increased from 2.4 % for patients with DCIS to 27.8 % for patients with stage II breast cancer (an absolute increase of 25.4 %). The probability of death from breast cancer in the absence of a local recurrence increased from 0.8 % for patients with DCIS to 19.8 % for patients with stage II cancer (absolute increase of 19 %), accounting for 75 % of the difference in breast cancer mortality (Table S3). Previous studies have also found that the risk of developing distant metastases or dying from breast cancer in the absence of a local recurrence increases with stage at diagnosis [3, 4, 15].
For patients with invasive breast cancer, tumour size refers to the greatest dimension (usually the diameter) of the largest area of contiguous invasion of stroma and does not include adjacent DCIS. For every doubling of tumour diameter, the number of cancer cells increases eight-fold. In this study, among patients with early-stage (non-metastatic) breast cancer treated with lumpectomy, the ratio of distant metastasis to local recurrence increased from 0.2 for patients with DCIS to 0.8 for patients with tumours of 1.0 cm or less in size, to 1.2 for patients with tumours of 1.1–2.0 cm in size and to 2.2 for patients with tumours of 2.1–5.0 cm in size (Figure S1). DCIS is an outlier in this analysis; the very low ratio of metastasis to recurrence is due to the combination of a relatively low mortality rate and a relatively high (unadjusted) rate of local recurrence for patients with DCIS.
We show here that the risk factors which predict local recurrence are not necessarily the same as those which predict death from breast cancer (Table 3). Notably, PR status was a strong prognostic factor for mortality but not for local recurrence. In contrast, high tumour grade and LVI were independent predictors of both local recurrence and mortality. Millar et al. also examined risk factors for local recurrence, distant recurrence and breast cancer mortality in women with early-stage breast cancer and also found them to be discordant [19].
Positive PR status was associated with an approximately 60 % reduced risk of death from breast cancer both for women with ER-positive and ER-negative tumours. In an unadjusted analysis, ER status was associated with a reduced risk of both local recurrence and breast cancer mortality, but after adjusting for PR status, the effect of ER status was not significant. This suggests that the effects of ER status and PR status are distinct and that both are relevant. Most studies compare the risk of recurrence or death for different molecular subtypes of breast cancer (triple-negative, luminal, etc.), wherein ER and PR status are treated as equivalent [19, 20]. This categorization obfuscates seminal differences in the prognostic effects of ER status and PR status.
We believe the five salient findings of this analysis to be the following: First, across stages, there is little correlation between the rates of local recurrence and the rates of breast cancer death; the risk of local recurrence is relatively constant with stage, whereas the risk of breast cancer death increases sharply with stage. Second, the risk of death from breast cancer following local recurrence increases with the initial stage at diagnosis. In the absence of a local recurrence, the risk of death also increases with stage at diagnosis. Third, the ratio of distant metastasis to local recurrence increases with tumour size. Fourth, PR status does not predict local recurrence but is a strong predictor of breast cancer mortality. Fifth, tumour grade and LVI are independent prognostic factors for both local recurrence and mortality (see Box 1).
This analysis has some limitations. The results are based on a relatively small number of patients with DCIS, and only six patients with DCIS died in the study period. We did not have information on many of the tumour characteristics for patients with DCIS (tumour size, tumour grade, LVI, HER2 status); therefore, some of the multivariable analyses are restricted to patients with stage I–II breast cancers. We did not have information on the use of HER2 targeted therapies (Herceptin); however, most patients in this study were treated before these therapies were introduced. We did not include margin status or multifocality in the model; the impact of these factors on local recurrence and breast cancer mortality will be the focus of a future study. There may have been some misclassification of the DCIS cases, i.e. some patients who were diagnosed with DCIS may have had undetected foci of stromal invasion or undetected nodal metastases, which, if detected, would have resulted in a diagnosis of stage I or stage II breast cancer. However, previous studies have found that only 1–2 % of patients with DCIS have lymph node metastases [21]. Further, it has not been shown that the presence of micro-invasion in patients with DCIS impacts on breast cancer mortality [21, 22]. For these reasons, we do not expect that a small degree of misclassification of DCIS would impact on our results.
There are two possible models to describe the relationship between local recurrence and distant recurrence/death in women with early-stage breast cancer (Fig. 5). In Model A, one path leads from the primary tumour to the local (in-breast) recurrence and a separate (parallel) path leads from the primary tumour to the distant recurrence. In Model B, one path leads from the primary tumour to the local recurrence and a second path in the sequence leads from the local recurrence to the distant recurrence. According to Model A, the primary tumour is the source of the metastases (and is ultimately the cause of death), and preventing the local recurrence does not prevent death. This is the conventional model for stage I and stage II breast cancers. According to Model B, the local recurrence is the source of the metastases and ultimately the cause of death and preventing the local recurrence prevents death. This has been the conventional model for DCIS until now. We believe that for both DCIS and invasive breast cancer, the relationship between local recurrence and distant metastasis is similar and is better represented by Model A. Many studies have shown that both for women with DCIS and invasive breast cancer, prevention of local (in-breast) recurrence with mastectomy (or radiotherapy) does not prevent death from breast cancer. In this and other studies, survival after local recurrence is shown to depend to a great extent on the prognosis of the primary tumour, which supports the position that the local recurrence is an indicator of the presence of metastatic disease and is not the cause [13, 15].
Two models for the relationship between local recurrence and distant recurrence/death in women with early-stage breast cancer. In Model A, one pathway leads from the primary tumour to the local (in-breast) recurrence and a separate pathway leads from the primary tumour to distant recurrence/death (mortality after distant recurrence approaches 100 %; therefore, the risk of distant recurrence may be approximated by the risk of breast cancer death). In Model B, one pathway leads from the primary tumour to the local recurrence and another pathway leads from the local recurrence to distant recurrence/death. If Model A is correct, preventing local recurrence does not prevent death from breast cancer. If Model B is correct, preventing local recurrence prevents death from breast cancer. The clinical situation for women with DCIS and early invasive breast cancer is more similar to Model A. Since the prevention of local recurrence does not prevent death from breast cancer, it follows that local recurrence in itself cannot metastasize/cause death (if it could, preventing it would prevent death), and therefore, the pathway from local recurrence to distant metastasis/death does not exist
In order for a breast cancer to cause death, it must have the potential for distant metastasis. It follows logically that the local recurrence in itself does not metastasize (if it could, preventing local recurrence would prevent death), and thus (for both DCIS and invasive breast cancer), the posited pathway from local recurrence to distant metastasis is illusory; that is, the primary tumour has metastatic potential, but the local recurrence does not. Although primary invasive tumours and local invasive recurrences may appear the same histologically, in this respect they behave differently.
We show here that the ratio of distant metastasis to local recurrence (an index of metastatic potential) increases with increasing tumour size. There are two possible explanations for this. Conventional thinking is that cancers increase in their propensity to metastasize as they enlarge (i.e. tumour size predicts tumour aggressiveness). An alternate explanation is that fast-growing cancers are inherently metastagenic (i.e. tumour aggressiveness predicts tumour size). If tumour size is a marker of tumour aggressiveness (like grade or ER status), then the benefits of early diagnosis are expected to be limited, and screening to detect smaller and smaller breast cancers will have little impact on preventing death.
In conclusion, the results of this and previous studies suggest that local recurrence and distant recurrence are distinct and separable events—one path leads from the primary tumour to local recurrence and a parallel path leads from the primary tumour to distant recurrence. Factors which predict local recurrence are different from factors which predict death (although they may overlap). The discovery of new biomarkers which predict local recurrence is not likely to be an effective strategy for reducing mortality from breast cancer.
References
Health Quality Ontario (2010) Gene expression profiling for guiding adjuvant chemotherapy decisions in women with early breast cancer: an evidence-based and economic analysis. Ont Health Technol Assess Ser 10(23):1–57
Wapnir I, Dignam J, Fisher B et al (2011) Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst 103:478–488
Anderson S, Wapnir I, Dignam J et al (2009) Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol 27:2466–2473
Wapnir I, Anderson S, Mamounas E et al (2006) Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project protocols of node-positive breast cancer. Clin Oncol 24:2028–2037
Rakovitch E, Nofech-Mozes S, Hanna W et al (2015) A population-based validation score of the DCIS Score predicting recurrence risk in individuals treated by breast conserving surgery alone. Breast Cancer Res Treat 152:389–398
Liu F, Shi W, Done S et al (2015) Identification of a low-risk luminal A breast cancer cohort that may not benefit from breast radiotherapy. JCO 33(18):2035–2040
Fisher B, Anderson S, Bryant J et al (2002) Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347(16):1233–1241
McGale P, Taylor C, Correa C et al (2014) Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383:2127–2135
Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P (2015) Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol 1(7):888–896
Falk R, Hofvind S, Skaane P, Haldorsen T (2011) Second events following ductal carcinoma in situ of the breast: a register-based cohort study. Breast Cancer Res Treat 129:929–938
Warnberg F, Garmo H, Emdin S et al (2014) Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS trial. J Clin Oncol 32:3613–3618
Donker M, Litiere S, Werutsky G et al (2013) Breast-conserving treatment with or without radiotherapy in ductal carcinoma in situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol 31:4054–4059
Holzel D, Emeny R, Engel J (2011) True local recurrences do not metastasize. Cancer Metastasis Rev 30:161–176
Dent R, Valentini A, Hanna W et al (2014) Factors associated with breast cancer mortality after local recurrence. Curr Oncol 21(3):418–425
Engel J, Eckel R, Kerr J et al (2003) The process of metastasisation for breast cancer. Eur J Cancer 39:1794–1806
Veronesi U, Marubini E, Del Vecchio M et al (1995) Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. J Natl Cancer Inst 87:19–27
Park S, Han W, Kim J et al (2015) Risk factors associated with distant metastasis and survival outcomes in breast cancer patients with locoregional recurrence. J Breast Cancer 18(2):160–166
Yi M, Bucholz T, Meric-Bernstam F et al (2011) Classification of ipsilateral breast tumor recurrences after breast conservation therapy can predict patient prognosis and facilitate treatment planning. Ann Surg 253:572–579
Millar E, Graham P, O’Toole S et al (2009) Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol 27(28):4701–4708
Kummel A, Kummel S, Barinoff J et al (2015) Prognostic factors for local, loco-regional and systemic recurrence in early-stage breast cancer. Geburtshilfe Frauenheilkd 75(7):710–718
De Mascarel I, MacGrogan G, Mathoulin-Pelissier S, Soubeyran I, Picot V, Coindre JM (2002) Breast ductal carcinoma in situ with microinvasion. A definition supported by a long-term study of 1248 serially sectioned ductal carcinomas. Cancer 94:2134–2142
Parikh R, Haffty B, Lannin D, Moran M (2012) Ductal carcinoma in situ with microinvasion: prognostic implications, long-term outcomes, and role of axillary evaluation. Int J Radiat Oncol Biol Phys 82:7–13
Acknowledgments
We thank Katarzyna Jerzak, Javaid Iqbal and Vasily Giannakeas for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sopik, V., Nofech-Mozes, S., Sun, P. et al. The relationship between local recurrence and death in early-stage breast cancer. Breast Cancer Res Treat 155, 175–185 (2016). https://doi.org/10.1007/s10549-015-3666-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3666-y