Abstract
Observational studies have suggested that metformin decreases the incidence of several common cancers. However, findings regarding breast cancer have been mixed. In order to explore this issue, a systematic literature review and meta-analysis were performed with a focus on potential biases. We conducted a comprehensive literature search for all pertinent studies addressing metformin use and breast cancer risk by searching PubMed, Cochrane Library, and Scopus (which includes Embase, ISI Web of Science) using the Mesh terms: “metformin” or “biguanides” or “diabetes mellitus, type 2/therapy” and “cancer” or “neoplasms.” When multiple hazard ratios (HR) or odds ratio (OR) were reported, the most adjusted estimate was used in the base-case analysis. We pooled the adjusted HR and performed sensitivity analyses on duration of metformin use (> or ≤3 years use), study quality (assessed using the GRADE system), and initial observation year of the cohort (before vs after 1997). From a total of 443 citations, 18 full-text articles were considered, and seven independent studies were included. All were observational (four cohort and three case control). Our combined OR of all seven studies was 0.83 (0.71–0.97). Stronger associations were found when analyses were limited to studies estimating the impact of longer metformin use (OR = 0.75. 95 % CI 0.62, 0.91) or among studies that began observing their cohort before 1997 (OR = 0.68. 95 % CI 0.55–0.084). Stratification according to study quality did not affect the combined OR but higher quality studies had smaller CI and achieved statistical significance. Interpretation is limited by the observational nature of reports and different comparison groups. Our analyses support a protective effect of metformin on breast cancer risk among postmenopausal women with diabetes. Clinical trials are needed for definitive determination of the role of metformin in breast cancer risk reduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Both obesity [1, 2] and diabetes have been associated with increased breast cancer risk with insulin as a potential mediating factor [3–5]. Metformin is a biguanide, increasingly used for type 2 diabetes management, that increases insulin sensitivity and improves glycemic control [6, 7]. In addition, preclinical studies have identified its properties suggestive of anti-cancer activity independent of insulin effects [8, 9]. Consequently, observational studies have examined associations between metformin use and cancer incidence.
An early meta-analysis of studies in men and women with diabetes found a 30 % lower incidence of all cancers associated with metformin use compared with other therapies for diabetes [10]. However, a meta-analysis of the three studies examining breast cancer and metformin [11–13] provided only suggestive, non-statistically significant evidence of an association with this disease [10]. As new studies have examined the metformin and breast cancer association, we searched the literature to identify pertinent reports addressing this issue and combined identified results in a meta-analysis.
Methods
We conducted a comprehensive literature search for all studies addressing the relationship between metformin use and breast cancer risk. Electronic databases searched include Pubmed, Cochrane Library, and Scopus (which includes Embase and ISI Web of Science). We used the Mesh terms: “metformin” or “biguanides” or “diabetes mellitus, type 2/therapy” and “cancer” or “neoplasms.” In addition, a manual search was completed for references of included studies and all studies reporting recently published meta-analyses on metformin and cancer risk [10, 14, 15].
With the first reports of metformin and breast cancer risk published in 2009, and a published meta-analysis covering the period January 1, 1966 to May 31, 2009, our electronic search focused on the period January 1, 2009 to May 31, 2012. There were no restrictions on language or type of article. Data extracted included information about the study design, study population, treatment exposure, minimum duration of use, comparison groups, year of cohort inception, number of breast cancer cases, variables considered in their analysis (confounders), and study quality [16]. Abstracts and papers were independently reviewed and abstracted by two coauthors (NC and LO).
Statistical analyses
When multiple hazard ratios (HR) or odds ratio (OR) were reported, the most adjusted estimate was used in the base-case analysis. When multiple durations of use were reported, we chose the longest duration of use. We pooled the adjusted HR using DerSimonian and Laird statistic. We performed sensitivity analyses on predetermined subgroups based on duration of metformin use (> or ≤3 years use), study quality, and initial observation year of the cohort (before vs after 1996).
Criteria for including a study in the analysis included: (1) published original observational study or clinical trial evaluating the impact of treatment with metformin as compared to a comparison group on the risk of incident breast cancer; (2) reported HR, OR, or relative risk (RR), and 95 % confidence intervals to estimate breast cancer risk for women with diabetes using metformin in relation to the identified comparison group, commonly women with diabetes using other drug therapy for diabetes. Studies were pooled and weighted according to inverse variance using random effects model of Dersimonian Laird.
All analyses were done using Meta XL Version 1.2.
Results
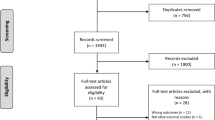
A total of 443 studies were identified and reviewed by title and abstract for inclusion (Fig. 1). Of these, 18 full-text articles were considered for inclusion, and seven met inclusion criteria. 11 were excluded: three were systematic reviews of metformin and cancer risk in diabetic patients [10, 14, 15]; six did not report HR on breast cancer incidence but reported instead on overall cancer incidence [17–22]; one did not disaggregate metformin from insulin treatment [23]; one addressed the impact of metformin on patients with breast cancer [24].
All the seven studies included in our analyses were observational. Four were cohort [11, 12, 25, 26] studies and three were case–control [27–29] studies. Three studies were conducted in the United Kingdom (UK) and one each in the US [26], Netherlands [29], Scotland [12], and Denmark [27]. The number of breast cancer cases included varied from 65 to 1,461.
All studies examined metformin use in comparison to other therapies for diabetes, but different comparators were used across studies. Four studies compared metformin to any other drug used for diabetes therapy, one compared metformin to sulfonylureas, and two contained multiple comparison groups (one compared metformin use to sulfonylurea, insulin, and a combination of metformin and sulfonylurea; the other compared metformin to thiazolidinediones, insulin, sulfonylurea, or any other treatment). Three studies did not require a minimum duration of metformin use to consider a metformin user. Five studies included only invasive breast cancers, one reported separate OR for invasive versus all types of breast cancer (including ductal carcinoma in situ), and only one further proved analyses by subtypes of breast cancer (hormone receptor status and HER2 status) (Table 1) [26].
All studies performed adjusted multivariate analyses, though the variables adjusted for differed across studies. Four adjusted for body mass index (BMI), four adjusted for age, four adjusted for smoking, three adjusted for A1c (average blood glucose), three adjusted for severity/duration of diabetes, three adjusted for use of menopausal hormone therapy, two adjusted for socioeconomic status, two adjusted for prior cancer or biopsy, and two adjusted for renal impairment. Three studies did dose–response analyses of duration of use.
Study quality was assessed using the GRADE system [16]. In the GRADE system, studies are categorized as high (4), moderate (3), low (2), or very low quality (1), with observational studies considered to be of low or very low quality. Ratings are affected by the risk of bias, consistency, directness, precision, publication bias, effect size, dose response, and handling of potential confounders. Studies were graded for quality independently by three coauthors (VS, NC, and LO). Any discrepancies were reviewed and discussed until consensus was achieved.
Of the seven studies, three were rated as low quality (the highest quality the GRADE system affords observational studies) and three as very low quality. The four studies rated as very low quality were given this rating due to their small number of breast cancer cases [12, 13], inadequate adjustment for critical confounders such as BMI [11, 29], or absence of dose–response relationship analyses [11, 29].
Six of seven studies reported an association between metformin use and lower breast cancer risk with respect to the comparison group and three reported that the association was statistically significant [13, 26, 29]. The combined OR of all seven studies for metformin use and breast cancer incidence was 0.83 (95 % CI, 0.71–0.97) (Fig. 2). We found weak evidence for heterogeneity among individual study findings (p for heterogeneity = 0.06). Most of the heterogeneity resulted from a single, relatively small, cross-sectional study [13]. Omission of this study from the meta-analysis substantially reduced the heterogeneity (p for heterogeneity 0.28) and slightly decreased the summary OR (0.90, 95 % CI 0.81–0.99).
Stronger associations were found when analyses were limited to studies with longer duration of metformin use (0.75 [95 % CI, 0.62, 0.91] vs 0.94 [95 % CI, 0.84, 1.06]) and for those who began MF before 1996 (0.68 [95 % CI, 0.55–0.084] vs after 1997 [95 % CI, 0.95 0.91–0.98]). Stratification according to quality did not affect the combined OR but higher quality studies had smaller confidence intervals (Table 2).
To assess the presence of publication bias, we created a funnel plot using the logarithm of the OR to ensure that positive and negative effects are equally spaced (Fig. 3). However, the small number of studies makes interpretation difficult and inconclusive.
Because some small negative studies that are most subject to publication bias might be missing, we excluded studies with less than 50 breast cancer cases exposed to metformin [12, 13] and obtained similar summary OR (0.93, 0.87–0.99; Q = 4.26, p = 0.37) of breast cancer incidence in women with diabetes using metformin in relation to the comparison group.
Discussion
This meta-analysis of published studies finds metformin use in women with diabetes associated with a lower invasive breast cancer incidence compared to incidence in women using other therapies for diabetes. While this finding, based on observational studies alone, could reflect confounding or bias, the stronger effect size seen in studies of longer duration metformin use suggest a real association.
Emerging clinical studies support a potential metformin influence on breast cancer [30]. Two pre-operative studies involving women with diabetes with invasive breast cancer have randomized participants to metformin or placebo [31, 32]. In one report, women assigned to metformin for 2 weeks had reduced Ki-67 [30], a measure of tumor proliferation [33], compared with non users. In a larger study, 200 women with breast cancer but without diabetes were randomly allocated to either metformin 850 mg twice per day (n = 100) or placebo (n = 100). Reduction in Ki-67 was seen only in women with insulin resistance (homeostasis model assessment [HOMA] index >2.8) [32]. In a retrospective analyses of neoadjuvant chemotherapy administered at MD Anderson Hospital, breast cancer patients with diabetes who were on metformin had a significantly higher frequency of complete response (24 %) compared to patients with diabetes who did not use metformin (8 %), as well as compared to patients without diabetes (16 %; p = 0.02) [34]. Finally, in a retrospective report, metformin use was associated with improved breast cancer-specific survival in diabetes patients [35]. Taken together, these proof-of-principal studies suggest that metformin may influence breast cancer proliferation.
The findings of this meta-analysis are limited by the observational nature of the study designs which preclude causal interpretation. Only one study [26] described the association of metformin use with the type and characteristics of breast cancers. These studies were conducted in different countries using different entry criteria and durations of metformin use. In addition, breast cancer incidence rates are influenced by frequency of mammography screening and only one study [26] controlled for this variable.
The mechanism by which metformin may influence mammary cancer is not established. Proposed mechanisms include direct effects on cancer cells through influence on AMPk with associated inhibition of the mammalian target of rapamycin (mTOR) pathway as well as indirect, insulin-mediated effects [36–38]. It is not clear that at the dosage of metformin used in clinical practice, mTOR inhibition is achieved. However, another agent targeting mTOR inhibition, everolimus, has demonstrated strong interaction in enhancing the effectiveness of both tamoxifen [39] and aromatase inhibitors [40] in randomized clinical trials involving advanced breast cancer patient management. In this regard, in preclinical studies co-administration of metformin and everolimus intensified the inhibition of breast cancer cell proliferation by chemotherapy [41].
An important question is if indirect insulin-mediating effects predominate, will clinical activity against breast cancer be limited to women with impaired glucose homeostasis. In this regard, while Bonanni [32] found anti-proliferative effects of metformin limited to breast cancer patients with insulin resistance or high BMI, Chlebowski et al. [26] reported no interaction between BMI and metformin influence on breast cancer incidence in their cohort analyses. Further information on this question is needed to inform clinical study designs.
Analyses of the potential association of metformin use with breast cancer incidence in observational studies are challenging [26]. In prospective cohort studies with several years of observation, a number of postmenopausal women will become newly diagnosed with diabetes over time. Clinical diabetes management also changes over time, not only reflecting disease progression and/or severity, but also based on evolving concepts regarding optimal management [42]. In addition, commonly used therapies such as insulin may increase cancer incidence while other classes of agents such as the thiazolidinediones increase insulin sensitivity and improve glucose homeostasis [42] and may also potentially influence breast cancer [43]. These factors require complex analytical approaches to deal with changes in disease classification (diabetes or not) and changes in therapies used by women with diabetes over the course of observation.
The current analyses have been based on the best currently available data and were performed according to accepted recommendations to minimize the problems associated with meta-analyses of observational studies [44]. In addition, the funnel plot analyses, and analyses excluding small studies, suggest that publication bias in the identified reports was not substantial.
There is an ongoing, full scale clinical trial evaluating the potential influence of metformin on breast cancer. In the National Cancer Institute of Cancer (NCIC), MA 32 study, women with early stage and resected breast cancer receiving standard breast cancer therapy are being randomized to metformin or placebo [45]. In addition, at least 14 other clinical studies are proceeding, largely pilot and feasibility efforts, to address metformin and cancer issues [30]. The current findings support such efforts.
In conclusion, our meta-analyses of published studies find that metformin use in women with diabetes is associated with a lower risk of invasive breast cancer. Because this finding is based upon observational studies, it may reflect bias or confounding. The finding of a stronger effect size associated with studies of longer duration of metformin use and those that had longer observation periods suggest that the finding may be real. If this result is confirmed in prospective studies with a large number of breast cancer events, clinical trials should assess whether metformin can reduce breast cancer risk.
References
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M (2008) Body mass index and incidence of cancer: a systemic review and meta-analyses of prospective observational studies. Lancet 371:569–578
Hvidtfeldt UA, Gunter MJ, Lange T et al (2012) Quantifying mediating effects of endogenous estrogen and insulin in the relation between obesity, alcohol consumption and breast cancer. Cancer Epidemiol Biomarkers Prev. May 7 [Epub ahead of print]
Wolf I, Sadetzki S, Catane R et al (2005) Diabetes mellitus and breast cancer. Lancet Oncol 6:103–111
Larsson SC, Mantzoros CS, Wolk A (2007) Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 121:856–862
Cohen H, Letoith D (2012) Obesity, type 2 diabetes and cancer: the insulin and insulin-like growth factor connection. Endocr Relat Cancer. May 16 [Epub ahead of print]
Nathan DM, Buse JB, Davidson MB et al (2009) Medical management of hyperglycemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 52:17–30
Goodwin PJ, Pritchard KI, Ennis M et al (2008) Insulin lowering effects of metformin in women with early breast cancer. Clin Breast Cancer 8:501–505
Gonzalez-Angulo AM, Meric-Bernstam F (2010) Metformin a therapeutic opportunity in breast cancer. Clin Cancer Res 16:1695–1700
Zhou G, Myers R, Li et al (2001) Role of AMP-activated protein kinase mechanism of metformin action. J Clin Invest 108:1167–1174
DeCensi A, Puntoni M, Goodwin PJ et al (2010) Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res 3:1451–1461
Currie CJ, Poole CD, Gale EA (2009) The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 52:1766–1777
Libby G, Donnelly LA, Donnan PT et al (2009) New users of metformin are at low risk of incident cancer. Diabetes Care 32:1620–1625
Bodmer M, Meier C, Krahenbuhl S et al (2010) Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care 33:1304–1308
Noto H, Goto A, Tsujimoto T, Noda M (2012) Cancer risk in diabetic patients treated with metformin: a systemic review and meta-analysis. PLoS One 7:e33411
Peairs KS, Barone BB, Snyder CF et al (2011) Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol 29:40–46
Guyatt GH, Oxman AD, Vist G et al (2011) GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiology 64:407–415
Monami M, Colombi C, Balzi D et al (2011) Metformin and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care 34:129–131
Yang X, So WY, Ma RCW et al (2011) Low HDL cholesterol, metformin use, and cancer risk in type 2 diabetes. Diabetes Care 34:375–380
Bo S, Benso A, Durazzo M, Ghigo E (2012) Does use of metformin protect against cancer in type 2 diabetes mellitus? J Endocrinol Invest 35:231–232
Yang X, So WY, Ma RC et al (2012) Diabetes and cancer: the mechanistic implications of epidemiological analyses from the Hong Kong Diabetes Registry. Diabetes Metab Res Rev. Feb 8 [Epub ahead of print]
Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R et al (2009) Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 373:2125–2135
Home PD, Kahn SE, Jones NP, Noronha D, Beck-Nielsen H et al (2010) Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 53:1838–1845
Morden NE, Liu SK, Smith J, Mackenzie TA, Skinner J et al (2011) Further exploration of the relationship between insulin glargine and incident cancer: a retrospective cohort study of older Medicare patients. Diabetes Care 34:1965–1971
Bayraktar S, Hernadez-Aya LF, Lei X et al (2012) Effect of metformin on survival outcomes in diabetic patients with triple receptor-negative breast cancer. Cancer 118:1202–1211
Staa Van, Patel D, Gallagher AM, de Bruin ML (2012) Glucose-lowering agents and the patterns of risk for cancer: a study with the General Practice Research Database and secondary care data. Diabetologia 55:654–665
Chlebowski RT, McTiernan A, Wactawski-Wende J et al (2012) Diabetes, metformin and breast cancer in postmenopausal women. J Clin Oncol. doi:10.1200/JCO.2011.39.7505
Bosco JLF, Antonsen S, Sorensen HT et al (2011) Metformin and incident breast cancer among diabetic women: a population-based case-control study in Denmark. Cancer Epidemiol Biomarkers Prev 20:101–111
Bodmer M, Meier C, Krahenbuhl S et al (2012) Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care 33:1304–1308
Ruitter R, Visser LE, Van Herk-Sukel MPP et al (2012) Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives. Diabetes Care 35:119–1243
Dowling RJ, Niraula S, Stambolic V, Goodwin PJ (2012) Metformin in cancer: translational challenges. J Mol Endocrinol 48:R31–R43
Hadad S, Iwamoto T, Jordan L et al (2011) Evidence for biological effects of metformin in operable breast cancer: a pre-operative, window-of-opportunity, randomized trial. Breast Cancer Rest Treat. Jun 8 [Epub ahead of print]
Bonanni B, Puntoni M, Cazzaniga M et al (2012) Dual effect of metformin on breast cancer proliferation in a randomized pre-surgical trial. J Clin Oncol. May 7 [Epub ahead of print]
Yerushalmi R, Woods R, Ravdin PM et al (2010) Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 11:174–183
Jiralerspong S, Palla SL, Giordano SH et al (2009) Metformin and pathologic complete response to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 27:3297–3302
He X, Esteva FJ, Ensor J et al (2011) Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2 + breast cancer. Ann Oncol. Nov 22 [Epub ahead of print]
Alimova IN, Liu B, Fan Z et al (2009) Metformin inhibits breast cancer cell growth, colony formation and reduces cell cycle arrest in vitro. Cell Cycle 8:909–915
Ben-Sahra I, Regazzetti C, Robert C et al (2011) Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. Jun 28 [epub ahead of print]
Martin M, Marais R (2012) Metformin: a diabetes drug for cancer, or a cancer drug for diabetes?. J Clin Oncol. May7 [Epub ahead of print]
Bachelot T, Bourgier C, Cropet C et al (2012) Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 002-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. May 7 [Epub ahead of print]
Baselga J, Campone M, Piccart M et al (2012) Everolimus in postmenopausal hormone receptor-positive advanced breast cancer. N Engl J Med 366:520–529
Liu H, Scholz C, Zang C et al (2012) Metformin and the mTOR Inhibitor Everolimus (RAD001) sensitize breast cancer cells to the cytotoxic effect of chemotherapeutic drugs in vitro. Anticancer Res 32:1627–1637
Tahrani AA, Bailey CJ, Del Prato S, Barnett AH (2011) Management of type 2 diabetes: new and future developments in treatment. Lancet 378:182–197
Yang X, So WY, Ma RC et al (2012) Use of thiazolidinedione and cancer risk in type 2 diabetes: the Hong Kong diabetes registry. Diabetes Res Clin PRact. Apr 11 [Epub ahead of print]
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology (MOOSE) group l (a proposal for reporting). JAMA 283:2008–2012
Goodwin PJ, Stambolic V, Lemieux J et al (2011) Evaluation of metformin in early breast cancer: a modification of the traditional paradigm for clinical testing of anti-cancer agents. Breast Cancer Res Treat 126:215–220
Conflicts of interest
The authors declare that they have no conflicts of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Col, N.F., Ochs, L., Springmann, V. et al. Metformin and breast cancer risk: a meta-analysis and critical literature review. Breast Cancer Res Treat 135, 639–646 (2012). https://doi.org/10.1007/s10549-012-2170-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2170-x