Abstract
Inborn errors of metabolism (IEMs) that present with abnormal imaging findings in the second half of pregnancy are mainly lysosomal storage disorders (LSDs), cholesterol synthesis disorders (CSDs), glycogen storage disorder type IV (GSD IV), peroxisomal disorders, mitochondrial fatty acid oxidation defects (FAODs), organic acidurias, aminoacidopathies, congenital disorders of glycosylation (CDGs), and transaldolase deficiency. Their biological investigation requires fetal material. The supernatant of amniotic fluid (AF) is useful for the analysis of mucopolysaccharides, oligosaccharides, sialic acid, lysosphingolipids and some enzyme activities for LSDs, 7- and 8-dehydrocholesterol, desmosterol and lathosterol for CSDs, acylcarnitines for FAODs, organic acids for organic acidurias, and polyols for transaldolase deficiency. Cultured AF or fetal cells allow the measurement of enzyme activities for most IEMs, whole-cell assays, or metabolite measurements. The cultured cells or tissue samples taken after fetal death can be used for metabolic profiling, enzyme activities, and DNA extraction. Fetal blood can also be helpful. The identification of vacuolated cells orients toward an LSD, and plasma is useful for diagnosing peroxisomal disorders, FAODs, CSDs, some LSDs, and possibly CDGs and aminoacidopathies. We investigated AF of 1700 pregnancies after exclusion of frequent etiologies of nonimmune hydrops fetalis and identified 108 fetuses affected with LSDs (6.3 %), 29 of them with mucopolysaccharidosis type VII (MPS VII), and six with GSD IV (0.3 %). In the AF of 873 pregnancies, investigated because of intrauterine growth restriction and/or abnormal genitalia, we diagnosed 32 fetuses affected with Smith-Lemli-Opitz syndrome (3.7 %).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An inborn error of metabolism (IEM) can be suspected in the antenatal period when abnormal ultrasound findings are observed, usually during the third trimester of pregnancy. Fetal autopsy, when performed, is particularly helpful to orient the biological investigations. IEMs that can be suspected in the antenatal period are lysosomal storage disorders (LSDs), disorders of postsqualene cholesterol synthesis, glycogen storage disorders, peroxisomal disorders, mitochondrial fatty acid oxidation disorders, organic acidurias, aminoacidopathies, congenital disorders of glycosylation (CDGs), transaldolase deficiency, and respiratory chain disorders. This review describes the biological investigations that can be performed to reach a correct diagnosis.
Possible biological investigations in pregnancies at risk of an inborn error of metabolism
Biological investigation requires fetal material, which can be sampled during pregnancy or after fetal abortion or termination of pregnancy. Amniotic fluid (AF) is the easiest material to collect during pregnancy. A minimum amount (15 ml) is needed, sampled in sterile packaging. It must be sent at room temperature to a cell culture laboratory in order to centrifuge it in sterile conditions, separate the supernatant, and grow the pellet of AF cells. The supernatant must then be frozen until metabolic investigations. In case of nonimmune hydrops fetalis (HF), the most frequent etiologies—such as antenatal malformations, viral infections, or karyotype abnormalities—have to be eliminated first. As soon as these etiologies have been eliminated—and especially in case of recurrence of antenatal signs in the family and/or consanguinity—an IEM can be suspected and must be searched for. IEMs are mainly due to enzyme or transporter deficiency. Global tests that allow detection of most IEMs in AF supernatant can be used to identify and quantify metabolites that accumulate upstream of the metabolic block. During fetal life, renal tubular maturation begins after the 14th week of pregnancy. After 20 weeks, the kidneys provide >90 % of the AF volume (Benoist et al. 2007). Therefore, AF supernatant is representative of fetal urine, and all methods used to diagnose IEM in urine can theoretically be applied to AF. Measurement of several lysosomal enzyme activities can also be performed using the supernatant of AF. Cultured AF cells are needed to measure enzyme activities, which is not possible using AF supernatant, or for performing whole-cell assays (such as fatty acid oxidation studies). Moreover, these cultured cells can be used for DNA extraction to confirm a diagnosis at the molecular level. In case of HF, ascites or pleural fluids can also be sampled: the supernatant can be used for metabolite analyses and/or enzymes activities, and a cell culture can be initiated.
Fetal blood is not usually sampled, although it can be of great help for diagnosing an IEM. When fetal anomalies are so severe that termination of pregnancy is proposed, it is possible to collect a blood sample before termination. One milliliter of ethylenediaminetetraacetate (EDTA) blood can be used for blood-cell count: anemia can orientate toward Pearson syndrome and the presence of vacuolated lymphocytes toward an LSD. Plasma can be used to measure nondiffusible metabolites that are not cleared by the placenta. It is especially useful for diagnosing disorders of peroxisomal metabolism, fatty acid oxidation, postsqualene cholesterol biosynthesis, and possibly some aminoacidopathies and organic acidurias. Fetal serum or plasma can also be used to measure enzyme activities for diagnosing LSDs.
After fetal death, fetal samples are taken by the fetopathologist. Blood and urine samples are usually impossible to obtain. Even if blood samples can be collected, postmortem modifications do not allow accurate interpretation of metabolite profiles or measurements. Therefore, the available materials are generally tissue samples. When sampled in sterile conditions, some can be used for cell culture: skin (except if the fetus is macerated), lung, kidney, or muscle. The cultured cells can be used for measuring enzyme activities, whole-cell assays, or DNA (and eventually RNA) extraction. Tissue biopsies (muscle, liver, heart, thymus, lung…) can also be deep frozen in cryotubes immediately after sampling at −80 °C or, better, in liquid nitrogen. Frozen tissues can be used for measuring enzyme activities or for DNA extraction. The drawback of postmortem samples is that diagnostic orientation by measuring metabolites is not possible.
Lysosomal storage disorders (LSDs)
LSDs are the most well-known disorders that can present with antenatal signs. They usually present with hydrops fetalis (HF) starting from the second trimester of pregnancy associated or not with fetal ascites. In some cases, isolated ascites can be found. According to a large series of patients, LSDs would be the etiology of 1.4 % of HF (Machin 1989). More recently, Bellini et al. (2015) and Gimovsky et al. (2015) performed a systematic review of the literature to evaluate the incidence and types of LSD in a case series of nonimmune hydrops (NIH). The overall incidence of LSDs varies from 1.3 % (Bellini et al. 2015) to 5.2 % (Gimovsky et al. 2015). The three more common LSDs identified, in order of decreasing incidence, were mucopolysaccharidosis type VII (MPS VII), Gaucher disease, and GM1 gangliosidosis (Gimovsky et al. 2015).
The diagnosis of LSDs relies on (1) metabolite analyses in the AF supernatant (or ascites or pleural fluids), cultured cells, and—when applicable—fetal serum; (2) measuring enzyme activities in the AF supernatant (or pleural and ascites fluids), cultured amniocytes, cultured fetal cells, fetal serum/plasma, or frozen tissue samples; (3) mutation analysis of the corresponding gene(s), after identifying an enzyme or sialic acid transporter defect, in DNA extracted from amniocytes, cultured fetal cells, or frozen tissue samples. Metabolites that can be analyzed in AF supernatant are mainly glycosaminoglycans, oligosaccharides, and sialic acid (Piraud et al. 1996). Electrophoresis and quantification of glycosaminoglycans allow diagnosing MPS VII and MPS IVA. The dramatic increase in chondroitin sulfate fraction is indicative of MPS VII, sometimes associated with abnormal presence of dermatan sulfate and/or heparan sulfate, while the abnormal presence of keratan sulfate orientates toward MPS IVA. The diagnosis of sialidosis, galactosialidosis, and GM1 gangliosidosis can be suspected by oligosaccharides analysis, classically performed by thin-layer chromatography but recently replaced by liquid chromatography–tandem mass spectrometry (LC-MS/MS), a more sensitive technique. Qualitative analysis by thin-layer chromatography of free sialic acid or by quantitative measurement by MS/MS is used for diagnosing infantile sialic acid storage disorder (ISSD). Several lysosomal enzyme activities can be measured in AF supernatant: β-D-glucuronidase activity (deficient in MPS VII) and α-L-fucosidase and total hexosaminidase activities, which may be increased (together with β-D-glucuronidase activity) in mucolipidosis type II. Recently, we developed an assay of plasma lysosphingolipids using LC-MS/MS (Pettazzoni et al. 2015a); lysoglucosylceramide (analyzed as lysohexosylceramide because it is indistinguishable from lysogalactosylceramide increase in Krabbe disease) was increased in plasma from patients affected with Gaucher disease (Rolfs et al. 2013). We retrospectively applied this assay in the AF supernatant from five pregnancies affected with Gaucher disease previously diagnosed by showing a deficiency of glucocerebrosidase activity in cultured fetal cells. We found a significant increase in lysohexosylceramide in AF of these five patients with Gaucher when compared with controls (unpublished data).
Cultured amniocytes or cultured fetal cells are useful to measure enzyme activities to confirm a diagnosis suspected after AF supernatant analysis, such as MPS VII, MPS IVA or oligosaccharidoses, including sialidosis (α-D-neuraminidase deficiency), galactosialidosis (combined α-D-neuraminidase and β-galactosidase deficiency), and GM1 gangliosidosis (β-galactosidase deficiency). They are also needed to measure other enzyme activities that cannot be measured in AF supernatant, e.g., glucocerebrosidase for diagnosing Gaucher disease, acid lipase for Wolman disease, sphingomyelinase for Niemann-Pick type A disease, or acid ceramidase for Farber disease (Kattner et al. 1997; Burin et al. 2004). Free sialic acid content can also be measured in cultured cells to confirm or establish ISSD diagnosis (Froissart et al. 2005). When a Niemann-Pick type C disease is suspected because of isolated ascites and, in some cases, splenomegaly (Spiegel et al. 2009), filipin staining can be performed in cultured cells to visualize the abnormal accumulation of unesterified cholesterol in the late endosomal/lysosomal compartment (Vanier and Latour 2015).
Complete blood count of fetal blood can reveal vacuolated cells, leading to suspicion of an LSD. The presence of such cells in peripheral blood smears has been described after birth in several LSDs, such as GM1 gangliosidosis, ISSD, sialidosis, mucolipidosis type II, MPS VII, and Wolman disease (Anderson et al. 2005). Recently, the presence of vacuolated cells in fetal ascites fluid has been observed in fetuses affected by various LSDs (Dugan et al. 2014; Dreux et al. 2015). Two classes of plasmatic biomarkers, oxysterols [cholestane-3β, 5α, 6β-triol, and 7-ketocholesterol (Jiang et al. 2011; Pagan et al. 2015)] and lysosphingolipids [lysophingomyelin and isoform 509 (Chuang et al. 2014; Giese et al. 2015; Pettazzoni et al. 2015a)], have recently emerged for diagnosing Niemann-Pick disease types C and A/B (sphingomyelinase deficiency). Oxysterols are also a marker of Wolman disease (Pagan et al. 2015). One can speculate that measuring oxysterols in fetal plasma could allow diagnosing these disorders. Once the diagnosis of an LSD has been determined by metabolite analysis and/or measuring enzyme activity, it is recommended to perform mutation analysis of the corresponding gene using DNA extracted from cultured amniocytes, cultured fetal cells, or frozen fetal tissue.
Since 1990, we have investigated ~1700 AF for LSDs after exclusion of the most frequent etiologies of nonimmune HF; 108 (6.3 %) fetuses affected with LSD were identified (Table 1). The most common LSDs identified, in order of decreasing incidence, were MPS VII, ISSD, then galactosialidosis, sialidosis, GM1 gangliosidosis, and Gaucher disease. This incidence is different from that reported in the literature (Gimovsky et al. 2015); however, it is possible that analysis of oligosaccharides and sialic acid were not performed in all reported studies. Interestingly, MPS VII represents more than one fourth of the diagnosed cases (27 %), although it is rarely diagnosed in the postnatal period. Similarly, antenatal presentation is the most common form of LSDs affecting sialic acid metabolism (95 % for sialic acid storage disorders, 93 % for galactosialidosis, 68 % for sialidosis) when compared with the number of postnatal diagnoses performed during the same period. Consanguinity has been reported in 39 % of affected fetuses.
Disorders of postsqualene cholesterol biosynthesis
Cholesterol biosynthesis is a complex pathway that can be divided into two main parts. The presqualene part leads to the biosynthesis of isoprenoids (including the intermediate precursor named squalene) and sterols. The postsqualene metabolic steps lead to the synthesis of cholesterol and vitamin D (Fig. 1). All disorders of postsqualene cholesterol biosynthesis are characterized by multiple congenital abnormalities, including significant skeletal involvement (Rossi et al. 2015).
The most frequent disorder is Smith-Lemli-Opitz (SLO) syndrome, which is due to 7-dehydrocholesterol reductase deficiency (DHCR7 gene). Inheritance is autosomal recessive. In a series of 30 cases diagnosed prenatally (10:30) or postnatally (20:30), Goldenberg et al. (2004) reported that intrauterine growth restriction (IUGR) was the most frequent detectable trait (20:30) and that it was isolated in nine cases. The key metabolite for diagnosing SLO syndrome is 7-dehydrocholesterol. In the same analytical run, 8- dehydrocholesterol is also usually quantified as an indirect biomarker, since the Δ8,Δ7 isomerase activity is maintained in SLO patients (Fig. 1). Measurement of sterol precursors is usually performed using gas chromatography mass spectrometry (GC-MS) because of its high chromatographic resolution, which is useful to discriminate isobaric components. It can be performed in AF supernatant, in cultured amniotic or fetal cells, as well as in fetal blood (plasma or serum). Table 2 summarizes our experience of 873 pregnancies at risk for SLO syndrome investigated between 2007 and 2014 because of IUGR and/or abnormal genitalia: 32 affected fetuses were diagnosed (3.7 %), and pregnancy was terminated in most cases. Fetal plasma was available in one of the affected fetuses, sampled at 32 weeks of gestation. Concentration of 7-dehydrocholesterol was 328 μmol/L (reference values in newborns, <3 μmol/L), 8-dehydrocholesterol was 402 μmol/L (reference values in newborn ~<0.5 μmol/L), and cholesterol was 176 μmol/L (reference values in newborn: 2600–4200 μmol/L). In AF, concentration of 7-dehydrocholesterol and 8-dehydrocholesterol were 26.4 μmol/L and 12.9 μmol/L, respectively (see Table 2 for reference values). Therefore, as amniocentesis is currently performed for chromosome analysis, when IUGF is associated with other malformations, measuring 7-dehydrocholesterol must be performed when karyotype is normal.
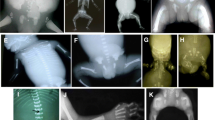
Measuring sterols in AF supernatant is also helpful for diagnosing three other inborn errors of post-squalene cholesterol biosynthesis: Conradi-Hünermann-Happle syndrome, desmosterolosis, and lathosterolosis. Conradi-Hünermann-Happle syndrome, also called X-linked dominant chondrodysplasia punctata (CDPX2), is caused by mutations in the EBP gene, which encodes for sterol Δ8−Δ7 isomerase. This syndrome is usually lethal early in pregnancy in hemizygous male fetuses, whereas high clinical variability has been described in affected female fetuses (Cañueto et al. 2012; Lefebvre et al. 2015). Few data are available in the literature concerning metabolite analysis in severe antenatal presentation of CDPX2. Table 3 summarizes our experience of eight fetuses affected with Conradi-Hünermann-Happle syndrome diagnosed between 2004 and 2015 on the basis of ultrasound findings. We found disproportionate and asymmetric shortening of long bones and abnormally early calcification of proximal and distal epiphyses of the humerus and femur. Clinical features of these female fetuses were reported in Lefebvre et al. (2015). Interestingly, all these severe cases, diagnosed at a mean age of 22 weeks of gestation, were due to de novo mutations of EBP, except for two fetuses with an affected mother and one case of germinal mosaicism. Lathosterolosis (Rossi et al. 2007) and desmosterolosis (Brunetti-Pierri et al. 2002) are very rare autosomal recessive conditions caused by sterol Δ5 desaturase deficiency (SC5D) and sterol Δ24 reductase deficiency (DHCR24), respectively. Their clinical phenotype is very similar to SLO syndrome, and <20 patients have been reported (Rossi et al. 2007 and 2015). An increase in lathosterol has been reported in plasma of a patient affected with lathosterolosis at 22 months of age (lathosterol = 81.6 μmol/L; reference values <18 μmol/L) (Ho et al. 2014). An increase in desmosterol has been reported in the plasma of patients affected with desmosterolosis (117–328 μmol/L; controls 1.3 ± 0.8 μmol/L) (Andersson et al. 2002; Zolotushko et al. 2011). To our knowledge, an increase in these compounds has not been reported in the AF supernatant of pregnancies with fetuses affected with these conditions, but it can be expected to be present. Therefore, these metabolites must be measured in AF supernatant together with 7-dehydrocholesterol when SLO syndrome is suspected. In all cases (CDPX2, lathosterolosis, desmosterolosis), the diagnosis must be confirmed by mutation analysis of the corresponding genes (EBP, SC5D, and DHCR24, respectively) using DNA extracted from cultured amniocytes or from cultured fetal fibroblasts or tissues.
Diagnosing the other disorders of postsqualene cholesterol synthesis—Antley-Bixler syndrome, Greenberg dysplasia, and congenital hemidysplasia with ichthyosiform erythroderma and limb defects (CHILD) syndrome—is highly oriented by ultrasound findings and fetopathological examination (Konstantinidou et al. 2008; Rossi et al. 2015). Sterols that accumulate in these conditions are difficult to measure. Therefore, their diagnosis relies on molecular analysis of the corresponding genes: POR (coding for a cytochrome P450 oxidoreductase) for autosomal recessive Antley-Bixler syndrome, FGFR2 (coding for fibroblast growth factor receptor 2) for autosomal dominant Antley-Bixler syndrome, LBR (coding for the lamin B receptor, affecting its enzymatic domain Δ14 reductase) for Greenberg dysplasia, and NSDHL [coding for nicotinamide adenine dinucleotide phosphate, reduced (NADPH) steroid dehydrogenase-like protein involved in the sterol C-4 demethylase complex] for CHILD syndrome.
Glycogen storage disorders
Glycogen storage disease type IV (GSD IV) can be suspected in nonimmune HF, especially if it is associated with fetal akinesia, arthrogryposis, and hydramnios (L’herminé-Coulomb et al. 2005; Pettazzoni et al. 2015b). So far, there is no metabolite analysis that allows identifying this disorder. The diagnosis, evocated by positive periodic acid–Schiff and diastase-resistant inclusions in histology, relies on measuring glycogen branching enzyme (GBE) in cultured amniocytes or cultured fetal cells and mutation analysis of GBE1. In our series of 1700 AFs investigated because of HF, six cases of GSD IV were identified (0.3 %).
Peroxisomal disorders
Defects in human genes encoding peroxisomal proteins can result in different peroxisomal disorders of variable severity. Some present with early antenatal manifestations. As described in the two previous reviews in this issue, two main clinical antenatal presentations can orientate toward a peroxisomal disorder: rhizomelic chondrodysplasia punctata (RCDP) and the Zellweger syndrome spectrum (ZSS). All these peroxisomal disorders are inherited autosomal recessively.
RCDP is characterized by severe proximal shortening of limbs, coronal clefts of vertebral bodies, and widespread calcific splitting of the epiphyses. Five types of RCDP have been described; all but one have the same clinical presentation in the antenatal period (reviewed by Waterham et al. 2016). RCDP type I is caused by mutations in PEX7 encoding the PTS2-protein receptor. As a consequence, the PTS2-targeted peroxisomal enzymes are not imported into peroxisomes, resulting in a defect in plasmalogen biosynthesis and α-oxidation of phytanic acid. RDCP type 2 is caused by mutations in GNPAT coding dihydroxyacetone phosphate acyltransferase (DHAPAT), an enzyme involved in plasmalogen biosynthesis. RDCP type 3 is caused by mutations in AGPS encoding another enzyme of plasmalogen biosynthesis, alkyl-DHAP synthetase. RCDP type 5 has been recently described (Baroy et al. 2015) and is caused by loss of the PEX5 long isoform, resulting in deficient PEX7-mediated import of PTS2-targeted proteins, causing a similar phenotype as in RCDP type 1. Conversely, RDCP type 4 (fatty acyl-CoA reductase 1 deficiency; FAR1) has a different clinical phenotype (Buchert et al. 2014). The common biochemical trait of all RCDP types is a defect in plasmalogen biosynthesis. Therefore, their biochemical diagnosis relies on measuring plasmalogens in cultured amniotic cells or cultured fetal cells by gas chromatography (Dacremont and Vincent 1995) or by LC–MS/MS (Zemski Berry and Murphy 2004). To our knowledge, measuring plasmalogen levels has never been performed in fetal erythrocytes, but it can be expected to be decreased in fetuses affected with RCDP, as it is in erythrocytes of RCDP patients after birth. It is also possible to measure DHAPAT activity in cultured amniotic or fetal cells. DHAPAT activity is lowered in all RCDP types, including RDCP type 3. This is because DHAPAT is only stable when it forms an intraperoxisomal complex with alkyl-DHAP synthetase (de Vet et al. 1999). The definitive diagnosis relies on mutation analysis of the corresponding genes (PEX7, GNPAT, AGPS, PEX5, and eventually FAR1) using DNA extracted from cultured fetal cells or tissue biopsies.
ZSS can be suspected on ultrasound scan and mainly on brain magnetic resonance imaging (MRI) because of cerebral gyration anomalies, with polymicrogyria and germinolysis cysts, echogenic kidneys, and epiphyses stippling. This clinical phenotype can be due to a peroxisome biogenesis disorder (PBD-ZSS) or to a single enzyme deficiency of peroxisomal fatty acid oxidation (acyl-CoA oxidase coded by ACOX1, and D-bifunctional protein coded by HSD17B4) (reviewed in Waterham et al. 2016). Patients with PBD-ZSS are a genetically heterogeneous group, with a generalized defect in peroxisomal functions due to mutations in any of the 13 different PEX genes encoding the peroxins involved in the import of peroxisomal membranes and/or matrix proteins: PEX1, PEX2, PEX3, PEX5, PEX6, PEX10, PEX11β, PEX12, PEX13, PEX14, PEX16, PEX19, and PEX26. The common biochemical feature to the ZSS is impairment of peroxisomal fatty acid oxidation. Therefore, measuring very-long-chain fatty acids (VLCFA) by GC-MS or LC-MS/MS in fetal plasma or cultured amniocytes or cultured fetal cells allows detection of all these defects: hexacosanoic acid (C26:0), and the ratios C26:0/C22:0 and C24:0/C22:0 are elevated in all cases. Table 4 illustrates the VLCFA levels in plasma from two fetuses affected with PBD-ZSS. VLCFA can be elevated in AF, but this measurement is not recommended because of the report of normal levels in affected fetuses (Verhoeven et al. 1995). It is also possible to perform an oxidation study of VLCFA in cultured cells by measuring the production of [1-14C] acetyl-CoA from [1-14C] C24:0 or C26:0. A decreased oxidation rate is in agreement with a defective peroxisomal fatty acid oxidation. Measurement of DHAPAT activity in cultured cells is important to differentiate PBD-ZSS from single-enzyme deficiencies: DHAPAT activity is decreased in PBD-ZSS and normal in acyl-CoA oxidase and D-bifunctional protein deficiency. It should be noted that pristanic acid level, which reflects a defect in peroxisomal β-oxidation; and phytanic acid level, which reflects a defect in peroxisomal α-oxidation in PBD-ZSS, are normal in fetal blood, since they both derive from dietary sources, and do not allow detection of these disorders (Table 4). Interestingly, pipecolic acid, an intermediate in lysine degradation and usually elevated in plasma and urine of patients with PBD-ZSS, was not elevated in the plasma of a fetus affected with PBD-ZSS (Table 4). It has also been previously reported that pipecolic acid levels can be normal in the AF of fetuses affected with a PBD-ZSS (Verhoeven et al. 1995). Pipecolic acid is a small molecule, and it can be hypothesized that it is cleared from fetal circulation by the placenta. Once one of these disorders is suspected on biochemical analyses, it is necessary to confirm the diagnosis by mutation analysis of the possible genes involved. A classic Sanger sequencing approach can be used for ACOX1 and HSD17B4 genes if a single enzyme deficiency is suspected. For the PBD, 13 PEX genes have been identified: whole-exome sequencing or analysis of a gene panel by next-generation sequencing (NGS) is more appropriate, although a “PEX Gene Screen” has been developed for the systematic screening of exons in the six PEX genes most commonly defective in PBD-ZSS (Steinberg et al. 2004).
Mitochondrial fatty acid oxidation disorders
Only two disorders of mitochondrial fatty acid oxidation have been described as presenting with antenatal manifestations: carnitine palmitoyltransferase 2 deficiency (CPT2) (Taroni et al. 1994, reviewed by Boemer et al. 2016), and multiple acyl-CoA dehydrogenase deficiency (MADD) due to electron transfer flavoprotein (ETF) or to electron transfer flavoprotein ubiquinone oxidoreductase (ETFQO) (Vianey-Saban et al. 2000). Measurement of acylcarnitines by MS/MS in AF supernatant allows detecting MADD by exhibiting an increase in short- and medium-chain acylcarnitines, as well as glutarylcarnitine (Fig. 2). Conversely, since long-chain acylcarnitines are not eliminated in urine but in bile, CPT2 deficiency cannot be reliably diagnosed by acylcarnitine analysis in AF (Boemer et al. 2016). Although it has never been performed, it is most probable that acylcarnitine profiling in fetal plasma or blood is diagnostic. Therefore, if fetal plasma or blood is not available, screening for these disorders is done by using whole-cell assays in cultured amniocytes or fetal cells (Nada et al. 1996) or by measuring CPT2 activity for CPT2 deficiency. The assay used to measure ETF and ETFQO activity necessitates the use of purified medium-chain acyl-CoA dehydrogenase (MCAD) and ETF from pig liver and is no longer available except in one lab. Mutation analysis of the corresponding genes, ETFA, ETFB, and ETFDH for MADD, and CPT2 for CPT2 deficiency, are then necessary to confirm the diagnosis. Because fetuses affected by severe cerebral malformations are frequently aborted, Boemer et al. (2016) suggest that fatty acid oxidation disorders may be underestimated and should be considered when faced with a fetus with Dandy-Walker or another brain dysgenesis, especially in cases with intrafamilial recurrence or in the presence of associated cystic kidney malformation and liver steatosis.
Acylcarnitine profile in amniotic fluid from a fetus affected with multiple acyl-CoA dehydrogenase deficiency (MADD) compared with a control amniotic fluid. C0 free carnitine, C2 acetylcarnitine, C3 propionylcarnitine, C4 butyryl(isobutyryl)carnitine, C5 isovaleryl(2-methylbutyryl)carnitine, C6 hexanoylcarnitine, C5DC glutarylcarnitine
Organic acidurias
Measurement of glutarylcarnitine by MS/MS (together with the ratio of glutarylcarnitine to propionylcarnitine) (Shigematsu et al. 1996) and of glutaric acid by GC-MS (Jakobs 1989) in AF supernatant are reliable indicators in pregnancies with an established risk of glutaric aciduria type I. However, it has never been demonstrated whether glutarylcarnitine was increased in fetal plasma from affected pregnancies. These metabolites can also be used in pregnancies at risk of glutaric aciduria type I on ultrasound findings (Mellerio et al. 2008). Abnormal results must be confirmed by measuring glutaryl-CoA dehydrogenase activity in cultured amniotic fluid of fetal cells or mutation analysis of GCDH.
Barth syndrome is considered in the differential diagnosis of unexplained male hydrops, dilated cardiomyopathy, endothelial fibroelastosis, left ventricular noncompaction, or pregnancy loss (Steward et al. 2010). An increase of 3-methylglutaconic aciduria has been reported in urinary organic acids of patients with Barth syndrome, but this is not a constant finding (Schmidt et al. 2004; Rigaud et al. 2013). Therefore, its measurement in AF cannot be a reliable marker of the disease. The diagnosis relies on cardiolipin analysis in cultured fetal cells or tissues or TAZ gene testing (Rigaud et al. 2013).
Mevalonic aciduria was diagnosed postnatally in a patient who presented abnormal prenatal ultrasound findings starting at 23 weeks of gestation: polyhydramnios, ascites, hyperechogenic bowel with mild dilation, agenesis of ductus venosus and skeletal dysmorphia (Schwarzer et al. 2008). Measurement of mevalonic acid in AF supernatant by stable isotope dilution assay using GC-MS can be used to detect affected fetuses. Determining mevalonate kinase activity or mutation analysis of MVK in cultured amniotic or fetal cells confirms the diagnosis (Rolland et al. 2005).
In a systematic review of all published patients with holocarboxylase synthetase deficiency, Bandaralage et al. (2016) reported abnormal antenatal imaging findings during the late second or early third trimester of pregnancy, the most common being subependymal cysts, ventriculomegaly, intraventricular hemorrhage, and often IUGR. The diagnosis of holocarboxylase synthetase deficiency can be suspected by measuring methylcitric and 3-hydroxyisovaleric acids (Suormala et al. 1998) and probably by an increase in propionylcarnitine (C3) and 3-hydroxyisovalerylcarnitine (C5OH) in AF supernatant. It is confirmed by measuring holocarboxylase synthetase activity in cultured AF cells and mutation analysis of HLCS. Prompt biotin supplementation, especially when commenced antenatally, is associated with disease regression and good clinical outcome (Thuy et al. 1999).
Aminoacidopathies
Fetal hyperechogenic colon (FHC) in the third trimester of gestation has been reported to be associated with cystinuria (Brasseur-Daudruy et al. 2006). In a retrospective study, Benoist et al. (2007) demonstrated that concentrations of cystine (median 72 μmol/L; controls 20–45), lysine (median 305 μmol/L; controls 60–180), ornithine (median 60 μmol/L; controls 8–30), and arginine (median 82 μmol/L; controls 10–45) in AF were all above the reference values in a group of six pregnancies with FHC, whereas these amino acids were in the reference intervals for gestational age in a group of 12 pregnancies with hyperechogenic small bowel. Diagnosis of cystinuria was confirmed at birth, suggesting that FHC and measurement of amino acids in AF are useful diagnostic indicators of cystinuria. Cystinuria is caused by impaired transport system for cystine and dibasic amino acids in renal proximal tubule and small intestine. The intestinal hyperechogenicity is probably due to accumulation of cystine crystals in the intestinal tract (Benoist at al 2007). Progressive closure of the anal sphincter only starts after 22 weeks of pregnancy, explaining why the occurrence of FHC only occurs after 26 weeks.
Other aminoacidopathies can present with signs in the antenatal period. Disorders of serine biosynthesis (3-phosphoglycerate dehydrogenase deficiency, 3-phosphoserine aminotransferase deficiency, 3-phosphoserine phosphatase deficiency) (de Koning et al. 2002; de Koning 2013), and asparagine synthetase deficiency (Alfadhel et al. 2015) are responsible for congenital microcephaly and IUGR. Brain abnormalities detectable in the antenatal period have been described in glutamine synthetase deficiency [cerebral and cerebellar atrophy, agyria, paraventricular cysts (Häberle et al. 2005)] , sulfite oxidase, and molybdenum cofactor deficiency [multiple subcortical cavities (Carmi-Nawi et al. 2011)], and nonketotic hyperglycinemia [hypoplasia of the corpus callosum (Paupe et al. 2002)]. Measurement of amino acids in AF is unreliable, at least for sulfite oxidase, molybdenum cofactor deficiency, and nonketotic hyperglycinemia (personal experience), and most probably for the amino acid synthesis defects that are undiagnosable in urine of affected patients. To our knowledge, measuring amino acids in fetal plasma has never been performed in these disorders, which probably explains why all patients with such disorders are diagnosed at birth. It must not be forgotten that maternal phenylketonuria or hyperphenylalaninemia leads to fetal microcephaly (Prick et al. 2012). Neonatal screening for phenylketonuria or hyperphenylalaninemia is performed in all industrialized countries, but because of increasing emigration, not all mothers are investigated. The diagnosis relies on measuring phenylalanine in plasma or a dried blood spot of the mother.
Congenital disorders of glycosylation
Congenital disorders of glycosylation (CDGs) are due to defects in the synthesis of glycans and the attachment of glycans to proteins and lipids, presenting with heterogeneous multisystemic clinical manifestations. To date, >60 different types of CDG have been reported. Most are due to defects in N-glycosylation, but disorders of O-glycosylation (especially O-mannosylation) have been reported. Both types of CDG can present with symptoms in the antenatal period.
The main clinical feature of defects of N-glycosylation is nonimmune HF. Léticée et al. (2010) reported a case and provided a systematic revue of 12 reported cases with CDG and HF. Nine cases were due to phosphomannomutase 2 congenital disorder of glycosylation (PMM2-CDG), the most frequent form of CDG, and is caused by PMM deficiency and mutations in PMM2. A frequent mutation (p.Arg141His) has been identified in PMM2, and the carrier rate is ~1:100 in Caucasian population. Based on this allele frequency, the expected incidence of PMM2-CDG should be 1:20,000 (Schollen et al. 2000). The discrepancy between the expected and observed incidence (estimated at 1:40,000–1:80,000) could be explained by early antenatal death or mild underdiagnosed phenotype. The other cases of CDG with HF were asparagine-linked glycosylation homologs (ALG) ALG1-CDG, ALG8-CDG, ALG12-CDG, and an unclassified one. Isoelectric focusing or Western blot glycosylation pattern of transferrin is the screening method of choice to detect N-glycosylation disorders associated with sialic acid deficiency. However, while transferrin abnormalities have been identified in fetal serum of two fetuses affected with PMM2-CDG at 27 weeks and 30 weeks of pregnancy, respectively (Edwards et al. 2006; Léticée et al. 2010), it has been demonstrated as unreliable in another case (Clayton et al. 1993). Further studies are needed to confirm these findings. The diagnosis is most often suspected at pathological examination. PMM activity can be measured in cultured amniocytes or fetal cells but can show intermediate values (Matthijs et al. 1998). Measurement of PMM activity in parents’ leucocytes has been proposed (Léticée et al. 2010) but, in our experience, there is an overlap between PMM activity in heterozygotes and controls. The clue is probably the mutational screening of the PMM2 gene and the development of gene panels by NGS. Recently, Lepais et al. (2015) reported two siblings affected with ALG3-CDG presenting antenatally with short long bones, IUGR, and cerebellar vermis hypoplasia.
Defects of protein O-mannosylation (POMT) are responsible for autosomal recessive Walker-Warburg syndrome (POMT1/POMT2-CDG) and muscle-eye-brain disease (POMGNT1-CDG) (Wopereis et al. 2006 ; Lacalm et al. 2016). They are suspected by ultrasound findings and pathological examination, but their diagnosis relies on mutation analysis of the corresponding genes: POMT1, POMT2, and POMGnT1.
Other inborn errors of metabolism
Transaldolase (TALDO) deficiency, an inborn error in the pentose phosphate pathway, can present with a multimalformation syndrome, HF, oligohydramnios, and IUGR (Valayannopoulos et al. 2006). The retrospective analysis of AF of an affected fetus sampled at 27 weeks of gestation exhibited elevated concentrations of sedoheptulose (13.5 μmol/L; reference values <1.2 μmol/L) and, to a lesser extent, ribitol (7 μmol/L; reference values 1–4 μmol/L). Conversely, erythritol and arabitol, which are found elevated in urine samples from TALDO patients, were normal in the AF of this affected pregnancy (Wamelink et al. 2008). TALDO activity is severely decreased in fibroblasts of patients (Valayannopoulos et al. 2006) and can probably be measured in cultured amniocytes or fetal cells—although, to our knowledge, it has never been reported—as well as mutation analysis of the TALDO gene in DNA extracted from fetal tissues.
Primary mitochondrial respiratory chain disorders (MRCD) are a heterogeneous group of pathologies caused by genetic alterations affecting mitochondrial energy production. Epidemiological studies have shown that average prevalence is about 1:20,000, indicating that MRCD is the most common group of inherited metabolic diseases (Schaefer et al. 2004). There is limited information on the antenatal manifestations of MRCD. Experiments in mice have shown that mitochondrial activity varies during fetal life. In the first trimester of pregnancy, the embryo develops in a low-oxygen environment, whereas in the third trimester, high oxidative metabolism takes place through the mitochondria to sustain the rapid fetal growth (reviewed in DiMauro and Garone 2011; Yanicostas et al. 2011). Retrospective studies of pediatric patients with MRCD showed that IUGR was the most frequent antenatal feature observed (von Kleist-Retzow et al. 2003; Tavares et al. 2013). However, due to the lack of characteristic antenatal findings, such diseases are usually diagnosed at birth. Moreover, there is no specific metabolite that can be measured in the supernatant of AF. Other inborn errors of energy metabolism can present with antenatal ultrasound abnormalities. Fumarase deficiency, an inborn error that affects Krebs cycle, can present with polymicrogyria soon after birth (Ottolenghi et al. 2011) and pyruvate dehydrogenase deficiency with agenesis of corpus callosum (Patel et al. 2012; Al Kaissi et al. 2014), but these abnormalities are not specific enough to allow suspicion of these disorders in the antenatal period.
Conclusion
IEM diagnosed in the second half of pregnancy on ultrasound findings are mainly LSDs and cholesterol biosynthesis defects (SLO). This is probably because they are the most frequent IEM presenting in fetal life but also because they are easy to diagnose. Biochemical testing is available for a wider range of IEMs (Table 5).
Why is it important to diagnose an IEM in the antenatal period? In the vast majority of these disorders, there are no options for treatment. This explains why there is a need to arrive at a diagnosis as soon as possible to eventually propose a termination of pregnancy. Moreover, in most cases, genetic counselling can be proposed to the families for future pregnancies. A prenatal diagnosis can be performed in uncultured chorionic villi, in most cases by mutation analysis or by measuring enzyme activity but sometimes by measuring metabolite(s), thus allowing early diagnosis—before 13 weeks. If the diagnosed IEM has a severe prognosis, an early termination of pregnancy can be proposed or an intrauterine treatment can be attempted. Treating the mother of a fetus affected with a disorder of serine synthesis (3-phosphoglycerate dehydrogenase deficiency) with L-serine during the last trimester of pregnancy had a beneficial effect: in contrast to her severely affected sibs, this girl was born normocephalic with a normal psychomotor development (de Koning 2013). The same beneficial effect has been observed in holocarboxylase synthetase deficiency by early treatment of the mother with biotine (Thuy et al. 1999). Preimplantation genetic diagnosis is now available in an increasing number of countries and allows exclusion of an affected fetus, and for some treatable disorders (e.g., cystinuria), prenatal diagnosis allows appropriate medical care at birth.
Some clues can be proposed to improve diagnosing IEM on ultrasound findings. A systematic retrospective review of antenatal signs of patients presenting with clinical signs early in the neonatal period can perhaps allow identification of more (or even new) specific ultrasound findings. The development of fetal spectroscopic MRI could be helpful by identifying an increased signal for N-acetylaspartic acid in Canavan disease, an increase of lactic acid in respiratory chain disorders and other disorders of energy metabolism, and eventually a decrease in creatine in creatine transporter defect. A tandem mass-spectrometry-based metabolomics study of AF, as it has been developed in urine (Pitt et al. 2002), could allow detection in a same run of abnormal metabolites. Whole-exome sequencing or development of gene panels according to ultrasound findings (HF, chondrodysplasia, …) will probably be the more promising way to reach a diagnosis. This approach can be applied using DNA from the fetus or, if it is not available, from the parents. However, biochemical investigation will remain helpful to confirm the deleterious effect of undescribed variants, and this emphasizes the need to collect fetal material.
References
Al Kaissi A, Kurz H, Bock W, Pärtan G et al (2014) Agenesis of the corpus callosum and skeletal deformities in two unrelated patients: Analysis via MRI and Radiography. Case Rep Orthop 2014:186973
Alfadhel M, Alrifai MT, Trujillano D et al (2015) Asparagine synthetase deficiency: new inborn errors of metabolism. JIMD Rep 22:11–16
Anderson G, Smith VV, Malone M, Sebire NJ (2005) Blood film examination for vacuolated lymphocytes in the diagnosis of metabolic disorders; retrospective experience of more than 2,500 cases from a single centre. J Clin Pathol 58:1305–1310
Andersson HC, Kratz L, Kelley R (2002) Desmosterolosis presenting with multiple congenital anomalies and profound developmental delay. Am J Med Genet 113(4):315–319
Bandaralage SP, Farnaghi S, Dulhunty JM, Kothari A (2016) Antenatal and postnatal radiologic diagnosis of holocarboxylase synthetase deficiency: a systematic review. Pediatr Radiol 46:357–364
Baroy T, Koster J, Stromme P et al (2015) A novel type of rhizomelic chondrodysplasia punctata, RCDP5, is caused by loss of the PEX5 long isoform. Hum Mol Genet 24:5845–5854
Bellini C, Donarini G, Paladini D et al (2015) Etiology of non-immune hydrops fetalis: An update. Am J Med Genet A 167A:1082–1088
Benoist JF, Imbard A, Dreux S et al (2007) Antenatal biochemical expression of cystinuria and relation to fetal hyperechogenic colon. Clin Chem 53:149–150
Boemer F, Deberg M, Schoos R et al (2016) Diagnostic pitfall in antenatal manifestations of CPT II deficiency. Clin Genet 89:193–197
Brasseur-Daudruy M, Garel C, Brossard V et al (2006) Hyper-echogenic colon: a prenatal sign of cystinuria? Prenat Diagn 26:1254–1255
Brunetti-Pierri N, Corso G, Rossi M et al (2002) Lathosterolosis, a novel multiple-malformation / mental retardation syndrome due to deficiency of 3-beta-hydroxysteroid-delta-5-desaturase. Am J Hum Genet 71:952–958
Buchert R, Tawamie H, Smith C et al (2014) A peroxisomal disorder of severe intellectual disability, epilepsy, and cataracts due to fatty acyl-CoA reductase 1 deficiency. Am J Hum Genet 95:602–610
Burin MG, Scholz AP, Gus R et al (2004) Investigation of lysosomal storage diseases in nonimmune hydrops fetalis. Prenat Diagn 24:653–657
Cañueto J, Girós M, Ciria S et al (2012) Clinical, molecular and biochemical characterization of nine Spanish families with Conradi-Hünermann-Happle syndrome: new insights into CDPX2 with a comprehensive review of literature. Br J Dermatol 166:830–838
Carmi-Nawi N, Malinger G, Mandel H et al (2011) Prenatal brain disruption in molybdenum cofactor deficiency. J Child Neurol 26:460–464
Chuang WL, Pacheco J, Cooper S et al (2014) Lyso-sphingomyelin is elevated in dried blood spots of Niemann-Pick B patients. Mol Genet Metab 111:209–211
Clayton P, Winchester B, Di Tomaso E et al (1993) Carbohydrate-deficient glycoprotein syndrome: normal glycosylation in the fetus. Lancet 341:956
Dacremont G, Vincent G (1995) Assay of plasmalogens and polyunsaturated fatty acids (PUFA) in erythrocytes and fibroblasts. J Inherit Metab Dis 18(Suppl 1):84–89
de Koning TJ (2013) Amino acid synthesis deficiencies. Handb Clin Neurol 113:1775–1783
de Koning TJ, Duran M, Van Maldergem L et al (2002) Congenital microcephaly and seizures due to 3-phosphoglycerate dehydrogenase deficiency: outcome of treatment with amino acids. J Inherit Metab Dis 25:119–125
de Vet EC, IJlst L, Oostheim W et al (1999) Ether lipid biosynthesis. Alkyl-dihydroxyacetonephosphate synthase protein deficiency leads to reduced dihydroxyacetonephosphate acyltransferase activities. J Lipid Res 40:1998–2003
Dimauro S, Garone C (2011) Metabolic disorders of fetal life: glycogenoses and mitochondrial defects of the mitochondrial respiratory chain. Semin Fetal Neonatal Med 16:181–189
Dreux S, Salomon LJ, Rosenblatt J et al (2015) Biochemical analysis of ascites fluid as an aid to etiological diagnosis: a series of 100 cases of nonimmune fetal ascites. Prenat Diagn 35:214–220
Dugan RB, Pletneva MA, Salari K et al (2014) Nonimmune fetal hydrops and lysosomal storage disease: the finding of vacuolated lymphocytes in ascitic fluid in two cases. Prenat Diagn 34(2):199–201
Edwards M, McKenzie F, O’callaghan S et al (2006) Prenatal diagnosis of congenital disorder of glycosylation type Ia (CDG-Ia) by cordocentesis and transferrin isoelectric focussing of serum of a 27-week fetus with non-immune hydrops. Prenat Diagn 26:985–988
Froissart R, Cheillan D, Bouvier R et al (2005) Clinical, morphological, and molecular aspects of sialic acid storage disease manifesting in utero. J Med Genet 42:829–836
Giese AK, Mascher H, Grittner U et al (2015) A novel, highly sensitive and specific biomarker for Niemann-Pick type C1 disease. Orphanet J Rare Dis 10:78
Gimovsky AC, Luzi P, Berghella V (2015) Lysosomal storage disease as an etiology of nonimmune hydrops. Am J Obstet Gynecol 212:281–290
Goldenberg A, Wolf C, Chevy F et al (2004) Antenatal manifestations of Smith-Lemli-Opitz (RSH) syndrome: a retrospective survey of 30 cases. Am J Med Genet A 124A:423–436
Häberle J, Görg B, Rutsch F et al (2005) Congenital glutamine deficiency with glutamine synthetase mutations. N Engl J Med 353:1926–1933
Ho AC, Fung CW, Siu TS et al (2014) Lathosterolosis: a disorder of cholesterol biosynthesis resembling Smith-Lemli-Opitz syndrome. JIMD Rep 12:129–134
Jakobs C (1989) Prenatal diagnosis of inherited metabolic disorders by stable isotope dilution GC-MS analysis of metabolites in amniotic fluid: review of four years experience. J Inherit Metab Dis 12(suppl2):267–270
Jiang X, Sidhu R, Porter FD et al (2011) A sensitive and specific LC-MS/MS method for rapid diagnosis of Niemann-Pick C1 disease from human plasma. J Lipid Res 52:1435–1445
Kattner E, Schafer A, Harzer K (1997) Hydrops fetalis: manifestation in lysosomal storage diseases including Farber disease. Eur J Pediatr 156:292–295
Konstantinidou A, Karadimas C, Waterham HR et al (2008) Pathologic, radiographic and molecular findings in three fetuses diagnosed with HEM/Greenberg skeletal dysplasia. Prenat Diagn 28:309–312
Lacalm A, Nadaud B, Massoud M et al (2016) Prenatal diagnosis of cobblestone lissencephaly associated with Walker-Warburg syndrome based on a specific sonographic pattern. Ultrasound Obstet Gynecol 47:117–122
Lefebvre M, Dufernez F, Bruel AL et al (2015) Severe X-linked chondrodysplasia punctata in nine new female fetuses. Prenat Diagn 35:675–684
Lepais L, Cheillan D, Collardeau-Frachon S et al (2015) ALG3-CDG: Report of two siblings with antenatal features carrying homozygous p.Gly96Arg mutation. Am J Med Genet A 167:2748–2754
Léticée N, Bessières-Grattagliano, Dupré T et al (2010) Should PMM2-deficiency (CDG Ia) be searched in every case of unexplained hydrops fetalis? Mol Genet Metab 101:253–277
L’herminé-Coulomb A, Beuzen F, Bouvier R et al (2005) Fetal type IV glycogen storage disease: clinical, enzymatic, and genetic data of a pure muscular form with variable and early antenatal manifestations in the same family. Am J Med Genet A 139A:118–222
Machin GA (1989) Hydrops revisited: literature review of 1,414 cases published in the 1980. Am J Med Genet 34:366–390
Matthijs G, Schollen E, Cassiman JJ et al (1998) Prenatal diagnosis in CDG1 families: beware of heterogeneity. Eur J Hum Genet 6:99–104
Mellerio C, Marignier S, Roth P et al (2008) Prenatal cerebral ultrasound and MRI findings in glutaric aciduria Type 1: a de novo case. Ultrasound Obstet Gynecol 31:712–714
Nada MA, Vianey-Saban C, Roe CR et al (1996) Prenatal diagnosis of mitochondrial fatty acid oxidation defects. Prenat Diagn 16:117–124
Ottolenghi C, Hubert L, Allanore Y et al (2011) Clinical and biochemical heterogeneity associated with fumarase deficiency. Hum Mutat 32:1046–1052
Pagan C, Latour P, Ruet S et al (2015) Contribution of plasmatic biomarkers to the diagnosis of Niemann-Pick type C. J Inherit Metab Dis 38(Suppl1):S57
Patel KP, O’Brien TW, Subramony SH et al (2012) The spectrum of pyruvate dehydrogenase complex deficiency: clinical, biochemical and genetic features in 371 patients. Mol Genet Metab 105:34–43
Paupe A, Bidat L, Sonigo P et al (2002) Prenatal diagnosis of hypoplasia of the corpus callosum in association with non-ketotic hyperglycinemia. Ultrasound Obstet Gynecol 20:616–619
Pettazzoni M, Piraud M, Froissart R et al (2015a) LS-MS/MS lysosphingolipids measurement in plasma for the screening and follow-up of lysosomal storage diseases. J Inherit Metab Dis 38(Suppl 1):S56
Pettazzoni M, Streichenberger N, Mezin P et al (2015b) Congenital and perinatal glycogen storage disease type IV: clinical, pathological and biological data in 5 cases. J Inherit Metab Dis 38(Suppl 1):S182
Piraud M, Froissart R, Mandon G, Bernard A, Maire I (1996) Amniotic fluid for screening of lysosomal storage diseases presenting in utero (mainly as non-immune hydrops fetalis). Clin Chim Acta 248:143–155
Pitt JJ, Eggington M, Kahler SG (2002) Comprehensive screening of urine samples for inborn errors of metabolism by electrospray tandem mass spectrometry. Clin Chem 48:1970–1980
Prick BW, Hop WC, Duvekot JJ (2012) Maternal phenylketonuria and hyperphenylalaninemia in pregnancy: pregnancy complications and neonatal sequelae in untreated and treated pregnancies. Am J Clin Nutr 95:374–382
Rigaud C, Lebre AS, Touraine R et al (2013) Natural history of Barth syndrome: a national cohort study of 22 patients. Orphanet J Rare Dis 8:70–82
Rolfs A, Giese AK, Grittner U et al (2013) Glucosylsphingosine is a highly sensitive and specific biomarker for primary diagnostic and follow-up monitoring in Gaucher disease in a non-Jewish, Caucasian cohort of Gaucher disease patients. PLoS One 8:e79732
Rolland MO, Cuisset L, Le Bozec J et al (2005) First-trimester enzymatic and molecular prenatal diagnosis of mevalonic aciduria. J Inker Metab Dis 28:1141–1142
Rossi M, d’Armento M, Parisi I et al (2007) Clinical phenotype of lathosterolosis. Am J Med Genet A 143:2371–2381
Rossi M, Hall CM, Bouvier R et al (2015) Radiographic features of the skeleton in disorders of post-squalene cholesterol biosynthesis. Pediatr Radiol 45:965–976
Schaefer AM, Taylor RW, Turnbull DM, Chinnery PF (2004) The epidemiology of mitochondrial disorders–past, present and future. Biochim Biophys Acta 1659:115–120
Schmidt MR, Birkebaek N, Gonzalez I, Sunde L (2004) Barth syndrome without 3-methylglutaconic aciduria. Acta Paediatr 93:419–421
Schollen E, Kjaergaard S, Legius E et al (2000) Lack of Hardy-Weinberg equilibrium for the most prevalent PMM2 mutation in CDG-Ia (congenital disorders of glycosylation type Ia). Eur J Hum Genet 8:367–371
Schwarzer V, Haas D, Hoffmann GF et al (2008) Abnormal prenatal ultrasound findings in mevalonic aciduria. Prenat Diagn 28:257–258
Shigematsu Y, Hata I, Nakai A et al (1996) Prenatal diagnosis of organic acidemias based on amniotic fluid levels of acylcarnitines. Pediatr Res 39:680–684
Spiegel R, Raas-Rothschild A, Reish O et al (2009) The clinical spectrum of fetal Niemann-Pick type C. Am J Med Genet 149A:446–450
Steinberg S, Chen L, Wei L et al (2004) The PEX Gene Screen: molecular diagnosis of peroxisome biogenesis disorders in the Zellweger syndrome spectrum. Mol Genet Metab 83:252–263
Steward CG, Newbury-Ecob RA, Hastings R et al (2010) Barth syndrome: an X-linked cause of fetal cardiomyopathy and stillbirth. Prenat Diagn 30:970–976
Suormala T, Fowler B, Jakobs C et al (1998) Late-onset holocarboxylase synthetase-deficiency: pre- and post-natal diagnosis and evaluation of effectiveness of antenatal biotin therapy. Eur J Pediatr 157:570–575
Taroni F, Gellera C, Cavadini P et al (1994) Lethal carnitine palmitoyltransferase (CPT) II deficiency in newborns: a molecular-genetic study. Am J Hum Genet 55:A265
Tavares MV, Santos MJ, Domingues AP et al (2013) Antenatal manifestations of mitochondrial disorders. J Inherit Metab Dis 36:805–811
Thuy LP, Belmont J, Nyhan WL (1999) Prenatal diagnosis and treatment of holocarboxylase synthetase deficiency. Prenat Diagn 19:108–112
Valayannopoulos V, Verhoeven NM, Mention K et al (2006) Transaldolase deficiency: a new cause of hydrops fetalis and neonatal multi-organ disease. J Pediatr 149:713–717
Vanier MT, Latour P (2015) Laboratory diagnosis of Niemann-Pick disease type C: the filipin staining test. Methods Cell Biol 126:357–375
Verhoeven NM, Kulik W, van den Heuvel CM, Jakobs C (1995) Pre- and postnatal diagnosis of peroxisomal disorders using stable-isotope dilution gas chromatography--mass spectrometry. J Inherit Metab Dis 18(Suppl 1):45–60
Vianey-Saban C, Bouvier R, Cochat P et al (2000) Antenatal expression of multiple acyl-CoA dehydrogenase deficiency. J Inherit Metab Dis 23:345–348
von Kleist-Retzow JC, Cormier-Daire V, Viot G et al (2003) Antenatal manifestations of mitochondrial respiratory chain deficiency. J Pediatr 143:208–212
Wamelink MM, Struys EA, Valayannopoulos V et al (2008) Retrospective detection of transaldolase deficiency in amniotic fluid: implications for prenatal diagnosis. Prenat Diagn 28:460–462
Waterham HR, Ferdinandusse S, Wanders RJ (2016) Human disorders of peroxisome metabolism and biogenesis. Biochim Biophys Acta 1863:922–933
Wopereis S, Lefeber D, Morava E, Wevers R (2006) Mechanisms in protein O-glycan biosynthesis and clinical and molecular aspects of protein O-glycan biosynthesis: a review. Clin Chem 52:574–600
Yanicostas C, Soussi-Yanicostas N, El-Khoury R et al (2011) Developmental aspects of respiratory chain from fetus to infancy. Semin Fetal Neonatal Med 16:175–180
Zemski Berry KA, Murphy RC (2004) Electrospray ionization tandem mass spectrometry of glycerophosphoethanolamine plasmalogen phospholipids. J Am Soc Mass Spectrom 15:1499–1508
Zolotushko J, Flusser H, Markus B et al (2011) The desmosterolosis phenotype: spasticity, microcephaly and micrognathia with agenesis of corpus callosum and loss of white matter. Eur J Hum Genet 19:942–946
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Details of funding for all research studies
The authors confirm independence from the sponsors; the content of the article has not been influenced by the sponsors.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of Helsinki 1975, as revised in 2000. Informed consent was obtained from all patients for inclusion in the study.
Additional information
Communicated by: Carlo Dionisi-Vici
Rights and permissions
About this article
Cite this article
Vianey-Saban, C., Acquaviva, C., Cheillan, D. et al. Antenatal manifestations of inborn errors of metabolism: biological diagnosis. J Inherit Metab Dis 39, 611–624 (2016). https://doi.org/10.1007/s10545-016-9947-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-016-9947-8