Abstract
Although the upregulated expression of Anxa2 has been implicated in carcinogenesis, cancer progression, and poor prognosis of cancer patients, the detailed molecular mechanisms involved in these processes remain unclear. In this study, we investigated the effect of Anxa2 downregulation with small interference RNA on breast cancer proliferation. To explore molecular mechanisms underlying Anxa2-mediated cancer cell proliferation. We analyzed cell cycle distribution and signaling pathways using semi-quantitative real-time PCR and Western blotting. Anxa2 depletion in breast cancer cells significantly inhibited cell proliferation by decelerating cell cycle progression. The retarded G1-to-S phase transition in Anxa2-silenced cells was attributed to the decreased levels of cyclin D1, which is a crucial promoting factor for cell proliferation because it regulates G1-to-S phase transition during cell cycle progression. We provided evidence that Anxa2 regulates epidermal growth factor-induced phosphorylation of STAT3. The reduced expression of phosphorylated STAT3 is the main factor responsible for decreased cyclin D1 levels in Anxa2-silenced breast cancer cells. Our results revealed the direct relationship between Anxa2 and activation of STAT3, a key transcription factor that plays a pivotal role in regulating breast cancer proliferation and survival. This study provides novel insights into the functions of Anxa2 as a critical molecule in cellular signal transduction and significantly improves our understanding of the mechanism through which Anxa2 regulates cell cycle and cancer cell proliferation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anxa2, also known as annexin A2, annexin II, or p36, is a multifunctional and multicompartmental protein; Anxa2, which was first purified from membrane vesicles of intestinal epithelial cells, is identified as a substrate of the protein tyrosine kinase v-Src [16, 17]. Anxa2 exists as a monomer or heterotetramer and is distributed intracellularly and extracellularly [3, 27, 48, 60]. Extracellular Anxa2 primarily functions as an important receptor and activator of plasminogen, which is critical for many biological activities, such as vascular thrombolysis, angiogenesis, and tissue remodeling [3, 27, 48, 60]. The physiological function of intracellular Anxa2 is associated with membrane-related events, such as exocytosis, endocytosis, membrane formation, and vesicular trafficking [3, 27, 48, 60]. Intracellular Anxa2 also plays an important role in RNA binding, DNA synthesis, and cellular signal transduction [12, 35, 56]. As such, Anxa2 modulates many cellular activities, including cell proliferation, apoptosis, cell migration, invasion, and angiogenesis [3, 27, 48, 56, 60]. Moreover, the deregulated expression of Anxa2 is involved in several diseases, such as cancer, inflammation, and anti-phospholipid syndrome [3, 27, 48, 60]. Nevertheless, the underlying mechanism through which Anxa2 contributes to these diseases remains largely unknown.
Accumulated evidence supported the pivotal role of Anxa2 in carcinogenesis and cancer progression [3, 27, 48, 60]. Anxa2 overexpression has been observed in many human malignancy tumors, including breast cancer [41], colorectal cancer [8, 10], pancreatic cancer [11, 45], renal cell carcinoma [32], hepatocellular carcinoma [29, 53], glioma [13], gastric cancer [9], and lung cancer [28]. The increased expression levels of Anxa2 in cancer tissues are associated with rapid recurrence [21, 45], metastasis [32, 52], poor response to chemotherapy [6, 20], and poor prognosis [32, 45]. Importantly, Anxa2 promotes cell adhesion [44], migration [40, 44, 59, 61, 62], invasion [51, 57, 59, 61], EMT [63, 64], and angiogenesis [26, 38], which are crucial for cancer metastasis. In vitro studies demonstrated that Anxa2 downregulation significantly blocks cell cycle progression, cell division, and cell proliferation in cell lines derived from multiple myeloma [2], breast cancer [42, 58], and lung cancer [47]; conversely, Anxa2 upregulation promotes cancer cell proliferation [51], these studies presented the implication of Anxa2 in cell cycle progression and cell proliferation. Two recent studies have shown that perturbing Anxa2 function may be a potential approach for cancer treatment [5, 39]. Thus, the mechanism through which Anxa2 regulates these processes must be elucidated.
In this study, we investigated the effect of Anxa2 knockdown on breast cancer proliferation. Anxa2 depletion in breast cancer cells significantly inhibited cell proliferation by decelerating cell cycle progression. The retarded G1-to-S phase transition in Anxa2-silenced cells was attributed to the reduced levels of cyclin D1, which is a crucial promoting factor for cell proliferation because it regulates G1-to-S phase transition during cell cycle progression [31, 34]. We provided evidence that the reduced expression of phosphorylated STAT3 is the main factor responsible for the decreased cyclin D1 levels in Anxa2-silenced breast cancer cells. Our results revealed the direct relationship between Anxa2 and activation of STAT3, a key transcription factor that plays a pivotal role in regulating breast cancer proliferation and survival.
Materials and methods
Cell lines, reagents, and antibodies
Human breast cancer cell lines MDA-MB-231 and T47D were obtained from the American Type Culture Collection. Cells were cultured in RPMI-1640 (Hyclone, Logan, USA) culture medium supplemented with 10 % fetal bovine serum (Gibco, Carlsbad, USA) in an incubator humidified at 37 °C with 5 % CO2. All the cells used in this study were passaged less than 3 months. Enhanced chemiluminescence reagents were purchased from Millipore (Darmstadt, Germany). Protease inhibitor (EDTA-free) was purchased from Roche Diagnostics (Mannheim, Germany). Lipofectamine 2000 and Click-iT EdU cell proliferation assay kit were purchased from Invitrogen (Carlsbad, USA). Mouse monoclonal antibodies against Anxa2, cyclin D1, cyclin E, and β-actin, was purchased from Santa Cruz Inc. (CA, USA). Rabbit monoclonal antibodies against Akt, phosphor-Akt (Thr308), p-S6K1 (Thr 389), Erk1/2, p-Erk1/2 (Thr202/Tyr204), and p-STAT3 (Tyr 705) were purchased from Cell Signaling Technology (Danvers, USA). Rabbit monoclonal antibodies against p70 S6K1 and STAT3 were purchased from Epitomics (Burlingame, USA).
Plasmid construction, lentivirus packaging, and stable cell line generation
Lentiviral vector pLKO.1-puro was constructed to express human Anxa2-specific shRNAs or human STAT3-specific shRNAs. The shRNA target sequences are listed in Table 1. A scrambled sequence that does not target any known human coding sequence was used for negative control. Packaging of lentiviral particles was performed as described previously [56]. Briefly, lentiviral vectors were cotransfected with two packaging plasmids into 293T cells using Lipofectamine 2000 according to the manufacturer’s instructions. Virus-containing media were collected at 48 and 72 h after transfection and then centrifuged at 4000 rpm to remove cell debris. The supernatant was ultrafiltrated through centrifugation in ultrafiltration columns (Millipore) at 3000 rpm for 1 h at 4 °C to concentrate lentiviral particles. The cells were infected in the presence of 8 µg/mL polybrene, and Anxa2 or STAT3 stable knockdown cells were obtained through selection with 6 µg/mL puromycin for 10 days.
Western blotting assay
Western blotting assay was performed as described previously [58]. Briefly, the cells were lysed in 1 × SDS cell lysis buffer. Protein lysates were quantified with BCA reagent, and 20 µg of the lysates were separated through SDS-PAGE and transferred onto a PVDF membrane. The membrane was blocked with 5 % nonfat milk and incubated with primary antibodies against Anxa2 (1:2000), cyclin D1 (1:500), cyclin E (1:1000), Akt (1:1000), p-Akt (1:1000), S6K1 (1:1000), p-S6K1 (1:1000), Erk1/2 (1:3000), p-Erk1/2 (1:2000), p38 (1:1000), p-p38 (1:500), STAT3 (1:1000), p-STAT3 (1:1000), and β-actin (1:5000) at 4 °C overnight. Immunodetection was subsequently performed with HRP-linked secondary antibodies and enhanced chemiluminescence reagents according to the manufacturer’s protocol.
Reverse transcription and quantitative PCR
Total RNA was extracted with Trizol reagent (Invitrogen, Carlsbad, USA). Reverse transcription was performed with a PrimeScript RT kit (Takara, Dalian, P.R. China) according to the manufacturer’s protocol. Semi-quantitative real-time PCR was performed using SYBR Premix Ex Taq (Takara, Dalian, P. R. China). PCR primers and reaction conditions are described in Table 2. The experiments were performed using four replicates for each group and repeated three times.
MTT assay
MTT assay was performed as described previously [58]. Briefly, control and Anxa2 knockdown cells were seeded into 96-well plates at a density of 2 × 103 cells per well. At each time point, 20 µL of MTT solution (5 mg/mL) in PBS was added into each well and the cells were stained for 4 h at 37 °C. The supernatant was then aspirated carefully. The formazan in the plate was dissolved by adding 200 µL of DMSO. Absorbance was determined at 570 nm on a micro-ELISA reader, with a reference wavelength at 630 nm. The assays were performed using five replicates for each time point and repeated three times.
Colony formation assay
Control and Anxa2 knockdown cells were seeded in 6 cm dishes at a density of 1000 cells per dish and then cultured for 14 days. The medium was removed. The cells were washed three times with PBS, fixed with methanol for 10 min, and stained with 0.5 % crystal violet solution at room temperature for 2 h. The number of colonies containing more than 50 cells was counted. The assay was performed in triplicate and repeated three times.
Cell cycle assay
Cell cycle assay was performed through flow cytometry. Briefly, control and Anxa2 knockdown cells were cultured to 70–80 % confluence. The cells were trypsinized, collected, and centrifuged at 1000 rpm for 5 min, washed three times with PBS, and fixed with ice-cold 70 % ethanol at 4 °C overnight. The cells were washed with ice-cold PBS three times and stained with 500 µL of propidium iodide (50 µg/mL) containing 1 µg/mL RNase at 37 °C for 30 min. Flow cytometric analysis was performed on a Beckman Coulter EPICS analyzer, and cell cycle phase distribution was analyzed with the MultiCycle AV software. The assay was performed in triplicate and repeated three times.
EdU incorporation assay
EdU staining was performed with a Click-iT EdU imaging kit with Alexa Fluor-488 according to the manufacturer’s protocol. Briefly, control and Anxa2 knockdown cells were seeded on coverslips and cultured for 24 h. The cells were incubated with EdU solution at a final concentration of 10 µM for 12 h at 37 °C, washed three times with PBS, and fixed with 4 % PFA/PBS for 10 min at room temperature. The cells were washed with PBS and permeabilized with 0.5 % Triton X100 in PBS for 15 min at room temperature. Subsequently, the cells were incubated with Click-iT reaction cocktail for 30 min at room temperature. Nuclear staining was performed with DAPI, coverslips were mounted, and EdU-positive staining cells were visualized and counted with an inverted fluorescence microscope. The assay was performed in triplicate and repeated three times.
Statistical analysis
All data are presented as mean ± SD. Statistical analyses were performed using the Graphpad Prim 6.00 software. Differences between groups were analyzed by one-way or two-way ANOVA. P values less than 0.05 (two-tailed) were considered as statistically significant. The asterisk means the P value is less than 0.05.
Results
Knockdown of Anxa2 inhibits breast cancer cell proliferation
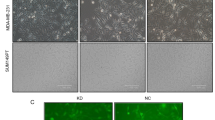
We previously reported that upregulation of Anxa2 in breast cancer cells enhances cell proliferation [51]. In the present study, we investigated the effect of Anxa2 silencing on the proliferation rate of breast cancer cells. As shown in Fig. 1a, b, the expression levels of Anxa2 in the two breast cancer cell lines significantly decreased after infection with lentivirus expressing two specific shRNAs targeting Anxa2 compared with wild type cells and cells infected with virus expressing control shRNA. The results of MTT based cell proliferation assay showed that proliferation of Anxa2 knockdown cells was inhibited compared with that of control or parental cancer cells (Fig. 1c, d). Similarly, cell colony formation was reduced after deletion of Anxa2 (Fig. 1e, f). These results suggest that Anxa2 may play an essential role in cell proliferation of breast cancer cells in vitro.
Knockdown of Anxa2 expression inhibits cell proliferation ability in human breast cancer cells. a and b Western blotting analysis of Anxa2 expression in T47D and MDA-MB-231 breast cancer cells infected with control or shAnxa2-expressing lentivirus. The numbers below the blots show the relative values from the densitometric analysis, normalized to β-actin, expressed as fold change over control. c and d The MTT assay showed that proliferation of Anxa2 knockdown cells was inhibited compared with that of control or parental cancer cells. Cells (2 × 103) were seeded in 96-well plates, at each time point, 20 µL of MTT solution (5 mg/mL) in PBS was added into each well and the cells were stained for 4 h at 37 °C. The formazan in the plate was dissolved by adding 200 µL of DMSO, and the absorbance was determined at 570 nm on a micro-ELISA reader. The assays were performed using five replicates for each time point and repeated three times. Statistical analysis was performed by a two-way ANOVA. *P < 0.05 compared to the control. e and f Cell colony formation was reduced after deletion of Anxa2 in T47D and MDA-MB-231 cells. Control and Anxa2 knockdown cells were seeded in 60 mm dishes at a density of 1000 cells per dish and cultured for 14 days. The number of colonies was quantified under an inverted microscope, and only colonies of more than 50 cells were counted. The assays were performed in triplicate for each group and repeated three times. Statistical analysis was performed by a one-way ANOVA followed by Tukey’s multiple comparison test. *P < 0.05 compared to the control
Knockdown of Anxa2 affects cell cycle distribution
As cell cycle progression is critical for cell proliferation, we assessed cell cycle distribution of the two breast cancer cell lines after knockdown of Anxa2 through flow cytometry assay. The number of Anxa2-silenced T47D and MDA-MB-231 cells significantly increased in the G0/G1 phase and decreased in the S phase compared with control cells (Fig. 2a, b). EdU incorporation assay was conducted to determine the effect of Anxa2 knockdown on cell proliferation. Consistent with the results obtained through flow cytometry assay, silencing of Anxa2 decreased the percentage of cells that incorporated EdU (Fig. 2c), thereby indicating a reduction of cell number in the S phase. Collectively, these results suggest that Anxa2 may play an important role in G1-to-S phase transition in breast cancer cells.
Knockdown of Anxa2 retards cell cycle distribution by downregulating cyclin D1 expression. a and b Knockdown of Anxa2 expression in T47D and MDA-MB-231 cells induced an increase in the proportion of the G0/G1 phase and a decrease in the proportion of the S phase compared with control cells. The experiments were performed in triplicates and repeated three times. c Silencing of Anxa2 in T47D and MDA-MB-231 cells decreased the percentage of cells that incorporated EdU. Control and Anxa2 knockdown cells were seeded on coverslips and incubated with EdU solution at a final concentration of 10 µM for 12 h at 37 °C, and then the cells were fixed, permeabilized, incubated with Click-iT reaction cocktail for 30 min at room temperature. Subsequently, the cells were stained with DAPI, and EdU-positive staining cells were visualized and counted with an inverted fluorescence microscope. The experiments were performed in triplicates and repeated three times. d Western blotting analysis of cyclin D1 and cyclin E expression in control and Anxa2-knockdown breast cancer cells. The numbers below the blots show the relative values from the densitometric analysis, normalized to β-actin, expressed as fold change over control. e Quantitative PCR analysis of expression of cyclin D1 mRNA in control and Anxa2-knockdown breast cancer cells. The experiments were performed using four replicates for each group and repeated three times. All statistical analysis was performed by a one-way ANOVA followed by Tukey’s multiple comparison test. *P < 0.05 compared to the control
Knockdown of Anxa2 reduces Cyclin D1 abundance
Cyclin-CDK complexes control cell cycle progression. Cyclin D1 and cyclin E are key regulators for G1-to-S-phase transition because they interact with Cdk4/6 to mediate Cdk4/6 activation by forming complexes. We assessed the effects of Anxa2 knockdown on cyclin D1 abundance. Compared with that of control cells, the expression level of the cyclin D1 protein decreased after downregulation of Anxa2 in breast cancer cells (Fig. 2d). However, Anxa2 knockdown did not affect cyclin E expression, which also plays a critical role in G1-to-S-phase transition. To investigate whether the decreased expression level of the cyclin D1 protein in Anxa2 knockdown cells is attributed to the reduced transcription of cyclin D1 messenger RNA (mRNA), we determined the expression of cyclin D1 mRNA in Anxa2 stable knockdown cells through real-time PCR. As shown in Fig. 2e, the expression of cyclin D1 mRNA also reduced after Anxa2 downregulation in human breast cancer cells.
Knockdown of Anxa2 does not affect the Akt/S6K1 pathway
Previous studies demonstrated that cyclin D1 abundance is also regulated at the translational level, and activation of Akt signaling to the S6 K pathway is responsible for the synthesis of the cyclin D1 protein [14, 33]. As such, we performed Western blotting assay to determine the effect of Anxa2 knockdown on the phosphorylation of Akt and the downstream mediator S6 K. As shown in Fig. 3a, b, downregulation of Anxa2 did not significantly affect EGF-induced phosphorylation of Akt and S6 K compared with that in control cells. These results suggest that the reduced levels of cyclin D1 in Anxa2 knockdown cells are not attributed to translational repression.
Knockdown of Anxa2 has no significant effect on Akt/S6K1 and MAPK pathway. a and b Western blotting analysis of expression of phosphorylation of Akt and S6 K in control and Anxa2 knockdown breast cancer cells. Control and Anxa2-knockdown breast cancer cells were grown to 80–90 % confluence, starved for 12 h, and stimulated with EGF for 0, 5, 15, and 30 min, then total cellular protein were extracted and subjected to Western blotting analysis. The numbers below the blots show the relative values from the densitometric analysis. The levels of p-Akt and p-S6 K were normalized to the corresponding total Akt and S6 K, values are expressed as fold changes compared to the zero-time control. c and d Western blotting analysis of expression of phosphorylation of Erk1/2 and p38 in control and Anxa2 knockdown breast cancer cells. The numbers below the blots show the relative values from the densitometric analysis. The levels of p-Erk1/2 and p-p38 were normalized to the corresponding total Erk1/2 and p38, values are expressed as fold changes compared to the zero-time control
Knockdown of Anxa2 has varying effects on the MAPK pathway in two breast cancer cell lines
Activation of MAPK signaling regulates transcription of cyclin D1 mRNA in several cell lines [1, 23, 43]. Two recent studies have demonstrated that Anxa2 may be involved in MAPK signaling [5, 51]. In this regard, we determined the potential role of Anxa2 in MAPK activation. As shown in Fig. 3d, the expression levels of phospho-Erk1/2 decreased by approximately 20–30 % in Anxa2 knockdown MDA-MB-231 cells compared with that in control cells; moreover, the expression levels of phospho-p38 in Anxa2-silenced MDA-MB-231 cells did not change (Fig. 3d). Our results also showed that Anxa2 knockdown does not significantly affect Erk1/2 and p38 MAPK phosphorylation in T47D cells (Fig. 3c). These data imply that Anxa2 expression may exhibit varied effects on MAPK activation in breast cancer cells.
Knockdown of Anxa2 inhibits STAT3 phosphorylation
Several studies have shown that cyclin D1 is transcriptionally regulated by STAT3 signaling in breast cancer cells [19, 25, 37]. Given that STAT3 activation is dependent on tyrosine phosphorylation at Y705 in response to various cytokines or growth factors [4], we investigated the effect of Anxa2 knockdown on EGF-induced tyrosine phosphorylation of STAT3. As shown in Fig. 4a, b, downregulation of Anxa2 inhibited EGF-induced phosphorylation of STAT3, and thus inactivated STAT3 in Anxa2-depleted cancer cells. As previous studies also demonstrated that IL-6 is a potent STAT3 activator and a growth factor for breast cancer cells [36], we assessed the involvement of Anxa2 in IL6-induced STAT3 activation. Consistently, IL-6-induced phosphorylation of STAT3 was also inhibited in Anxa2 knockdown cells (Fig. 4c, d). These findings suggest that Anxa2 downregulation in breast cancer cells results in defects of STAT3 activation.
Knockdown of Anxa2 inhibits STAT3 phosphorylation in breast cancer cells. a and b Western blotting analysis of EGF-induced tyrosine phosphorylation of STAT3 at Tyr 705 site in control and Anxa2 knockdown breast cancer cells. Control and Anxa2-knockdown breast cancer cells were starved for 12 h, and stimulated with EGF for 0, 5, 15, and 30 min, then total cellular protein were extracted and subjected to Western blotting analysis. The numbers below the blots show the relative values from the densitometric analysis. The levels of p-STAT3 were normalized to the corresponding total STAT3, values are expressed as fold changes compared to the zero-time control. c and d Western blotting analysis of IL-6-induced phosphorylation of STAT3 at Tyr 705 site in control and Anxa2 knockdown breast cancer cells. The numbers below the blots show the relative values from the densitometric analysis. The levels of p-STAT3 were normalized to the corresponding total STAT3, values are expressed as fold changes compared to the zero-time control
Knockdown of STAT3 significantly decreases the expression levels of cyclin D1 mRNA in breast cancer cells
To determine whether STAT3 regulates cyclin D1 expression and breast cancer cell proliferation, we stably downregulated the expression levels of STAT3 in T47D and MDA-MB-231 cells through lentivirus-mediated shRNAs. As shown in Fig. 5a, two different shRNA sequences targeting STAT3 effectively silenced STAT3 expression in the two breast cancer cell lines. Western blotting analysis results further showed that the expression levels of the cyclin D1 protein in STAT3-silenced cells were significantly lower than those in control cells (Fig. 5a). Consistently, the results from real-time RT-PCR also showed a notable decrease in cyclin D1 mRNA expression after STAT3 depletion (Fig. 5b). We then determined the effect of STAT3 knockdown on breast cancer proliferation. MTT assay results showed that the cell proliferation rate significantly decreased in STAT3-silenced T47D and MDA-MB-231 cells compared with that in control cells (Fig. 5c). Similarly, the reduction in STAT3 expression in the two cell lines inhibited cell colony formation (Fig. 5d), which indicates that STAT3 plays a critical role in breast cancer proliferation. Taken together, these results support the critical role of STAT3 in controlling breast cancer proliferation by regulating cyclin D1 expression.
Knockdown of STAT3 significantly inhibits cell proliferation and decreases the expression levels of cyclin D1 mRNA in breast cancer cells. a Western blotting analysis of cyclin D1 and STAT3 expression in T47D and MDA-MB-231 breast cancer cells infected with control or shSTAT3-expressing lentivirus. The numbers below the blots show the relative values from the densitometric analysis, normalized to β-actin, expressed as fold change over control. b Quantitative PCR analysis of expression of cyclin D1 mRNA in control and STAT3-knockdown breast cancer cells. The experiments were performed using four replicates for each group and repeated three times. c The MTT assay showed that proliferation of STAT3 knockdown cells was inhibited compared with that of control cells. The assays were performed using five replicates for each time point and repeated three times. Statistical analysis was performed by a two-way ANOVA. *P < 0.05 compared to the control. d Cell colony formation was reduced after deletion of STAT3 in T47D and MDA-MB-231 cells. The experiments were performed in triplicate, and repeated three times. Statistical analysis was performed by a one-way ANOVA followed by Tukey’s multiple comparison test. *P < 0.05 compared to the control
Inhibition of Erk1/2 slightly decreases the expression levels of cyclin D1 in breast cancer cells
To clarify whether the attenuated Erk1/2 phosphorylation after Anxa2 knockdown reduces cyclin D1 expression in MDA-MB-231 cells, we examined the inhibitory effects of PD98059, a potent and specific inhibitor of Erk1/2 kinases, on MDA-MB-231 cells. As shown in Fig. 6a, pretreatment with PD98059 significantly inhibited phosphorylation of Erk1/2 in a dose-dependent manner, which indicates the inhibition of Erk1/2 activation. In cells treated with a high concentration of PD98059 (20 μM), which resulted in more than 90 % inhibition of Erk1/2 phosphorylation, the expression levels of the cyclin D1 protein decreased by approximately 40 %, whereas the levels of cyclin D1 mRNA reduced by approximately 20 % compared with those in control cells. However, in cells treated with a low concentration of PD98059 (5 μM), which blocked Erk1/2 phosphorylation by approximately 60 %, the levels of the cyclin D1 protein marginally decreased by 10 %, whereas the expression levels of cyclin D1 mRNA did not change (Fig. 6a). These data indicate that activation of Erk1/2 signaling is partially responsible for cyclin D1 expression. Given that knockdown of Anxa2 only resulted in approximately 20–30 % inhibition of Erk1/2 phosphorylation in MDA-MB-231 cells (Fig. 3d), our results demonstrated that the partial inhibition of Erk1/2 phosphorylation may not be the main factor responsible for the reduction in cyclin D1 abundance in Anxa2 knockdown cells.
Inhibition of Erk1/2 slightly decreases the expression levels of cyclin D1 in breast cancer cells. a Inhibition of Erk1/2 partially decreases the expression levels of cyclin D1 protein in MDA-MB-231 cells. Western blotting analysis of expression of cyclin D1 and Erk1/2 phosphorylation in control and cells treated with different doses of PD98059, an Erk1/2 kinases inhibitor. The numbers below the blots show the relative values from the densitometric analysis. The levels of p-Erk1/2 were normalized to the corresponding total Erk1/2, the levels of cyclin D1 were normalized to β-actin, values are expressed as fold change over the untreated control. b Quantitative PCR analysis of expression of cyclin D1 mRNA in control and cells treated with different doses of PD98059. The experiments were performed using four replicates for each group and repeated three times. Statistical analysis was performed by a one-way ANOVA followed by Tukey’s multiple comparison test. *P < 0.05 compared to the untreated control
Discussion
The deregulated expression of Anxa2 has been reported in many types of tumors, including breast cancer, and high expression of Anxa2 is correlated with cancer progression and poor prognosis of cancer patients [27, 48, 60]. Accumulated evidences showed that Anxa2 is a critical regulator in promoting angiogenesis, cancer cell proliferation, cell cycle progression, migration, invasion, and metastasis [5, 50, 51, 59, 61–64]. Nevertheless, the detailed molecular mechanisms involved in these processes remain elusive. In the present study, we demonstrated that Anxa2 silencing inhibited cell proliferation by retarding the G1-S phase transition through downregulation of cyclin D1 expression in a STAT3-dependent manner. We presented evidence that Anxa2 regulates EGF and IL-6-induced phosphorylation of STAT3, and inactivation of STAT3 is the major factor that affects the reduction in cyclin D1 level in Anxa2 knockdown cells. Overall, this study provides novel insights into the functions of Anxa2 as a critical molecule in cellular signal transduction. Our findings also significantly improve our understanding of the mechanism through which Anxa2 regulates cancer cell proliferation.
Two recent studies have shown that blocking the function of Anxa2 through neutralizing antibodies can inhibit breast cancer cell proliferation in vitro and breast tumor growth in vivo [5, 39]. In agreement with these observations, our results showed that silencing Anxa2 in breast cancer cells inhibited cell proliferation as evidenced through the results of MTT and colony formation assays (Fig. 1). These results indicate a critical function of Anxa2 in promoting cancer cell proliferation. Nevertheless, minimal information is known regarding the precise molecular mechanism through which Anxa2 regulates cancer cell proliferation. In this paper, we showed that Anxa2 depletion reduced cell proliferation by retarding cell cycle at the G1 phase as examined through flow cytometry and EdU incorporation assay (Fig. 2a–c). Cyclin D1 and cyclin E are critical regulators for G1-to-S-phase transition. We showed that the blockage of cell cycle progression in Anxa2 knockdown cells was attributed to the reduction in cyclin D1 abundance without affecting the expression level of cyclin E (Fig. 2d). The deregulated expression of cyclin D1 has been proposed to mediate mammary tumorigenesis [49, 55]. Intensive studies have also shown that elevated expression of cyclin D1 frequently occurs in human breast cancer and is correlated with disease aggravation [30, 46]. Thus, our finding that Anxa2 regulates cyclin D1 abundance provides new evidence that Anxa2 is associated with breast cancer progression.
The abundance of cyclin D1 are regulated through several complex mechanisms, including transcription, translation, and posttranslational modifications [30]. Translation of cyclin D1 mRNA is regulated by mTORC1/S6K1 signaling downstream of the protein kinase Akt [14, 15]. A recent study reported that Anxa2 knockdown by shRNA enhanced EGF-induced phosphorylation of Akt [7]. By contrast, another two recent studies have shown that functional inhibition of Anxa2 with antibodies or siRNA inhibited EGF-induced AKT pathway in breast cancer cells [5, 42]. These contradicting results suggest that defining the accurate role of Anxa2 in regulating Akt signaling is urgently needed. Additionally, the information about the effect of Anxa2 silencing on S6k1 phosphorylation is limited. Thus, we investigated the effect of Anxa2 silencing on the Akt/S6K1 pathway in our established models. The results demonstrated that knockdown of Anxa2 expression with shRNA did not affect EGF-induced Akt phosphorylation at threonine 308 site in human breast cancer cells (Fig. 3a, b). The phosphorylation of S6K1, which functions downstream of Akt, was not affected in Anxa2-silenced cells. Hence, Akt/mTORC1/S6K1 pathway is not affected in Anxa2-silenced breast cancer cells. Given that Akt/mTORC1/S6 K signaling is required for translation of cyclin D1 mRNA [14, 15], we revealed that the reduction in the cyclin D1 protein in Anxa2-depleted cells may be attributed to the decrease in transcription of its mRNA. Consistent with this hypothesis, our data showed that the expression of cyclin D1 mRNA significantly decreased in Anxa2-silenced breast cancer cells (Fig. 2e).
Transcription of cyclin D1 mRNA has been reported to be induced by growth factor-mediated signaling cascades that involve the Ras-Raf-MAPK pathway [22]. As previous studies demonstrated that Anxa2 may participate in MAPK signal transduction [5, 7, 42, 51]; we speculate that Anxa2 may regulate cyclin D1 transcription by influencing MAPK activation. However, results from Fig. 3c revealed that Anxa2 deletion in T47D cells did not significantly affect EGF-induced Erk1/2 and p38 MAPK phosphorylation. Moreover, in Anxa2-silenced MDA-MB-231 cells, Erk1/2 phosphorylation was minimally reduced, whereas, p38 MAPK phosphorylation remained unchanged (Fig. 3d). These data indicate that the reduced transcription of cyclin D1 mRNA in Anxa2-silenced cells may not be attributed to the slight inhibition of Erk1/2 signaling. Accordingly, the expression levels of cyclin D1 mRNA in cells treated with low doses of Erk1/2 inhibitor, which slightly inhibited Erk1/2 phosphorylation, did not evidently changed compared with those in control cells (Fig. 6b). Moreover, the phenomenon, in which high doses of inhibitor markedly reduced cyclin D1 protein expression compared with mRNA in MDA-MB-231 cells, may be explained by a recent finding that Erk1/2 signaling plays a role in cyclin D1 translation regulation [33]. Taken together, our results suggest that the partial inhibition of Erk1/2 phosphorylation by Anxa2 knockdown may not be the main factor responsible for the reduction of cyclin D1 abundance in breast cancer cells.
In addition to Erk1/2 signaling, cyclin D1 mRNA transcription is also regulated by STAT3 in several types of carcinomas, particularly in breast cancer [19, 25]. As a transcription factor, STAT3 is phosphorylated by either receptor tyrosine kinase, such as EGFR, or nonreceptor tyrosine kinase, such as Src and Jak2. Phosphorylation leads to STAT3 dimerization, nuclear translocation and then activation. We previously reported that Anxa2 is a binding protein of STAT3 in breast cancer cells [50]. In this paper, we showed that silencing of Anxa2 inhibited EGF- or IL-6-induced tyrosine phosphorylation of STAT3, indicating that Anxa2 may mediate STAT3 activation in breast cancer cells. Consistent with this result, a recent study has shown that knockdown of Anxa2 with siRNA inhibited EGF-induced STAT3 phosphorylation in breast cancer cells [42]. However, the detailed function of STAT3 suppression in Anxa2-silenced cells remains unknown. In the present study, we further demonstrated that depletion of STAT3 through shRNA significantly reduced the expression levels of the cyclin D1 protein, as well as the levels of its mRNA. Consistently, the reduced STAT3 expression in the two cell lines inhibited cell proliferation. Taken together, these results support a notion that STAT3 is responsible for breast cancer proliferation by regulating the expression of cyclin D1 in Anxa2-silenced cells.
In summary, our study revealed that Anxa2 significantly functions in breast cancer proliferation by regulating cyclin D1 expression at the transcriptional level in a STAT3-dependent manner. Activated STAT3 and elevated levels of Anxa2 are frequently observed in a large number of human malignancies, particularly in breast cancer, and are correlated with tumor progression [6, 18, 24, 40–42, 54]. Thus, our findings reveal the direct relationship between Anxa2 and STAT3 activation and the important role of Anxa2 in tumorigenesis as evidenced by its involvement in key cellular functions, such as cell cycle and cell proliferation.
References
Balmanno K, Cook SJ (1999) Sustained MAP kinase activation is required for the expression of cyclin D1, p21Cip1 and a subset of AP-1 proteins in CCL39 cells. Oncogene 18:3085–3097. doi:10.1038/sj.onc.1202647
Bao H, Jiang M, Zhu M, Sheng F, Ruan J, Ruan C (2009) Overexpression of Annexin II affects the proliferation, apoptosis, invasion and production of proangiogenic factors in multiple myeloma. Int J Hematol 90:177–185. doi:10.1007/s12185-009-0356-8
Bharadwaj A, Bydoun M, Holloway R, Waisman D (2013) Annexin A2 heterotetramer: structure and function. Int J Mol Sci 14:6259–6305. doi:10.3390/ijms14036259
Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE Jr (1999) Stat3 as an oncogene. Cell 98:295–303. doi:10.1016/S0092-8674(00)81959-5
Chaudhary P, Thamake SI, Shetty P, Vishwanatha JK (2014) Inhibition of triple-negative and Herceptin-resistant breast cancer cell proliferation and migration by Annexin A2 antibodies. Br J Cancer 111:2328–2341. doi:10.1038/bjc.2014.542
Chuthapisith S, Bean BE, Cowley G, Eremin JM, Samphao S, Layfield R, Kerr ID, Wiseman J, El-Sheemy M, Sreenivasan T, Eremin O (2009) Annexins in human breast cancer: possible predictors of pathological response to neoadjuvant chemotherapy. Eur J Cancer 45:1274–1281. doi:10.1016/j.ejca.2013.12.002
De Graauw M, Cao L, Winkel L, van Miltenburg MH, le Devedec SE, Klop M, Yan K, Pont C, Rogkoti VM, Tijsma A, Chaudhuri A, Lalai R, Price L, Verbeek F, van de Water B (2013) Annexin A2 depletion delays EGFR endocytic trafficking via cofilin activation and enhances EGFR signaling and metastasis formation. Oncogene. doi:10.1038/onc.2013.219
Duncan R, Carpenter B, Main LC, Telfer C, Murray GI (2008) Characterisation and protein expression profiling of annexins in colorectal cancer. Br J Cancer 98:426–433
Emoto K, Sawada H, Yamada Y, Fujimoto H, Takahama Y, Ueno M, Takayama T, Uchida H, Kamada K, Naito A, Hirao S, Nakajima Y (2001) Annexin II overexpression is correlated with poor prognosis in human gastric carcinoma. Anticancer Res 21:1339–1345
Emoto K, Yamada Y, Sawada H, Fujimoto H, Ueno M, Takayama T, Kamada K, Naito A, Hirao S, Nakajima Y (2001) Annexin II overexpression correlates with stromal tenascin-C overexpression: a prognostic marker in colorectal carcinoma. Cancer 92:1419–1426. doi:10.1002/1097-0142
Esposito I, Penzel R, Chaib-Harrireche M, Barcena U, Bergmann F, Riedl S, Kayed H, Giese N, Kleeff J, Friess H, Schirmacher P (2006) Tenascin C and annexin II expression in the process of pancreatic carcinogenesis. J Pathol 208:673–685. doi:10.1002/path.1935
Filipenko NR, MacLeod TJ, Yoon CS, Waisman DM (2004) Annexin A2 is a novel RNA-binding protein. J Biol Chem 279:8723–8731. doi:10.1074/jbc.M311951200
Gao H, Yu B, Yan Y, Shen J, Zhao S, Zhu J, Qin W, Gao Y (2013) Correlation of expression levels of ANXA2, PGAM1, and CALR with glioma grade and prognosis. J Neurosurg 118:846–853. doi:10.3171/2012.9.JNS112134
Gao N, Flynn DC, Zhang Z, Zhong XS, Walker V, Liu KJ, Shi X, Jiang BH (2004) G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3 K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am J Physiol Cell Physiol 287:C281–C291. doi:10.1152/ajpcell.00422.2003
Gao N, Zhang Z, Jiang BH, Shi X (2003) Role of PI3 K/AKT/mTOR signaling in the cell cycle progression of human prostate cancer. Biochem Biophys Res Commun 310:1124–1132
Gerke V, Weber K (1984) Identity of p36 K phosphorylated upon Rous sarcoma virus transformation with a protein purified from brush borders; calcium-dependent binding to non-erythroid spectrin and F-actin. EMBO J 3:227–233
Glenney JR Jr, Tack BF (1985) Amino-terminal sequence of p36 and associated p10: identification of the site of tyrosine phosphorylation and homology with S-100. Proc Natl Acad Sci USA 82:7884–7888
Hsieh FC, Cheng G, Lin J (2005) Evaluation of potential Stat3-regulated genes in human breast cancer. Biochem Biophys Res Commun 335:292–299. doi:10.1016/j.bbrc.2005.07.075
Ishii Y, Waxman S, Germain D (2008) Tamoxifen stimulates the growth of cyclin D1-overexpressing breast cancer cells by promoting the activation of signal transducer and activator of transcription 3. Cancer Res 68:852–860. doi:10.1158/0008-5472.CAN-07-2879
Jin L, Shen Q, Ding S, Jiang W, Jiang L, Zhu X (2012) Immunohistochemical expression of Annexin A2 and S100A proteins in patients with bulky stage IB-IIA cervical cancer treated with neoadjuvant chemotherapy. Gynecol Oncol 126:140–146. doi:10.1016/j.ygyno.2012.04.005
Kagawa S, Takano S, Yoshitomi H, Kimura F, Satoh M, Shimizu H, Yoshidome H, Ohtsuka M, Kato A, Furukawa K, Matsushita K, Nomura F, Miyazaki M (2012) Akt/mTOR signaling pathway is crucial for gemcitabine resistance induced by Annexin II in pancreatic cancer cells. J Surg Res 178:758–767. doi:10.1016/j.jss.2012.05.065
Klein EA, Assoian RK (2008) Transcriptional regulation of the cyclin D1 gene at a glance. J Cell Sci 121:3853–3857. doi:10.1242/jcs.039131
Lavoie JN, L’Allemain G, Brunet A, Muller R, Pouyssegur J (1996) Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem 271:20608–20616
Leslie K, Gao SP, Berishaj M, Podsypanina K, Ho H, Ivashkiv L, Bromberg J (2010) Differential interleukin-6/Stat3 signaling as a function of cellular context mediates Ras-induced transformation. Breast Cancer Res 12:R80. doi:10.1186/bcr2725
Leslie K, Lang C, Devgan G, Azare J, Berishaj M, Gerald W, Kim YB, Paz K, Darnell JE, Albanese C, Sakamaki T, Pestell R, Bromberg J (2006) Cyclin D1 is transcriptionally regulated by and required for transformation by activated signal transducer and activator of transcription 3. Cancer Res 66:2544–2552. doi:10.1158/0008-5472.CAN-05-2203
Ling Q, Jacovina AT, Deora A, Febbraio M, Simantov R, Silverstein RL, Hempstead B, Mark WH, Hajjar KA (2004) Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. J Clin Invest 113:38–48. doi:10.1172/JCI19684
Lokman NA, Ween MP, Oehler MK, Ricciardelli C (2011) The role of annexin A2 in tumorigenesis and cancer progression. Cancer Microenviron 4:199–208. doi:10.1007/s12307-011-0064-9
Luo CH, Liu QQ, Zhang PF, Li MY, Chen ZC, Liu YF (2013) Prognostic significance of annexin II expression in non-small cell lung cancer. Clin Trans Oncol 15:938–946. doi:10.1007/s12094-013-1028-y
Mohammad HS, Kurokohchi K, Yoneyama H, Tokuda M, Morishita A, Jian G, Shi L, Murota M, Tani J, Kato K, Miyoshi H, Deguchi A, Himoto T, Usuki H, Wakabayashi H, Izuishi K, Suzuki Y, Iwama H, Deguchi K, Uchida N, Sabet EA, Arafa UA, Hassan AT, El-Sayed AA, Masaki T (2008) Annexin A2 expression and phosphorylation are up-regulated in hepatocellular carcinoma. Int J Oncol 33:1157–1163
Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL (2011) Cyclin D as a therapeutic target in cancer. Nat Rev Cancer 11:558–572. doi:10.1038/nrc3090
Musgrove EA, Lee CS, Buckley MF, Sutherland RL (1994) Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle. Proc Natl Acad Sci USA 91:8022–8026
Ohno Y, Izumi M, Kawamura T, Nishimura T, Mukai K, Tachibana M (2009) Annexin II represents metastatic potential in clear-cell renal cell carcinoma. Br J Cancer 101:287–294
Parrales A, Lopez E, Lee-Rivera I, Lopez-Colome AM (2013) ERK1/2-dependent activation of mTOR/mTORC1/p70S6 K regulates thrombin-induced RPE cell proliferation. Cell Signal 25:829–838. doi:10.1016/j.cellsig.2012.12.023
Quelle DE, Ashmun RA, Shurtleff SA, Kato JY, Bar-Sagi D, Roussel MF, Sherr CJ (1993) Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev 7:1559–1571
Rescher U, Ludwig C, Konietzko V, Kharitonenkov A, Gerke V (2008) Tyrosine phosphorylation of annexin A2 regulates Rho-mediated actin rearrangement and cell adhesion. J Cell Sci 121:2177–2185
Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM (2007) Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J 21:3763–3770. doi:10.1096/fj.07-8832com
Saxena NK, Vertino PM, Anania FA, Sharma D (2007) leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to CYCLIN D1 promoter via activation of Stat3. J Biol Chem 282:13316–13325. doi:10.1074/jbc.M609798200
Semov A, Moreno MJ, Onichtchenko A, Abulrob A, Ball M, Ekiel I, Pietrzynski G, Stanimirovic D, Alakhov V (2005) Metastasis-associated protein S100A4 induces angiogenesis through interaction with Annexin II and accelerated plasmin formation. J Biol Chem 280:20833–20841. doi:10.1074/jbc.M412653200
Sharma M, Blackman MR, Sharma MC (2012) Antibody-directed neutralization of annexin II (ANX II) inhibits neoangiogenesis and human breast tumor growth in a xenograft model. Exp Mol Pathol 92:175–184. doi:10.1016/j.yexmp.2011.10.003
Sharma M, Ownbey RT, Sharma MC (2010) Breast cancer cell surface annexin II induces cell migration and neoangiogenesis via tPA dependent plasmin generation. Exp Mol Pathol 88:278–286
Sharma MR, Koltowski L, Ownbey RT, Tuszynski GP, Sharma MC (2006) Angiogenesis-associated protein annexin II in breast cancer: selective expression in invasive breast cancer and contribution to tumor invasion and progression. Exp Mol Pathol 81:146–156
Shetty PK, Thamake SI, Biswas S, Johansson SL, Vishwanatha JK (2012) Reciprocal regulation of annexin A2 and EGFR with Her-2 in Her-2 negative and herceptin-resistant breast cancer. PLoS One 7:e44299. doi:10.1371/journal.pone.0044299
Shi Y, Sharma A, Wu H, Lichtenstein A, Gera J (2005) Cyclin D1 and c-myc internal ribosome entry site (IRES)-dependent translation is regulated by AKT activity and enhanced by rapamycin through a p38 MAPK- and ERK-dependent pathway. J Biol Chem 280:10964–10973. doi:10.1074/jbc.M407874200
Shiozawa Y, Havens AM, Jung Y, Ziegler AM, Pedersen EA, Wang J, Lu G, Roodman GD, Loberg RD, Pienta KJ, Taichman RS (2008) Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem 105:370–380. doi:10.1002/jcb.21835
Takano S, Togawa A, Yoshitomi H, Shida T, Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Kato A, Tomonaga T, Nomura F, Miyazaki M (2008) Annexin II overexpression predicts rapid recurrence after surgery in pancreatic cancer patients undergoing gemcitabine-adjuvant chemotherapy. Ann Surg Oncol 15:3157–3168. doi:10.1245/s10434-008-0061-5
Velasco-Velazquez MA, Li Z, Casimiro M, Loro E, Homsi N, Pestell RG (2011) Examining the role of cyclin D1 in breast cancer. Future Oncol 7:753–765. doi:10.2217/fon.11.56
Wang CY, Chen CL, Tseng YL, Fang YT, Lin YS, Su WC, Chen CC, Chang KC, Wang YC, Lin CF (2012) Annexin A2 silencing induces G2 arrest of non-small cell lung cancer cells through p53-dependent and -independent mechanisms. J Biol Chem 287:32512–32524. doi:10.1074/jbc.M112.351957
Wang CY, Lin CF (2014) Annexin A2: its molecular regulation and cellular expression in cancer development. Dis Markers 2014:308976. doi:10.1155/2014/308976
Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV (1994) Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature 369:669–671. doi:10.1038/369669a0
Wang YQ, Zhang F, Tian R, Ji W, Zhou Y, Sun XM, Liu Y, Wang ZY, Niu RF (2012) Tyrosine 23 phosphorylation of Annexin A2 promotes proliferation, invasion, and Stat3 phosphorylation in the nucleus of human breast cancer SK-BR-3 Cells. Cancer Biol Med 9:248–253. doi:10.7497/j.issn.2095-3941.2012.04.005
Wu B, Zhang F, Yu M, Zhao P, Ji W, Zhang H, Han J, Niu R (2012) Up-regulation of Anxa2 gene promotes proliferation and invasion of breast cancer MCF-7 cells. Cell Prolif 45:189–198. doi:10.1111/j.1365-2184.2012.00820.x
Yao H, Zhang Z, Xiao Z, Chen Y, Li C, Zhang P, Li M, Liu Y, Guan Y, Yu Y, Chen Z (2009) Identification of metastasis associated proteins in human lung squamous carcinoma using two-dimensional difference gel electrophoresis and laser capture microdissection. Lung Cancer 65:41–48
Yu GR, Kim SH, Park SH, Cui XD, Xu DY, Yu HC, Cho BH, Yeom YI, Kim SS, Kim SB, Chu IS (2007) Kim DG (2007) Identification of molecular markers for the oncogenic differentiation of hepatocellular carcinoma. Exp Mol Med 39:641–652
Yu H, Pardoll D, Jove R (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9:798–809
Yu Q, Geng Y, Sicinski P (2001) Specific protection against breast cancers by cyclin D1 ablation. Nature 411:1017–1021. doi:10.1038/35082500
Zhang F, Liu Y, Wang Z, Sun X, Yuan J, Wang T, Tian R, Ji W, Yu M, Zhao Y, Niu R (2015) A novel Anxa2-interacting protein Ebp1 inhibits cancer proliferation and invasion by suppressing Anxa2 protein level. Mol Cell Endocrinol 411:75–85. doi:10.1016/j.mce.2015.04.013
Zhang F, Zhang H, Wang Z, Yu M, Tian R, Ji W, Yang Y, Niu R (2014) P-glycoprotein associates with Anxa2 and promotes invasion in multidrug resistant breast cancer cells. Biochem Pharmacol 87:292–302. doi:10.1016/j.bcp.2013.11.003
Zhang F, Zhang L, Zhang B, Wei X, Yang Y, Qi RZ, Ying G, Zhang N, Niu R (2009) Anxa2 plays a critical role in enhanced invasiveness of the multidrug resistant human breast cancer cells. J Proteome Res 8:5041–5047. doi:10.1021/pr900461c
Zhang W, Zhao P, Xu XL, Cai L, Song ZS, Cao DY, Tao KS, Zhou WP, Chen ZN, Dou KF (2013) Annexin A2 promotes the migration and invasion of human hepatocellular carcinoma cells in vitro by regulating the shedding of CD147-harboring microvesicles from tumor cells. PLoS One 8:e67268. doi:10.1371/journal.pone.0067268
Zhang X, Liu S, Guo C, Zong J, Sun MZ (2012) The association of annexin A2 and cancers. Clin Trans Oncol 14:634–640. doi:10.1007/s12094-012-0855-6
Zhao P, Zhang W, Tang J, Ma X, Dai J, Li Y, Jiang J, Zhang S, Chen Z (2010) Annexin II promotes invasion and migration of human hepatocellular carcinoma cells in vitro via its interaction with HAb18G/CD147. Cancer Sci 101:387–395
Zhao P, Zhang W, Wang SJ, Yu XL, Tang J, Huang W, Li Y, Cui HY, Guo YS, Tavernier J, Zhang SH, Jiang JL, Chen ZN (2011) HAb18G/CD147 promotes cell motility by regulating annexin II-activated RhoA and Rac1 signaling pathways in hepatocellular carcinoma cells. Hepatology 54:2012–2024. doi:10.1002/hep.24592
Zheng L, Foley K, Huang L, Leubner A, Mo G, Olino K, Edil BH, Mizuma M, Sharma R, Le DT, Anders RA, Illei PB, Van Eyk JE, Maitra A, Laheru D, Jaffee EM (2011) Tyrosine 23 phosphorylation-dependent cell-surface localization of annexin A2 is required for invasion and metastases of pancreatic cancer. PLoS One 6:e19390. doi:10.1371/journal.pone.0019390
Zhou S, Yi T, Liu R, Bian C, Qi X, He X, Wang K, Li J, Zhao X, Huang C, Wei Y (2012) Proteomics identification of annexin A2 as a key mediator in the metastasis and proangiogenesis of endometrial cells in human adenomyosis. Mol Cell Proteomics 11(M112):017988. doi:10.1074/mcp.M112.017988
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (Nos. 81372844, and 81472474), Tianjin Municipal Science and Technology Commission (Nos. 12JCZDJC24500 and 12JCQNJC07000), Changjiang Scholars and Innovative Research Team (IRT1076), 863 Project (2012AA020206-5), Specialized Research Fund for the Doctoral Program of Higher Education (20131202110002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Fei Zhang and Zhiyong Wang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, F., Wang, Z., Yuan, J. et al. RNAi-mediated silencing of Anxa2 inhibits breast cancer cell proliferation by downregulating cyclin D1 in STAT3-dependent pathway. Breast Cancer Res Treat 153, 263–275 (2015). https://doi.org/10.1007/s10549-015-3529-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3529-6