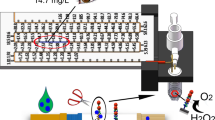
Abstract
Early diagnosis of C reactive protein (CRP) is critical to applying effective therapies for related diseases. Diagnostic technology in today's healthcare systems is mostly deployed in central laboratories, involves expensive and time-consuming processes, and is operated by specialized personnel. For example, the enzyme-linked immunosorbent assay (ELISA), considered the gold standard diagnostic method, is labor-intensive and requires complex procedures such as multiple washing and labeling steps. Due to these limitations of current diagnostic techniques, it is difficult for people to regularly monitor their health and ultimately the disease is more likely to be diagnosed at a later stage. The problem is exacerbated for economically disadvantaged people living in underdeveloped countries. To address these challenges in the traditional diagnostic field, point-of-care (POC) biosensors have emerged as a promising alternative. This allows patients to have their health checked regularly at or near their bedside without resorting to laboratory tests. Nanotechnology-based methods such as biosensors have been extensively researched and developed. Among biosensors, there are also label-free biosensors with high sensitivity that do not require complicated procedures and reduce test time. However, some drawbacks such as high cost, bulky size and need for trained personnel to operate have not been improved. In this review article, we provide an overview of routine methods in CRP diagnosis and then introduce biosensors as a modern, advanced alternative to older methods. Readers of this article can learn about biosensing and its benefits while being aware of the limitations of routine methods.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
C-reactive protein (CRP) was discovered in 1930 by Tillett and Francis (Zeller et al. 2022). The name CRP originates from its initial identification as a substance in the sera of patients with acute inflammation that reacts with the pneumococcal capsular saccharide antigen 'c'. CRP has both anti-inflammatory and pro-inflammatory properties (Manikandan et al. 2020). It plays a role in recognizing and removing damaged cells and foreign pathogens by binding to histones, phosphocholines, phospholipids, chromatin and fibronectin (Jimenez and Szalai 2021). It activates the classical complement pathway and activates phagocytic cells via Fc receptors to damaged or apoptotic cells, facilitate clearance of cell debris, and foreign pathogens (Baruah 2020). However, this can become pathological when activated by autoantibodies presenting the phosphocholine arm in autoimmune processes such as idiopathic thrombocytopenic purpura (ITP) (Baruah 2020). In some cases, CRP exacerbate tissue damage by activating the complement system and activating inflammatory cytokines (Enocsson et al. 2021). Compared to the erythrocyte sedimentation rate, an indirect test of inflammation, CRP levels increase and decrease rapidly with the onset and disappearance of inflammatory incentives (Enocsson et al. 2021; Perico et al. 2021). Sustained elevated CRP levels can be seen in chronic inflammatory diseases such as inflammatory arthritis such as rheumatoid arthritis and chronic infections (Bhagavatham et al. 2022). Elevated C-reactive protein has many causes. These include acute and chronic diseases of infectious or non-infectious origin. However, trauma can also lead to an increase in CRP (alarm response), although significant increases in CRP levels are usually associated with infectious causes (Pathogen-associated molecular patterns (PAMPs)) (Sahu et al. 2020; Souza Pires-Neto and E.d.S.G. Amoras, M.A.F. Queiroz, S. Demachki, S.R. da Silva Conde, R. Ishak, I.M.V. Cayres-Vallinoto, A.C.R. Vallinoto 2020). Low altitude tends to be associated with a wide range of etiologies, from sleep disturbances to periodontal disease. Due to the diagnostic importance of CRP as a biomarker of inflammation, there are many methods commonly used in the literature such as: and various types of biosensors (Wang et al. 2019; Qin et al. 2020). Most of these methods are expensive and difficult to apply in the medical field. ELISA tests are often expensive and time consuming to prepare. Furthermore, the sensitivity of the method depends on the ELISA kit used and is limited to elevated CRP concentrations (Xie et al. 2020). To minimize the problems of conventional CRP assays such as high false positive rate due to non-specific binding, biosensor technology was expansion in the past decade remarkably. Biosensors are one of the most important nanomaterial-based methods in detection of wide range biomarkers and biomolecules. Our goal in this paper is to provide the latest and comprehensive insights on biosensing techniques in the detection of CRP, focusing on the potential of biosensing technology in the determination of various biomarkers and targets.
2 C-reactive protein
C-reactive protein (CRP) is a well-known immune response protein belonging to the pentraxin family of proteins. C-reactive units appear at the very early stages of infection and have been found to be at high concentrations in the blood during inflammatory conditions (Sproston and Ashworth 2018; Avan et al. 2018). Unlike many other acute phase proteins, CRP levels increase more rapidly and have long been used for clinical purposes as a diagnostic and prognostic biomarker and as a response to therapy (Sproston and Ashworth 2018; Avan et al. 2018). Moreover, CRP synthesized in the liver and levels increase in response to inflammation (Ridker and Rane 2021). CRP is an acute-phase response protein that is primarily induced by the action of IL-6 on genes responsible for CRP transcription during the acute phase of inflammatory/infectious processes (Ridker and Rane 2021). It is questionable whether dysregulation of the role of CRP in clearing apoptotic cells and cellular debris is involved in the pathogenesis of systemic lupus erythematosus (SLE), and has not been conclusively demonstrated (Kosutova et al. 2023). Animal studies of lung tissue in alveolitis (an inflammation in the inner part of the lungs) have shown that CRP has several protective properties by reducing neutrophil-mediated damage to the alveoli and leakage of proteins into the lung (Blondonnet et al. 2016) (Fig. 1).
molecular structure of C reactive protein (Volanakis 2001)
3 Methods in detection of C-reactive protein
ELISA is an immunological assay commonly used to measure antigens, antibodies, proteins and glycoproteins in biological samples. ELISA was employed for determination of CRP in an original study (Hastuti et al. 2019). ELISA was advanced for detection of the monomeric conformation (mCRP) at inflammatory loci. This method showed acceptable linearity and analytical properties (Zhang et al. 2018). Serum CRP in systemic lupus erythematosus was evaluated in an original work. All the analytical results were acceptable (Williams et al. 2005). Saliva and serum samples were verified for CRP using a clinically validated hsCRP ELISA kit. Antigen–antibody interactions are generated in a monolayer at the bottom of the well, allowing more antibody molecules to capture and detect low concentrations of CRP (Christodoulides et al. 2005). CRP was assayed by the western blot method in a study. The blots were exposed to films numerous times to obtain a linear response with the enhanced chemiluminescence technique and band density was assessed by densitometry (Nakakuki et al. 2005). Additionally western blot method was applied for detection of CRP in a research work (Li et al. 2004).
As mentioned, there are various methods for identifying CRP, some of which are summarized in Table 1. Examining the results shows that routine methods have many limitations and weaknesses, including low sensitivity and specificity and high cost, so the development of advanced methods has been one of the goals of researchers in recent years. One of the interesting methods is nanotechnology-based methods, especially biosensors. In the following, biosensors will be introduced and biosensors developed for CRP detection will be discussed later.
4 Biosensors
Medical biosensors play a major and emergent role in medical diagnostics and patient monitoring (Baryeh et al. 2017). The use of tissues, biomolecules, and organisms has made biosensors exceptionally suited to numerous diagnostic and real-time detection trials, and the potential for high sensitivity and specificity promises that this trend will continue (Baryeh et al. 2017; Chen et al. 2020). Biosensors suffer from ever-present issues of biocompatibility, particularly with regard to in vivo use. Researchers are rapidly progressing the state-of-the-art in biofouling prevention, and have made dramatic advances in short-term implantable sensors (Chen et al. 2020). As biocompatibility is better understood and controlled, longer-term and more accurate in-vivo sensors will become commonplace. The use of nanomaterials in the development of medical biosensors to improve POC biosensing devices is one of the major topics currently being researched in this field (Pérez et al. 2019). Immobilization of nanostructures in thin films or directly on working electrodes can improve the electrical interaction between sensor components as well as direct electron transfer (DET) for enzyme-catalyzed redox processes (Pérez et al. 2019; Turner et al. 1991). In addition, labeling of specific probes with nanomaterials has enabled lower detection limits and more sensitive sensors through electrical signal amplification. Multiple labels with different nanomaterials have also enabled easy, rapid and inexpensive simultaneous detection of biomarkers (Vadgama 2017). This is important in diagnosing multiple diseases. Furthermore, electrochemical assays can be multiplexed through electrode arrays independent of bulky components such as optics. Therefore, they are suitable for used as POC devices. Conventional techniques due to the need for very low detection limits, high sensitivity and accuracy (Vadgama 2017; Weihs et al. 2021). The development of biosensors has been an important activity over the last 50 years. About 300 scientific papers and 200 patents were filed between 1984 and 1990. For biosensors, the number of patents doubled by 1997, with nanotechnology as a reference for biosensor technology leading to his over 6,000 patent registrations (Parkinson and Pejcic 2005). Typically, biosensors are comprised of three components: (1) the detector, which identifies the stimulus; (2) the transducer, which converts this stimulus to a useful output; and (3) the signal processing system, which involves amplification and display of the output in an appropriate format (Ridker and Rane 2021; Zamani et al. 2019) (Fig. 2).
Biosensors can be classified according to the mode of physicochemical transduction or the type of biorecognition element. Based on the transducer, biosensors can be classified as electrochemical, optical, thermal, and piezoelectric biosensors (Fig. 3).
In the following, the newest biosensors developed for CR detection will be reviewed. Analytical features, nanocomposite and some other features of the structure will also be discussed.
5 Biosensors for detection of C reactive protein
Conductive and biocompatible graphdiyne (GDY) nanosheets, antifouling agents, and specific MIPs are combined to construct highly sensitive protein MIP biosensors for sensitive and selective detection of human CRP. Dopamine has been used as a functional monomer due to its facile polymerization process, and the low-fouling properties. Additionally, polydopamine have been validated, forming stable complexes with template molecules through hydrogen bonding and multipoint electrostatic attraction. With high biocompatibility and conductivity, GDY was independently synthesized and incorporated into C-reactive protein-imprinted polymers (C-MIPs) to enhance electrochemical responses and make it suitable for bioactive protein molecules that provides a unique microenvironment (Cui et al. 2022). The application of indium tin oxide (ITO) electrodes in the field of biosensing is noteworthy for its potential for differential surface modification and enhancement (Azani et al. 2020; Isfahani et al. 2019). ITO substrates are classified by their stability evidenced by their low current capacity, high conductivity, electrochemical activity over a wide potential range, and electrochemical and physical properties. Due to the high adhesion between ITO and PET materials, ITO-PET materials are very stable and flexible (Azani et al. 2020; Isfahani et al. 2019). A novel silanization agent based ITO one-use sheets system was developed for detection of CRP as a potential Alzheimer's disease (AD) blood biomarker. Created impedimetric nano-platform have excellent reproducibility, repeatability, and reusability (Sonuç Karaboğa and Sezgintürk 2018).
Etching of silver-coated nanorods/nanobipyramids or gold nanorods/nanobipyramids to induce plasmonic changes represents an efficient way to extend the performance of ELISAs. The study proposed a multicolor ELISA that spans a broad linear range and a large number of color changes for highly sensitive and semi-quantitative naked eye detection (Weng et al. 2022).
An innovative platform of immunoassay for the detection of CRP has been developed using Resonant Acoustic Profiling™ (RAP™) with comparable sensitivity to a high sensitivity CRP ELISA (hsCRP) but with significant time efficiency (12 m turnaround time to result). Developed label-free immunoassay showed acceptable sensitivity and linearity (McBride and Cooper 2008). Sensitive detection of CRP, as a biomarker for heart disease, was developed using a carbon nanofiber-based biosensor platform. Up and down aligned carbon nanofibers were grown using plasma-enhanced chemical vapor deposition to construct nano-electrode arrays in a 3 × 3 configuration. In this research electrochemical impedance spectroscopy (EIS) and Cyclic voltammetry (CV) were used for CRP detection (Gupta et al. 2014). In clinical practice, testing for pentameric pCRP is frequently used as a prognostic indicator of a patient's risk of emerging Cardiovascular disease (CVD). Structural modifications of pCRP exhibit diverse biological activities in the body (Hu et al. 2006). In recent years, mCRP is considered to be a more potent inducer than pCRP, so mCRP determination has become a vital indicator for assessing risk of developing CVD. Surface plasmon resonance (SPR) biosensing technology can be used to improve detection accuracy and real-time response when detecting pCRP or mCRP (Hu et al. 2006). In a study, CRP three monoclonal antibodies (Mabs), 8D8, C8, and 9C9, are immobilized on a protein G layer for subsequent CRP discovery. Experimental results show that Mab C8 reacts with both mCRP, and pCRP, Mab 8D8 reacts with pCRP, and Mab 9C9 reacts with mCRP (Hu et al. 2006) (Fig. 4).
Developed immunosensor for detection of CRP monoclonal Ab, reproduced from ref (Hu et al. 2006)
A highly sensitive and label-free fiber optic SPR biosensor for the specific detection of CRP has been proposed and demonstrated. In this study, dopamine is a cross-linking agent for immobilizing anti-CRP monoclonal antibodies, an efficient and simple method to specifically modify SPR fiber optic sensors. By experimentally optimizing the fixation time of the anti-CRP monoclonal antibody and the reaction time of the antigen and antibody, the sensor exhibited a sufficiently linear response to CRP concentration in the range of 0.01–20 μg/mL, demonstrating the highest CRP indicates sensitivity (Wang et al. 2017).
6 Recent developed biosensors
An electrochemical aptasensor has been developed to detect CRP. The surface of CSPE was first modified with carbon nanofiber chitosan (CNFs-CHIT) nanocomposites. An amino-terminal RNA aptamer probe was then attached to the amino group of CHIT using glutaraldehyde as a cross linker. As a final point, methylene blue (MB) was self-assembled as a redox probe on the aptasensor surface. The results obtained showed that the CNFs-CHIT nanocomposites increased the surface coverage of aptamers by up to 5.9-fold (Amouzadeh Tabrizi and Acedo 2022) (Fig. 5).
SCPE/CNFs-based aptasensor for detection of CRP, reproduced from ref (Amouzadeh Tabrizi and Acedo 2022)
Surface-enhanced Raman scattering (SERS) can detect molecules even at the single-molecule scale on or near the surface of plasma nanostructures, greatly extending the scope of standard Raman spectroscopy (Kneipp et al. 1997). Additionally, the plasma biosensor enables user-friendly and rapid testing (within 5–15 min) due to its excellent sensitivity and multiple functions. To stably and reliably use SERS for single-cell detection in practical applications, it is necessary to control the nanostructure stability and the plasma effect around hotspots to limit the SERS signal fluctuations. To do this, we need a better understanding of the origin of the strongly amplified single-cell signal. As a powerful analytical technique, SERS technology relies heavily on substrate materials ranging from traditional precious metals to semiconductor nanomaterials or novel nanocomposites (Kneipp et al. 1997). SERS-based signal detection and molecular recognition offer unprecedented opportunities for research in biomedicine, life sciences, analytical chemistry, and other fields (Kneipp et al. 1997).
A new aptamer SERS biosensor for sensitive protein biomarker detection has been presented. This biosensor contains a SERS tag (reporter-labeled Au nano-bridge nano-gap particles, Au-NNPs) and an original magnetic capture substrate (Ag-coated Fe3O4-Au NPs, Ag-MNPs). SERS performance of this biosensor using C-reactive protein (CRP), a characteristic diagnostic biomarker. Aptamers against CRP were modified with both SERS tags and magnetic capture substrates for specific recognition by the Au-NNPs-CRP-Ag-MNPs sandwich assay. This platform achieved sensitive detection of CRP with an acceptable LOD that much lower than known analytical methods. The method also exhibits excellent selectivity and specificity for CRP in the presence of interference from other proteins and high accuracy in detection of real human serum samples (Hu et al. 2021). A simple pyrolytic method was developed to synthesize graphene quantum dots (GQDs) with a diameter of 2.2 nm. Subsequently, to fabricate Au/GQDs/anti-CRP electrodes, GQDs were successfully electrodeposited on the surface of bare Au electrodes, providing a matrix platform with nano-interfaces for effective anti-CRP immobilization. Using CV, we investigated the contact of anti-CRP and CRP on Fe (CN)63−/4− as mediators. Current signals recorded from differential pulse voltammetry measurements demonstrate that the binding interactions between CRP and anti-CRP are efficiently induced. Moreover, the performance figures of the developed sensor, including sensory response of less than 50 s with high sensitivity and good LOD, indicate that it is a potential candidate for point-of-care testing. Additionally, this sensor used the prepared Au/GQDs/anti-CRP biosensor to detect CRP in an artificial serum solution (Ringer's lactate) (Lakshmanakumar et al. 2022). A biosensor for SERS consisting of multifunctional DNA three-way junctions (DNA 3WJ), porous gold nanoplates (pAuNPs), and Au-Te structures have been advanced for the detection of CRP. pAuNP and Au-Te nanostructures were synthesized by galvanic exchange reaction, and morphology was confirmed by transmission electron microscopy, scanning electron microscopy, and DLS. To generate the SERS signal, Au-Te nanostructures were anchored to indium tin oxide substrates and thiol-modified CRP aptamers were self-assembled onto the modified substrates for CRP recognition. To amplify the SERS signal and identify the Raman tag, the multifunctional DNA 3WJ was conjugated to pAuNP and each fragment of 3WJ was conjugated to biotin (pAuNP binding), methylene blue (Raman reporter), and CRP aptamer (target binding) (Kim et al. 2022) (Fig. 6).
SERS-based biosensor for detection of CRP, with permission from ref (Kim et al. 2022)
An aptasensing method based on the integration of RNA on Cu-MOF was established for the detection of CRP. Cu-MOF displayed mimetic peroxidase enzymatic activity and stimulated fluorescence at the time and can be used as dual-signal transduction. CRP-binding RNA was immobilized on the Cu-MOF and used as a highly selective recognition element. The immobilized RNA can block the fluorescence of the signal traducer probe and peroxidase activity. Adding CRP to the RNA/Cu-MOF will release RNA from the surface of Cu-MOF and recover both the stimulated fluorescence and peroxidase activity (Ali and Omer 2022).
Forster resonance energy transfer (FRET) based biosensors generally consist of two fluorescent probes (donor and acceptor) fused to a central metabolite-binding protein (BP) (Liu et al. 2020). Under optimal conditions, FRET occurs between the two probes upon excitation of the donor, and energy is also transferred to the acceptor. As a result, both fluorescent probes exhibit different fluorescence intensities depending on the FRET effect (Liu et al. 2020; Yamao et al. 2016) (Fig. 7).
Schematic of the structure of the intramolecular FRET biosensor, which consists of the studied protein (sensor domain) fused with a ligand domain, which is sandwiched by the donor and acceptor fluorophores, e.g., cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP), reproduced from ref (Yamao et al. 2016)
A novel fluorescent aptasensor based on aptamers modified by both gold nanoparticles (AuNPs) and nitrogen-doped graphene quantum dots (N-GQDs) for the detection of C-reactive protein (CRP). The FRET effect is employed in our aptasensor by the change of aptamers conformation when binding with the target. A noticeable fluorescence quench of the N-GQDs can be detected when CRP appears in the assay due to electron transfer between the donor and accepter (Chen et al. 2022) (Fig. 8, Table 2).
Schematic of the structure of the intramolecular FRET biosensor (Chen et al. 2022)
Novel label-free biosensing system based on responsive hydrogels surface relief gratings developed for the determination of CRP at clinical concentration range achieved using a home-based measurement system. The developed method was successfully applied to determine CRP in certified human serum samples sensitively and specifically (Lucío et al. 2021).
7 Conclusion
Fast, sensitive, and specific diagnostic assays play an important role in measuring CRP and assessing the efficacy of cardiovascular treatments. To achieve these goals, many efforts have been made to develop biosensors based on specific interactions between bioreceptors and analytes. Several studies have been performed to detect CRP based on DNA hybridization or CRP-associated proteins. However, few reports on the detection of CRP are available in the literature. In fact, a number of indirect methods have been developed for early detection of CRP. Interest in nanotechnology has revealed new opportunities in the development of these biosensor assays. The high surface-to-volume ratio and electrical and optical properties of nanostructures enhance the sensitivity of biosensors. Carbon nanostructures, quantum dots, nanoclusters, and metal and metal oxide nanoparticles are just a few of the known materials that have emerged as canhisates for the development of highly sensitive CRP biosensors. Intelligent use of such nano-objects has led to significant performance gains with increased sensitivity and several orders of magnitude lower detection limits. Despite significant advances in CRP biosensing in recent years, POC is affordable, robust, easy to use, portable, and has sufficient quantitative accuracy to enable clinical decision-making, there is a great need to progress biosensors technology. Furthermore, the development of biosensors that allows simultaneous detection of cardiovascular biomarkers could achieve higher detection sensitivity. In summary, biosensors technology has become popular in CRP determination due to their low cost, non-destructive measurement, high performance features such as fast response, and simple operation. Extensive research on membrane development is crucial in order to improve the sensor performance in terms of sensitivity and selectivity.
Abbreviations
- CV:
-
Cyclic voltammetry
- EIS:
-
Electrochemical impedance spectroscopy
- CRP:
-
C reactive protein
- DPV:
-
Differential pulse voltammetry
- CVD:
-
Cardiovascular disease
- SPR:
-
Surface plasmon resonance
- CSPE:
-
Carbon screen-printed electrode
- CNFs-CHIT:
-
Carbon nanofiber chitosan
- SERS:
-
Surface-enhanced Raman scattering
- GQDs:
-
Graphene quantum dots
- DLS:
-
Dynamic light scattering
- AD:
-
Alzheimer's disease
- ELISA:
-
Enzyme-linked immunosorbent assay
References
G.K. Ali, K.M. Omer, Ultrasensitive aptamer-functionalized Cu-MOF fluorescent nanozyme as an optical biosensor for detection of C-reactive protein. Anal. Biochem. 658, 114928 (2022)
M. Amouzadeh Tabrizi, P. Acedo, Highly Sensitive RNA-Based Electrochemical Aptasensor for the Determination of C-Reactive Protein Using Carbon Nanofiber-Chitosan Modified Screen-Printed Electrode. Nanomaterials. 12 (3), 415 (2022)
A. Avan, S.B. Tavakoly Sany, M. Ghayour‐Mobarhan, H.R. Rahimi, M. Tajfard, G. Ferns, Serum C‐reactive protein in the prediction of cardiovascular diseases: Overview of the latest clinical studies and public health practice. J. Cell. Phys. 233 (11), 8508–8525 (2018)
M.R. Azani, A. Hassanpour, T. Torres, Benefits, problems, and solutions of silver nanowire transparent conductive electrodes in indium tin oxide (ITO)-free flexible solar cells. Adv. Energy Mater. 10 (48), 2002536 (2020)
M. Baruah, C-reactive protein (crp) and markers of oxidative stress in acute myocardial infarction. Clin. Signif. C-reactive Protein 95–115 (2020)
K. Baryeh, S. Takalkar, M. Lund, G. Liu, Introduction to medical biosensors for point of care applications, Medical biosensors for point of care (POC) applications. Elsevier, pp. 3–25 (2017)
S.K.S. Bhagavatham, D. Potikuri, V. Sivaramakrishnan, Adenosine deaminase and cytokines associated with infectious diseases as risk factors for inflammatory arthritis and methotrexate as a potential prophylactic agent. Med. Hypotheses 159, 110751 (2022)
R. Blondonnet, J.M. Constantin, V. Sapin, M. Jabaudon, A pathophysiologic approach to biomarkers in acute respiratory distress syndrome. Dis. Markers. 2016, 3501373 (2016)
Y.-T. Chen, Y.-C. Lee, Y.-H. Lai, J.-C. Lim, N.-T. Huang, C.-T. Lin, J.-J. Huang, Review of integrated optical biosensors for point-of-care applications. Biosensors 10 (12), 209 (2020)
X. Chen, X. Liu, C. Zhang, H. Meng, B. Liu, X. Wei, A rapid fluorescent aptasensor for point-of-care detection of C-reactive protein. Talanta 249, 123661 (2022)
N. Christodoulides, S. Mohanty, C.S. Miller, M.C. Langub, P.N. Floriano, P. Dharshan, M.F. Ali, B. Bernard, D. Romanovicz, E. Anslyn, Application of microchip assay system for the measurement of C-reactive protein in human saliva. Lab Chip 5 (3), 261–269 (2005)
M. Cui, Z. Che, Y. Gong, T. Li, W. Hu, S. Wang, A graphdiyne-based protein molecularly imprinted biosensor for highly sensitive human C-reactive protein detection in human serum. Chem. Eng. J. 431, 133455 (2022)
O. de Souza Pires-Neto, E.D.S.G. Amoras, M.A.F. Queiroz, S. Demachki, S.R. da Silva Conde, R. Ishak, I.M.V. Cayres-Vallinoto, A.C.R. Vallinoto, Hepatic TLR4, MBL and CRP gene expression levels are associated with chronic hepatitis C. Infect. Genet. Evol. 80, 104200 (2020)
H. Enocsson, J. Karlsson, H.-Y. Li, Y. Wu, I. Kushner, J. Wetterö, C. Sjöwall, The complex role of C-reactive protein in systemic lupus erythematosus. J. Clin. Med. 10 (24), 5837 (2021)
R.K. Gupta, A. Periyakaruppan, M. Meyyappan, J.E. Koehne, Label-free detection of C-reactive protein using a carbon nanofiber based biosensor. Biosens. Bioelectron. 59, 112–119 (2014)
P. Hastuti, D. Martantiningtyas, D. Karita, S.A. Tasmini, A. Sadewa, Association of-174 G> C Interleukin-6 gene polymorphism with interleukin-6 and c-reactive protein levels and obesity: a case–control study among people/residents of western Indonesia. Med. J. Malaysia 74 (5), 400–404 (2019)
W.P. Hu, H.Y. Hsu, A. Chiou, K.Y. Tseng, H.Y. Lin, G.L. Chang, S.J. Chen, Immunodetection of pentamer and modified C-reactive protein using surface plasmon resonance biosensing. Biosens. Bioelectron. 21 (8), 1631–1637 (2006)
Z. Hu, X. Zhou, J. Duan, X. Wu, J. Wu, P. Zhang, W. Liang, J. Guo, H. Cai, P. Sun, H. Zhou, Z. Jiang, Aptamer-based novel Ag-coated magnetic recognition and SERS nanotags with interior nanogap biosensor for ultrasensitive detection of protein biomarker. Sens. Actuators B Chem. 334, 129640 (2021)
V.B. Isfahani, N. Memarian, H.R. Dizaji, A. Arab, M.M. Silva, The physical and electrochromic properties of Prussian Blue thin films electrodeposited on ITO electrodes. Electrochim. Acta 304, 282–291 (2019)
R.V. Jimenez, A.J. Szalai, Therapeutic lowering of C-reactive protein. Front. Immunol. 11, 619564 (2021)
S.M. Kim, J. Kim, G. Yim, H.J. Ahn, M. Lee, T.-H. Kim, C. Park, J. Min, H. Jang, T. Lee, Fabrication of a surface-enhanced Raman spectroscopy-based analytical method consisting of multifunctional DNA three-way junction-conjugated porous gold nanoparticles and Au-Te nanoworm for C-reactive protein detection. Anal. Bioanal. Chem. 414 (10), 3197–3204 (2022)
K. Kneipp, Y. Wang, H. Kneipp, L.T. Perelman, I. Itzkan, R.R. Dasari, M.S. Feld, Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 78 (9), 1667 (1997)
P. Kosutova, P. Mikolka, D. Mokra, A. Calkovska, Anti-inflammatory activity of non-selective PDE inhibitor aminophylline on the lung tissue and respiratory parameters in animal model of ARDS. J. Inflamm. 20 (1), 10 (2023)
M. Lakshmanakumar, N. Nesakumar, S. Sethuraman, K.S. Rajan, U.M. Krishnan, J.B.B. Rayappan, Development of an Electrodeposited Graphene Quantum Dot Electrode for the Electrochemical Detection of C-Reactive Protein (CRP) Biomarker. ChemistrySelect 7 (14), e202104511 (2022)
L. Li, N. Roumeliotis, T. Sawamura, G. Renier, C-Reactive Protein Enhances LOX-1 Expression in Human Aortic Endothelial Cells. Circ. Res. 95 (9), 877–883 (2004)
L. Liu, F. He, Y. Yu, Y. Wang, Application of FRET biosensors in mechanobiology and mechanopharmacological screening. Front. Bioeng. Biotechnol. 8, 595497 (2020)
M.I. Lucío, A.H. Montoto, E. Fernández, S. Alamri, T. Kunze, M.-J. Bañuls, Á. Maquieira, Label-free detection of C-Reactive protein using bioresponsive hydrogel-based surface relief diffraction gratings. Biosens. Bioelectron. 193, 113561 (2021)
S.B. Manikandan, R. Manikandan, M. Arumugam, P. Mullainadhan, An overview on human serum lectins. Heliyon 6 (8), e04623 (2020)
J.D. McBride, M.A. Cooper, A high sensitivity assay for the inflammatory marker C-Reactive protein employing acoustic biosensing. J. Nanobiotechnol. 6 (1), 1–8 (2008)
T. Nakakuki, M. Ito, H. Iwasaki, Y. Kureishi, R. Okamoto, N. Moriki, M. Kongo, S. Kato, N. Yamada, N. Isaka, T. Nakano, Rho/Rho-Kinase Pathway Contributes to C-Reactive Protein–Induced Plasminogen Activator Inhibitor-1 Expression in Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 25 (10), 2088–2093 (2005)
G. Parkinson, B. Pejcic, Using biosensors to detect emerging infectious diseases (Prepared for The Australian Biosecurity Cooperative Research Centre, 2005), pp. 1–80
J.A.C. Pérez, J.E. Sosa-Hernández, S.M. Hussain, M. Bilal, R. Parra-Saldivar, H.M. Iqbal, Bioinspired biomaterials and enzyme-based biosensors for point-of-care applications with reference to cancer and bio-imaging. Biocatal. Agric. Biotechnol. 17, 168–176 (2019)
L. Perico, A. Benigni, F. Casiraghi, L.F. Ng, L. Renia, G. Remuzzi, Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat. Rev. Nephrol. 17 (1), 46–64 (2021)
L. Qin, F. Li, X. Gong, J. Wang, W. Huang, N. Hu, Combined measurement of D-dimer and C-reactive protein levels: highly accurate for diagnosing chronic periprosthetic joint infection. J. Arthroplasty 35 (1), 229–234 (2020)
P.M. Ridker, M. Rane, Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ. Res. 128 (11), 1728–1746 (2021)
B.R. Sahu, R.K. Kampa, A. Padhi, A.K. Panda, C-reactive protein: a promising biomarker for poor prognosis in COVID-19 infection. Clin. Chim. Acta 509, 91–94 (2020)
M.N. Sonuç Karaboğa, M.K. Sezgintürk, A novel silanization agent based single used biosensing system: Detection of C-reactive protein as a potential Alzheimer’s disease blood biomarker. J. Pharmaceut. Biomed. Anal. 154, 227–235 (2018)
N.R. Sproston, J.J. Ashworth, Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 9, 754 (2018)
R.F. Turner, D.J. Harrison, R.V. Rojotte, Preliminary in vivo biocompatibility studies on perfluorosulphonic acid polymer membranes for biosensor applications. Biomaterials 12 (4), 361–368 (1991)
P. Vadgama, Materials for improved point of care biosensor–tissue interfaces, Medical Biosensors for Point of Care (POC) Applications. Elsevier, pp. 45–66 (2017)
J.E. Volanakis, Human C-reactive protein: expression, structure, and function. Mol. Immunol. 38(2–3), 189–197 (2001)
W. Wang, Z. Mai, Y. Chen, J. Wang, L. Li, Q. Su, X. Li, X. Hong, A label-free fiber optic SPR biosensor for specific detection of C-reactive protein. Sci. Rep. 7 (1), 16904 (2017)
J. Wang, R. Niu, L. Jiang, Y. Wang, X. Shao, M. Wu, Y. Ma, The diagnostic values of C-reactive protein and procalcitonin in identifying systemic lupus erythematosus infection and disease activity. Medicine 98 (33) (2019)
F. Weihs, A. Anderson, S. Trowell, K. Caron, Resonance energy transfer-based biosensors for point-of-need diagnosis—progress and perspectives. Sensors 21 (2), 660 (2021)
G. Weng, X. Shen, J. Li, J. Wang, J. Zhu, J. Zhao, A plasmonic ELISA for multi-colorimetric sensing of C-reactive protein by using shell dependent etching of Ag coated Au nanobipyramids. Anal. Chim. Acta 1221, 340129 (2022)
R.C. Williams, M.E. Harmon, R. Burlingame, T.W. Du Clos, Studies of serum C-reactive protein in systemic lupus erythematosus. J. Rheumatol. 32 (3), 454–461 (2005)
J. Xie, M.-Q. Tang, J. Chen, Y.-H. Zhu, C.-B. Lei, H.-W. He, X.-H. Xu, A sandwich ELISA-like detection of C-reactive protein in blood by citicoline-bovine serum albumin conjugate and aptamer-functionalized gold nanoparticles nanozyme. Talanta 217, 121070 (2020)
M. Yamao, K. Aoki, N. Yukinawa, S. Ishii, M. Matsuda, H. Naoki, Two new FRET imaging measures: Linearly proportional to and highly contrasting the fraction of active molecules. PLoS ONE 11 (10), e0164254 (2016)
F.G. Zamani, H. Moulahoum, M. Ak, D.O. Demirkol, S. Timur, Current trends in the development of conducting polymers-based biosensors. TrAC Trends Anal. Chem. 118, 264–276 (2019)
J. Zeller, B. Bogner, J.D. McFadyen, J. Kiefer, D. Braig, G. Pietersz, G. Krippner, T.L. Nero, C.J. Morton, K.C.T. Shing, Transitional changes in the structure of C-reactive protein create highly pro-inflammatory molecules: Therapeutic implications for cardiovascular disease. Pharmacol. Therapeut. 108165 (2022)
L. Zhang, H.-Y. Li, W. Li, Z.-Y. Shen, Y.-D. Wang, S.-R. Ji, Y. Wu, An ELISA assay for quantifying monomeric C-reactive protein in plasma. Front. Immunol. 9, 511 (2018)
Acknowledgements
This study was supported by the Physical Medicine and Rehabilitation Research Center, Aging Research Institute, Faculty of Medicine, Tabriz University of Medical Sciences, Iran.
Author information
Authors and Affiliations
Contributions
Conceptualization & Supervision: Ahmad Mobed, Investigation: Hamidreza Hassanzadeh Khanmiri, Data curation: Fatemeh Rezamohammadi, Mehrnoush Rahmani,Writing – original draft: Fatemeh Yazdanfar, Tannaz Haghgouei, Writing & editing of revised draft: Fatemeh Rezamohammadi, Mehrnoush Rahmani.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khanmiri, H.H., Yazdanfar, F., Mobed, A. et al. Biosensors; noninvasive method in detection of C-reactive protein (CRP). Biomed Microdevices 25, 27 (2023). https://doi.org/10.1007/s10544-023-00666-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s10544-023-00666-y