Abstract
Objectives
To test the hypothesis that more frequent enzyme replacement therapy (ERT) slows the decline in kidney function in adult patients with Fabry disease.
Methods
A single center open label 10-year prospective clinical trial of 12 patients with advanced Fabry disease who, after having experienced an ongoing decline in renal function after 2-4 years of receiving ERT at the approved dose of 0.2 mg/kg agalsidase alfa every other week (EOW), were switched to weekly (EW) ERT at the same dose. We used linear regression to fit each individual patient’s longitudinal estimated glomerular filtration rate (eGFR) record in order to compare the deterioration rates between EOW and EW ERT.
Results
For the entire group, mean slope on agalsidase alfa every 2 weeks was -7.92 ± 2.88 ml/min/1.73 m2/year and 3.84 ± 4.08 ml/min/1.73 m2/year on weekly enzyme infusions (p = 0.01, two-tailed paired t test). Three patients (25 %) completed the entire study with relatively preserved renal function while 50 % of patients reached end-stage renal disease (ESRD) during the 10 years of this study. The estimated average delay to ESRD was 13.8 years [n = 11; 95 % CI 0.66, 27]. One patient had a positive eGFR slope on weekly infusions while the patient with the highest antibody titer had a steeper slope after switching. Mean globotriaosylceramide concentrations in urine and plasma as well as urine protein excretion remained unchanged.
Conclusions
Weekly enzyme infusions slow the decline of renal function in a subgroup of more severe patients thus showing that existing ERT can be further optimized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fabry disease is a progressive, X-linked metabolic vasculopathy due to the deficiency of the lysosomal enzyme alpha-galactosidase A (GALA) (Brady et al 1967). The incidence is estimated to be between 1:40,000 and 1:170,000 based on diagnostics for symptomatic patients. In newborn screening studies in Europe incidences of up to 1:1,250 were reported, although those studies frequently identify genetic variants with unknown clinical significance (van der Tol et al 2014). The enzymatic defect leads to the storage of alpha-d-galactosyl conjugates, such as globotriaosylceramide (Gb3), in a plethora of cells and tissues, e.g., vascular endothelial cells, pericytes, and smooth muscle cells of the vascular system, renal epithelial cells, myocardial cells, and dorsal root ganglion neuronal cells (Brady and Schiffmann 2000). The disease has its clinical onset in school age with pain in the extremities, recurrent febrile episodes, and recurrent abdominal pain (Ries et al 2003, 2005). With progression of the condition, patients develop cardiomyopathy, progressive renal failure, hearing loss, and recurrent strokes (Ries et al 2007). Patients with Fabry disease have a risk of premature death. Median age of death in males is 50 to 57 years and in females 70 to 72 years (El Dib et al 2013).
Enzyme replacement therapy plays an important role in the treatment of Fabry disease (Eng et al 2001; Schiffmann et al 2001). Two enzyme preparations are available, agalsidase alfa (Replagal®, Shire Human Genetic Therapies, Lexington, MA), given at 0.2 mg/kg every other week, approved in the European Union in 2001, and agalsidase beta (Fabrazyme®, Genzyme, Cambridge, MA), given at 1.0 mg/kg every other week, approved in the USA in 2003 and European Union in 2001 (EMA 2006, 2005; FDA 2003).
In children, prevention of late stage complications is the goal of therapy, whereas in adults at least a delay of disease progression should be achieved (Ries et al 2005; Wraith et al 2008; Borgwardt et al 2013; Anderson et al 2014). There are patients with advanced Fabry disease who continue to deteriorate despite enzyme replacement therapy (Rombach et al 2014), (Sirrs et al 2014). More frequent enzyme infusions were considered by the investigator, because intra-cellular half-life of the enzyme is between 24-48 hours, which covers only a limited time in the conventional biweekly infusion regimen (Schiffmann et al 2000). Despite one or more years of ERT, plasma and urinary sediment Gb3, while remaining reduced by approximately 50 % compared to baseline in most patients, tend to rise progressively toward patients’ pretreatment baseline, in part due to antibody development (Schiffmann et al. 2006). This might be taken to suggest that repeated enzyme infusions at the current dose and frequency mostly break down the Gb3 produced during the interval between ERT administrations rather than the previously accumulated storage material. The onset of renal insufficiency is significantly delayed in patients with a small but measurable residual enzyme activity (2–8 % of normal) (Branton et al 2002). This suggests that continuous presence of even low enzyme activity in cells may be sufficient to substantially reverse the metabolic defect and slow or delay the progression to renal failure. According to our clinical experience, many patients report experiencing more of their Fabry disease symptoms in the second week after their biweekly infusion compared with the first post-infusion week. Increased clinical benefit is seen with higher doses of ERT in Gaucher disease, another lysosomal storage disorder (Altarescu et al 2000).
We initiated a clinical trial to investigate the efficacy and safety of an intensified weekly enzyme replacement therapy instead of every other week infusions of 0.2 mg agalsidase alfa/kg body weight in 2003 in a subgroup of patients with progressive renal decline (Schiffmann et al 2007). Two-year results of this protocol were reported in 2007. The present paper summarizes the efficacy and safety data of 10 years of intensified weekly enzyme replacement, the first and the longest systematic investigation and follow-up of intensified enzyme replacement therapy with agalsidase alfa in Fabry disease. The goal of this clinical trial was to test the hypothesis that weekly infusions of agalsidase alfa at the standard dose of 0.2 mg/kg of body weight will significantly slow the decline in renal function in a subgroup of patients with rapid progressive renal failure who have previously been on the same dose of agalsidase alfa administered every two weeks. In order to ascertain acute effects of enzyme replacement as secondary goal, in some of the patients, was to assess whether sweat function would be similar before and two days following agalsidase alfa infusions. Additional secondary outcome measures include globotriaosylceramide concentrations (Gb3) in plasma and urinary sediment as well as urinary protein excretion.
Materials and methods
Study design
The study design, inclusion and exclusion criteria were published previously together with the 2-year results of the study (Schiffmann et al 2007). Briefly, this was a prospective open label, single center study conducted from 2003 to 2008 at the National Institute of Neurological Disorders and Stroke, Bethesda, MD, and from 2008 to 2013 at the Baylor Research Institute, Dallas, TX, USA. The trial was registered under clinicaltrials.gov identifier NCT00068107. Patients were eligible for this study if they showed a rate of decline of estimated glomerular filtration rate (eGFR) of 5 ml/min per 1.73 m2 or more per year while receiving conventional dosing, i.e., 0.2 mg/kg agalsidase alfa every other week. Concomitant medications for hypertension and/or angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB) for renoprotection were permitted for inclusion into the study if these treatments had remained stable for at least the 6 months before beginning weekly agalsidase alfa therapy. ACE inhibitors or ARB were not to be initiated during the study period unless rapid deterioration of kidney function mandated their use. The institutional review boards of the National Institute of Neurologic Disorders and Stroke and of Baylor Research Institute approved the protocol. All patients gave their written informed consent. This research adheres to the Declaration of Helsinki.
Intervention
Patients received weekly (i.e., every 7 ± 3 days) doses of agalsidase alfa at 0.2 mg/kg body weight administered over 40 min. The initial evaluation and the first weekly infusion were conducted at the National Institutes of Health. Thereafter, patients received their weekly infusions of agalsidase alfa close to their homes at their local clinic or hospital under physician supervision.
Efficacy assessments
As outlined previously, the primary endpoint of this study was the mean rate of change in eGFR during the weekly treatment period of up to 10 years compared with the rate of change of eGFR during the preceding 2 to 4 years of EOW infusions in the same patients (Schiffmann et al 2007). eGFR was determined using the four-variable Modification of Diet in Renal Disease (MDRD) formula (Levey 2002) and sweat function was measured with the quantitative sudomotor axon reflex test (QSART). Quantitative sensory testing (QST) was conducted as previously described (Schiffmann et al 2003). Patients had QST every 6 months. Blood samples were obtained every three months for estimation of GFR, determination of plasma Gb3 levels, and measurement of the presence of anti–agalsidase alfa antibodies. Twenty-four-hour urine collections were obtained at baseline and every three months thereafter for measurement of urine sediment Gb3 levels and urine protein levels. Plasma and urine sediment Gb3 levels as well as anti–agalsidase alfa antibodies were analyzed at Shire Human Genetic Therapies as described previously (Schiffmann et al 2001, 2006, 2007).
Safety assessments
Safety was assessed locally before, during, and after every weekly infusion (vital signs, weight, adverse event assessment, concomitant medication assessment), and more extensively every three months at the study center (physical and neurological examination, vital signs, weight, adverse event assessment, complete blood count with differential, comprehensive metabolic profile, general chemistry, urine analysis including assessment for proteinuria, EKG). All laboratory parameters with the exception of plasma Gb3 and anti–agalsidase alfa antibody determinations were done at the study centers (National Institutes of Health, Baylor University Medical Center).
Statistical analyses
The study analysis plan was conducted as outlined previously (Schiffmann et al 2007): we applied methods of descriptive statistics. All hypothesis testing was two-tailed at significance level of 0.05. All values are expressed as means ± SD unless otherwise noted. Survival curve (time-to-ESRD) was calculated with the Kaplan-Maier method.
Linear regression was used to fit each individual patient’s longitudinal eGFR record to assess the time trends, separately for the EOW and Weekly treatment plans. Slope and intercept fitted by the linear models during the EOW were used to calculate the estimated time-to-event (ESRD) which was defined as eGFR ≤ 10 ml/min/1.73 m2. For those patients who did not develop ESRD after switching to the weekly enzyme administration, expected time-to-event under the weekly treatment plan was estimated in the same fashion as for EOW.
With the expected time-to-event under the EOW and the expected/actual time-to-event under the EW treatment plan, the expected individual delay in time to ESRD due to switching the treatment plan could be calculated as the difference of the two above-mentioned quantities. Hence a sample of expected time-to-event delay was obtained for the ten patients, for which the sample average and 95 % confidence interval were used to estimate the population level expected delay of ESRD occurrence due to the change in frequency of ERT administration. An order statistics-based nonparametric confidence interval for population median was supplied because the distribution of the ten samples delay significantly deviated from the normal.
For quantitative sensory testing, the outcome variable (detection threshold score) was analyzed for all combinations of location (foot, hand, and thigh) and test (cold, vibration, and warm). Score values of “>25” were set to 25. Time, measured in years, was centered at the date in which patients switched treatment regimen. A linear mixed model analysis was used to test for differences in the linear association between time and detection threshold score pre-and-post ERT regimen change while accounting for the correlation among observations from the same individual. Specifically, the model contained a subject specific random intercept with year as a fixed effect and knot at time of the treatment change. The null hypothesis that slopes pre-and-post treatment changes were equal was tested to assess the altered therapy.
Patient population enrollment
Patients with the classic (most severe) form of Fabry disease who previously were treated 2-4 years with agalsidase alfa ERT at 0.2 mg/kg every two weeks were eligible for this study. All but one had progressive renal insufficiency with estimated glomerular filtration declining over 5 ml/min/1.73 m2/year. We screened 41 patients; 12 patients were initially entered into this study. One patient was found to have stable kidney function and no change in sweat function on QSART and was subsequently excluded. All patients were white males, ten of non-Hispanic and two of Hispanic ethnicity. The median age at baseline was 44 (range 24 to 53) years.
Results
Patient disposition (see also Table 1)
Twelve patients were enrolled in this trial. Patient 013 was screened initially but was found to have stable renal function and was therefore excluded from the rest of the study. Patient 004, who entered this study with a creatinine clearance of 15 ml/1.73 m2/min had two coronary angiographies two months into this protocol. These procedures precipitated a rapid decline in GFR followed by the need for renal dialysis and death a few months later from a cardiac event. Eleven patients with declining GFR completed two years on this protocol. One patient (005) with normal and stable kidney function left the protocol after two years for personal reasons. During the fifth year, patient 012 left the protocol for personal reasons and patient 002 reached end-stage kidney disease that was precipitated by his heart disease and the use of contrast material. Patient 011 received a renal transplantation from a live donor after 5 years. During year 6, patient 001 had severe renal decline and began kidney dialysis and was therefore taken off the study. Patient 006 reached end-stage renal disease and was started on dialysis. During year 8, patient 010, who had reached end-stage kidney disease and had received a renal transplant, was started on agalsidase beta enzyme replacement therapy and was therefore excluded from this study. During the 10th year patient 009 died suddenly at home, presumably from an acute cardiac event. No autopsy was performed. The study ended in December 2013 after 10 years. The three patients who completed this protocol have been transitioned to agalsidase beta enzyme replacement therapy. A survival analysis on weekly enzyme infusions for the study cohort is shown in Fig. 1.
Compliance remained high overall: in all but one patient (patient 006), less than 7 % of the infusions were missed.
-
a)
Efficacy
Effect of weekly infusions on eGFR (Table 1)
Mean slope on agalsidase alfa every 2 weeks was -7.92 ± 2.88 ml/min/1.73 m2/year and -3.84 ± 4.08 ml/min/1.73 m2/year on weekly enzyme infusions (p = 0.01, two-tailed paired t test). Seven out of ten patients had a positive estimated delay to ESRD events due to the treatment plan switching from EOW to EW enzyme infusions (Figs. 1 and 2 in Supplementary material). The decline of eGFR in patient 003 was reversed after switching to EW treatment plan (Fig. 3 Supplementary material). However, patient 006 experienced an expedited decline of eGFR slope after switching (-8.52 ml/min/1.73 m2/year (EOW) vs 13.2 ml/min/1.73 m2/year (EW), (Fig. 4 Supplementary material). Without considering patient 003’s very promising case, the mean estimated delay to ESRD was 13.8 years [n = 11; 95%CI 0.66, 27]. The median delay of time-to-end stage renal disease among the ten patients was 5 years (nonparametric 80 % confidence interval for median TTE delay as [3,49])
Despite the positive effect of weekly ERT, patients 001, 002, 006, 010, 011, and 012 reached end-stage renal disease during the 10 years of this study (Fig. 1). Three patients completed the entire 10 years study with relatively preserved renal function (Table 1).
Effect of weekly infusions on sweat function assessed by QSART
QSART response pre-or post-infusion did not change in a reliable direction. QSART testing was stopped in this protocol in year 4 (data not shown). Patient 005 with stable renal function was entered into this study because of low sweat output prior to enzyme infusion in the every two weeks regimen, with marked improvement post-infusion. His kidney function remained stable and normal, but there was no consistent improvement in the sweating patterns over most of the time points. At two years he did have a pre-infusion response that increased post-infusion (Schiffmann et al 2003).
Effect of weekly infusions on quantitative sensory testing
There was no significant change in the slopes of vibration and cold perception. However, the slopes for warm perception in the hand, thigh, and foot during EW infusions were changed significantly to positive (detection thresholds deteriorated over time) from negative (improving detection threshold) slopes during EOW infusions (Supplementary Table 1).
Metabolic effects and effects on urine protein excretion
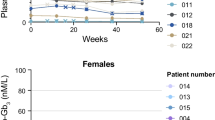
There was no significant change in Gb3 concentrations in both plasma (data not shown) and urine Gb3 concentrations as well as in urine protein excretion between baseline and last measured values (Figs. 2 and 3).
Safety
A total of 25 serious adverse events were reported during the study. All were thought to be unrelated to study drug and reflect the advanced Fabry disease stages of these patients. No infusion reactions to agalsidase alfa have been observed during this study. The adverse events that occurred were all expected manifestations or known complications of Fabry disease. Routine blood tests did not suggest any agalsidase alfa-related abnormality.
Anti-agalsidase alfa antibodies
Five patients had positive IgG antibody titers against agalsidase alfa. Patient 006 had the highest titer, which ranged between 1600 and 6400; patient 004’s titer increased to 1600. Both patients did poorly in this study. The antibody titer of patients 7 and 12 was below 1600 and tended to decrease over time. No patient tested positive for IgE antibodies against agalsidase alfa.
Discussion
This is the longest study assessing the effects of intensified enzyme replacement therapy with weekly administrations in advanced Fabry disease. Per the inclusion critieria of this study, the present patient cohort represents the spectrum of more severe renal disease when compared with the populations described by Rombach which may be due to the heavy proteinuria (Fig. 3) (Rombach et al 2014).
In general, this population of patients with advanced Fabry disease who did not respond sufficiently to biweekly ERT experienced continued decline in renal function on weekly enzyme infusions. However, it appears that the slope of decline could be decelerated and ESRD was significantly delayed in nine out of ten patients. The eGFR slope became positive in another patient. Overall, three out of 12 patients (25 %) completed the 10-year study with relatively preserved kidney function. As expected, weekly ERT was not able to reverse the advanced phenotype of Fabry disease and cardiovascular complications of the underlying condition continued to occur. This finding is not surprising since ERT in other lysosomal storage diseases, such as the mucopolysaccharidoses, has been shown to work better if administered weekly when compared directly with every other week administration (Muenzer et al 2006).
A short time comparison of three different doses of agalsidase alfa 0.2 mg/kg every other week, 0.1 mg/kg weekly and 0.2 mg/kg weekly each given for four weeks in a cross-over study in 18 patients showed no statistically significant difference between the doses (Hughes et al 2013). Other studies have shown no significant change when switching from agalsidase beta 1 mg/kg every other week to agalsidase alfa 0.2 mg/kg every other week (Pisani et al 2013; Lin et al 2014; Tsuboi and Yamamoto 2014). One study with non-randomized switch from agalsidase beta 1 mg/kg every other week to agalsidase alfa 0.2 mg/kg every other week or agalsidase beta 0.3 – 0.5 mg/kg every other week was associated with progression in microalbuminuria and Fabry related symptoms after 1 year (Weidemann et al 2014). The cumulative dose received by the present subjects is less than if they had been treated with 1.0 mg/kg EOW. This may have had an effect clearance of Gb3 in podocytes (Tondel et al 2013), but renal biopsies were not part of the present protocol. It is not currently possible to separate ERT dose from frequency of administration. Therefore, the value of the cumulative ERT dose in a chronic metabolic disorder is of unclear significance. A head-to-head comparison of the clinical outcome of biweekly enzyme infusions with agalsidase alfa at 0.2 mg/kg and agalsidase beta at 1 mg/kg every other week was conducted in an overall study of 362 patients during five years in Canada. In this large study population, clinical events, i.e., death, neurologic or cardiovascular events, development of end-stage renal disease or sustained increase in serum creatinine of 50 % from baseline were similar in the two treatment groups (Sirrs et al 2014). Advanced Fabry disease is difficult to treat. In a case-historical control study of patients with advanced Fabry disease (N = 40 in each group), the rate of clinical events (stroke, ESRD, sudden cardiac death) was not different between the matched natural history group and the group that received ERT for up to 6 years (Weidemann et al 2013). In the present study, Gb3 concentrations in urine and plasma did not predict clinical response in this study. This is in line with the previous finding, that Gb3 is a diagnostic and pharmacodynamics biomarker, but not a surrogate biomarker (Schiffmann et al 2013).
The greater decline in detection threshold for warm stimuli during EW infusions compared to EOW is not easily explained, particularly in view of the relative stability of cold and vibration perceptions. It is unlikely that weekly infusions are a direct cause of these changes, and therefore, we assume that other unknown concurrent confounders explain these changes. Furthermore, with small number of subjects, outliers are hard to assess and can heavily influence results.
This study has several limitations. The main outcome measure consisted of change over time of the eGFR (slope) rather than a clinical event such as ESRD. However, it has been recently shown that a more rapid decline in eGFR is strongly associated with the risk of ESRD (Coresh et al 2014). In addition, categorical endpoints would require larger sample sizes. The study was uncontrolled. A 10-year study design with an untreated control group was not considered feasible due to the commercial availability of enzyme replacement therapy. The sample size was small. This is due to the fact that the disease is rare and that the study design targeted a small subgroup with advanced disease. Since most patients were on concomitant treatment with ACE-inhibitors and/or ARBs it is difficult to estimate the net effect of any of these medications. However, as exemplified in patients 006 and 011, ACE-I/ARB did not prevent significant decline of renal function (Table 1). On the other hand, renal function in patients 003 and 008 stabilized without the use of such medications, probably facilitated by the low presence or absence of proteinuria in these patients. Because of ongoing deterioration, patients 002, 006, 011, 012 were started on double the weekly dose of 0.4 mg/kg at the 2 years’ time point in an attempt to rescue. Follow-up did not suggest a further slowing effect compared to the 0.2 mg/kg/week dose and all reached end-stage renal disease while in this study. It is noteworthy that all patients who were anti-agalsidase alfa antibody positive progressed to end stage renal disease and that the patients who remain in this study and therefore are the slowest progressors have always been antibody negative (4/5), or were transiently positive (1/5). However, antibody negativity did not protect from rapid renal deterioration (patients 001, 002, 011). Nevertheless we demonstrated some preservation of eGFR despite heavy proteinuria in our study population with twice the dose of initial enzyme replacement although administered at a higher frequency, i.e., weekly infusions.
The optimal time to start ERT, its dosing and frequency will very likely remain an open issue in the absence of conclusive evidence. Early diagnosis and therapy may provide better results than treatment initiated at a late stage of disease when end-organ damage may not be reversible anymore. Early systematic screening of pediatric populations with a clinical risk constellation for Fabry disease may facilitate instituting early therapy. This approach is being assessed in a multicenter pilot study of pediatric patients (clinicaltrials.gov identifier NCT02152189). Novel therapy approaches with increased efficacy are desirable.
References
Altarescu G, Schiffmann R, Parker CC et al (2000) Comparative efficacy of dose regimens in enzyme replacement therapy of type I Gaucher disease. Blood Cells Mol Dis 26:285–290
Anderson LJ, Wyatt KM, Henley W et al (2014) Long-term effectiveness of enzyme replacement therapy in Fabry disease: results from the NCS-LSD cohort study. J Inherit Metab Dis 27(6):969–978. doi:10.1007/s10545-014-9717-4
Borgwardt L, Feldt-Rasmussen U, Rasmussen AK, Ballegaard M, Meldgaard Lund A (2013) Fabry disease in children: agalsidase-beta enzyme replacement therapy. Clin Genet 83:432–438
Brady RO, Schiffmann R (2000) Clinical features of and recent advances in therapy for Fabry disease. JAMA 284:2771–2775
Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L (1967) Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N Engl J Med 276:1163–1167
Branton MH, Schiffmann R, Sabnis SG et al (2002) Natural history of Fabry renal Disease: influence of alpha- galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore) 81:122–138
Coresh J, Turin TC, Matsushita K et al (2014) Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311:2518–2531
El Dib RP, Nascimento P, Pastores GM (2013) Enzyme replacement therapy for Anderson-Fabry disease. Cochrane Database Syst Rev 2, CD006663
EMA Fabrazyme: EPAR - Product Information In Editor ed.^eds. Book Fabrazyme: EPAR - Product Information. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000370/WC500020547.pdf. Accessed 27 July 2014
EMA Replagal: EPAR - Product Information. In Editor ed.^eds. Book Replagal: EPAR - Product Information. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000369/WC500053612.pdf. Accessed 24 July 2014
Eng CM, Banikazemi M, Gordon RE et al (2001) A phase 1/2 clinical trial of enzyme replacement in fabry disease: pharmacokinetic, substrate clearance, and safety studies. Am J Hum Genet 68:711–722
FDA FABRAZYME, BLA no. 103979 In Editor ed.^eds. Book FABRAZYME, BLA no. 103979. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103979s5135lbl.pdf. Accessed 24 July 2014
Hughes DA, Deegan PB, Milligan A et al (2013) A randomised, double-blind, placebo-controlled, crossover study to assess the efficacy and safety of three dosing schedules of agalsidase alfa enzyme replacement therapy for Fabry disease. Mol Genet Metab 109:269–275
Levey AS (2002) Clinical practice. Nondiabetic kidney disease. N Engl J Med 347:1505–1511
Lin HY, Huang YH, Liao HC et al (2014) Clinical observations on enzyme replacement therapy in patients with Fabry disease and the switch from agalsidase beta to agalsidase alfa. J Chin Med Assoc 77:190–197
Muenzer J, Wraith JE, Beck M et al (2006) A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome). Genet Med 8:465–473
Pisani A, Spinelli L, Visciano B et al (2013) Effects of switching from agalsidase Beta to agalsidase alfa in 10 patients with anderson-fabry disease. JIMD Rep 9:41–48
Ries M, Ramaswami U, Parini R et al (2003) The early clinical phenotype of Fabry disease: a study on 35 European children and adolescents. Eur J Pediatr 162:767–772
Ries M, Gupta S, Moore DF et al (2005) Pediatric Fabry disease. Pediatrics 115:e344–e355
Ries M, Kim HJ, Zalewski CK et al (2007) Neuropathic and cerebrovascular correlates of hearing loss in Fabry disease. Brain 130:143–150
Rombach SM, Smid BE, Linthorst GE, Dijkgraaf MG, Hollak CE (2014) Natural course of Fabry disease and the effectiveness of enzyme replacement therapy: a systematic review and meta-analysis: effectiveness of ERT in different disease stages. J Inherit Metab Dis 37:341–352
Schiffmann R, Murray GJ, Treco D et al (2000) Infusion of alpha-galactosidase A reduces tissue globotriaosylceramide storage in patients with Fabry disease. Proc Natl Acad Sci U S A 97:365–370
Schiffmann R, Kopp JB, Austin HA 3rd et al (2001) Enzyme replacement therapy in fabry disease: a randomized controlled trial. JAMA 285:2743–2749
Schiffmann R, Floeter MK, Dambrosia JM et al (2003) Enzyme replacement therapy improves peripheral nerve and sweat function in Fabry disease. Muscle Nerve 28:703–710
Schiffmann R, Ries M, Timmons M, Flaherty JT, Brady RO (2006) Long-term therapy with agalsidase alfa for Fabry disease: safety and effects on renal function in a home infusion setting. Nephrol Dial Transplant 21:345–354
Schiffmann R, Askari H, Timmons M et al (2007) Weekly enzyme replacement therapy may slow decline of renal function in patients with Fabry disease who are on long-term biweekly dosing. J Am Soc Nephrol 18:1576–1583
Schiffmann R, Ries M, Blankenship D et al (2013) Changes in plasma and urine globotriaosylceramide levels do not predict Fabry disease progression over 1 year of agalsidase alfa. Genet Med 15:983–989
Sirrs SM, Bichet DG, Casey R et al (2014) Outcomes of patients treated through the Canadian Fabry disease initiative. Mol Genet Metab 111:499–506
Tondel C, Bostad L, Larsen KK et al (2013) Agalsidase benefits renal histology in young patients with Fabry disease. J Am Soc Nephrol: JASN 24:137–148
Tsuboi K, Yamamoto H (2014) Clinical course of patients with Fabry disease who were switched from agalsidase-beta to agalsidase-alpha. Genet Med 16:766–772
van der Tol L, Smid BE, Poorthuis BJ et al (2014) A systematic review on screening for Fabry disease: prevalence of individuals with genetic variants of unknown significance. J Med Genet 51:1–9
Weidemann F, Niemann M, Stork S et al (2013) Long-term outcome of enzyme-replacement therapy in advanced Fabry disease: evidence for disease progression towards serious complications. J Intern Med 274:331–341
Weidemann F, Kramer J, Duning T et al (2014) Patients with Fabry disease after enzyme replacement therapy dose reduction versus treatment switch. J Am Soc Nephrol 25:837–849
Wraith JE, Tylki-Szymanska A, Guffon N et al (2008) Safety and efficacy of enzyme replacement therapy with agalsidase beta: an international, open-label study in pediatric patients with Fabry disease. J Pediatr 152:563–570, 570 e561
Acknowledgments
This work was supported in part by the Intramural Program of the National Institute of Neurological Disorders and Stroke, by Shire Human Genetic Therapies and the Baylor Healthcare System.
Conflict of interest
Markus Ries was an employee of Shire HGT from 2006 to 2009; he has served on advisory boards for Amicus, Alexion, GSK, and Shire HGT, has received consultancy honoraria from Alexion, Oxyrane, and Shire HGT as well as unrestricted research grants from Shire HGT in compliance with the policy of the Hospital of the University of Heidelberg, Germany. Raphael Schiffmann received research funds and honoraria from Shire HGT and Amicus Therapeutics.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Marc Patterson
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 147 kb)
Rights and permissions
About this article
Cite this article
Schiffmann, R., Swift, C., Wang, X. et al. A prospective 10-year study of individualized, intensified enzyme replacement therapy in advanced Fabry disease. J Inherit Metab Dis 38, 1129–1136 (2015). https://doi.org/10.1007/s10545-015-9845-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-015-9845-5