Abstract
Heavy metals bioremediation by medicinal plants is an important research issue, which has yet to be investigated. Matricaria chamomilla accumulation of soil cadmium (Cd, 0, 10 and 40 mg/kg) and lead (Pb, 0, 60 and 180 mg/kg) affecting plant biochemical properties L. at different growth stages in the greenhouse and field was investigated. The 10-kg experimental pots (located in the greenhouse and field with 80% of field capacity moisture) were filled with the treated soils, and were planted with M. chamomilla L. seeds (three replicates). Plants were sampled to determine their biochemical properties including Cd and Pb contents, pigments, proline (Pro), leaf relative water (LRW), lipid peroxidation (LX), and superoxide dismutase (SOD, EC 1.15. 1.1), and catalase (CAT, EC 1.11.1.6) activities. Soil final concentration of Cd and Pb was also determined. Heavy metal stress significantly decreased plant pigment contents; however, it significantly increased plant PRO, LRW, LX and SOD, and not CAT. Heavy metal, growth stage, growth location, and their interactions significantly affected plant heavy metal concentrations. Interestingly, although significantly higher concentration of Cd was observed in plant aerial part under greenhouse conditions, plant roots had significantly higher concentrations of Cd under field conditions, and it was reverse for Pb. Increased concentration of Cd and Pb significantly enhanced plant Pro content and the highest one was resulted by Pb3 (913.46 mg/g fresh weight) significantly higher than other treatments including Cd3 (595.34 mg/g fresh weight). M. chamomilla is a suitable species for the bioremediation of soils polluted with Cd and Pb.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to globe industrialization, the concentrations of heavy metals are considerably increasing affecting people health and the environment including plants. The use of medicinal plants, for the promotion of people health and treatment of different diseases, is also increasing (Melkegna and Jonah 2020). However, at the same time medicinal plants, especially in the industrialized areas are subjected to the contamination of different heavy metals including cadmium (Cd) and lead (Pb) (Doostikhah et al. 2020; Georgieva et al. 2020).
Accordingly, investigating the accumulation of heavy metals in medicinal plants, affecting their biochemical properties, is of outmost significance (Miransari 2011). The plant M. chamomilla L. or German chamomile, from the Asteraceae/Compositae family, as an important medicinal plant. It is an annual herb from the composite family with: (1) a height of 50–80 cm, (2) pyramid-like roots, which are mostly accumulated on the soil surface, (3) high number of root hairs, and (4) thin and alternated leaves. The plant is grown by seed and is tolerant in heavy metal polluted soils (Pirzad et al. 2011).
Different methods including physical, chemical and biological ones have been used for the treatment of the contaminated areas. At the same time the use of tolerant plants is one of the healthiest and most efficient methods for the bioremediation of the contaminated environment. Plants used for the process of phytoremediation can act as hyperaccumulators, metal indicators and metal excluders (Pandey et al. 2019).
Plants, which are suitable for bioremediation, must be able to absorb heavy metals by their roots at the rates higher than 1000 mg/kg, with the translocation factor (shoot/root concentration) and bioconcentration factor (BCF, shoot/soil) higher than 1. However, up to now, there is not any plant with all the above-mentioned properties, and without the use of amendments it is not possible fulfilling all such characteristics. Tolerant plants are able to sequester Pb in and around their roots, and hence prevent the environment from being contaminated. Research has indicated, medical plants can be used for the process of phytoremediation, which is due to their biochemical properties (Egendorf et al. 2020).
Heavy metals may negatively affect plant growth and physiology by binding to the cellular proteins and replacing the essential cations for plant growth, which results in the malfunctioning of cellular organelles by oxidative stress (Asghari et al. 2017). Accordingly, the inactivation of enzymes and production of reactive oxygen species, may lead to the peroxidation of cellular membrane lipid, and subsequent damage of cellular RNA and DNA, amino acids and proteins. Plants alleviate oxidative stress by the production of enzymatic (i.e. superoxide dismutase, glutathione peroxidase, and catalase) and non-enzymatic (i.e. carotenoids) antioxidants (Sajedi et al. 2010, 2011).
However, the interesting point is the increased production of secondary biochemicals in heavy metal stress, which is a function of plant species, and the type and concentrations of heavy metals. The production of reactive oxygen species, under heavy metal stress, results in the production of polyunsaturated fatty acids hydroperoxide (PUFA-OOH) by the oxidation of poly unsaturated fatty acids (PUFA). The produced PUFA-OOH is ultimately converted to oxylipins, which results in the expression of the genes, required for the production and accumulation of secondary metabolites in plant cells (Maleki et al. 2017).
The present research is noticeable from two different aspects: (1) how the growth and biochemical properties of M. chamomilla L., as an important medicinal plant, may be affected under heavy metal stress of Cd and Pb, and (2) if bioremediation of heavy metal polluted areas with M. chamomilla L. negatively affect the biochemical properties including the secondary metabolites production (medicinal properties) of M. chamomilla L. One important method to increase plant tolerance under heavy metal stress is to induce plant production of antioxidants and secondary metabolites (Haque et al. 2020; Nabaei and Amooaghaie 2020).
With respect to the above-mentioned details, and because, to our knowledge, there is not any data on the accumulation of Cd and Pb affecting M. chamomilla L. biochemical properties, this research was conducted. The objective was to investigate soil Cd and Pb affecting heavy metal accumulation of M. chamomilla, and plant biochemical properties at different growth stages, in the greenhouse and in the field.
Materials and methods
Research site
The experiment was conducted in 2018–2019 in the research greenhouse (G) and research field (F) of Isfahan (Khorasgan) Islamic Azad University, Isfahan, Iran, with the eastern longitude of 51° and 23′ and northern latitude of 32°32′, and the altitude of 1590 m. The climate of the region is dry and semi dry with warm summers, the average yearly rainfall and temperature are equal to 130 mm and 14 °C, respectively. The physical and chemical properties of the soil (0–30 cm) were determined using the standard methods (Miransari et al. 2008) including EC = 4.5 dS/m, pH = 7.9, OC = 0.68%, CaSO4 = 0.05%, total N = 0.007%, available P = 34 mg/kg, SP = 52%, sand = 18%, silt = 51%, and clay = 31%.
Experimental design
The experiment is a three-way factorial including heavy metal concertation, plant growth stage, and plant growth site on the basis of a completely randomized block design with three replicates. The experimental treatments of lead (Pb) (0, 60 and 180 mg/kg) as Pb (NO3)2, and cadmium (Cd) (0, 10 and 40 mg/kg) as Cd (NO3)2, 4H2O were sprayed to pollute the experimental soil. The sprayed soils were stored in plastic bags for two weeks at 20 °C, and 53% humidity to achieve the equilibrium (Lindsay 1979). The plastic pots (10-kg) with the diameter and height of 25 cm were filled with the treated soils and used in the greenhouse and in the field. The soils of the pots were then examined for the concentration of heavy metals in the Laboratory of Soil and Water, Isfahan’s Research and Education Center for Agriculture and Natural Resources (Bremner and Mulvaney 1982) (Table 1).
Seeds of M. camomilla L. were obtained from Seed and Plant Research Improvement Institute, Karaj, Iran, and were planted in the pots. The pots were planted in the March of 2018 and 2019, each experiment was one year long. The amount of seeds per pot was 0.002 g, which was mixed with silt and planted, with the final number of 15 plants in each pot. The pots were irrigated to the soil moisture of 80% of field capacity, determined by weighing the collected soils samples. The plants were sampled to determine plant [aerial part (A) and roots (R)] Cd and Pb and biochemical properties at different growth stages including tillering (Ti), stemming (St), and flowering (Fl). The plants were then completely collected at harvest, and the roots were washed with distilled water.
Antioxidant enzymes
The activity of antioxidant enzymes was determined by the following: 100 mg of the plant seedling was treated with one milliliter of the mixed bufferic compound (polyvinylpyrrolidone 1%, triton X-100, K3PO4 with the pH of 7) and completely homogenized. The solution was centrifuged at 15,000g at 4 °C for 20 min. The supernatant was used to determine the activity of antioxidant enzymes.
Catalase
The bufferic compound (2.95 mL) including K3PO4 (50 mL, pH = 7), and H2O2 (15 mM) was mixed with 0.05 mL of enzyme extract. The specific activity of catalase was determined using the following formula (volumetric activity of catalase divided by protein concentration of extract)
U is a one unit of catalase activity, which is equal to the amount of the enzyme, which catalyses 1 µM of H2O2, and converts it to oxygen and water in 1 min, Δ is the absorbance difference at the wave length of 240 nm in 1 min, TV is the total volume (3 mL), EV is the extract volume (0.05 mL), ε is the catalase coefficient (39.4 mM−1 cm−1), D is the coefficient of dilution, for example, if during extracting, 1 mL of bufferic compound is mixed with 0.1 mL homogenized plant sample, D is equal to 10.
Superoxide dismutase
The activity of superoxide dismutase was measured according to the following: 50 µL of enzyme extract was treated with 3 mL of the bufferic solution of 50 mM buffereic phosphate (pH = 7.8), EDTA 75 nM, methionine 13 mM, Nitro blue tetrazolium chloride 63 µM and riboflavin 1.3 mM. The samples were subjected to light for 15 min and then their absorbance was determined at 560 nM using spectrophotometer. A control sample, which had not been subjected to light and another control sample containing all the components of the bufferic solution except the enzymatic extract were also used. The activity of SOD was determined using the following formula:
U is a one unit of SOD activity is the amount of enzyme, inhibiting 50% of photoreduction of nitro blue tetrazolium chloride, ΔAc is the difference of light absorbance (560 nm) of control sample (without enzymatic extract) in 1 min, ΔAc is the difference of light absorbance (560 nm) of main sample (with enzymatic extract) in one minute, D is the coefficient of dilution.
Bradford assay
The method of Bradford (1976) is a colorimetric method using spectrophotometer to determine sample protein. Bradford indicator was prepared according to the following: 100 mg Coomassie blue (G250) was dissolved in 50 mL ethanol 95%, and was then treated with 85% phosphoric acid. The solution was brought up to the volume (1 L) using distilled deionized water. The solution was then filtered using Whatman (#1) filter paper. The indicator was stored in the dark glass tubes.
Standard protein solution
One milligram of bovine serum albumin (BSA) was dissolved in 1 mL of distilled deionized water to get the standard protein solution of 1 mg/mL. The solution was stored at − 20 °C.
Standard curves
The standard curves were prepared according to the following: 10, 20, 40, 60, 80, and 100 µL of standard protein solutions were poured into autoclaved glass tubes and were brought up to the volume of 100 µL by distilled deionized water. A blank sample for the spectrophotometer was also prepared using 100 µL of distilled deionized water. Each glass tube was treated with 5 mL of Bradford indicator and was mixed thoroughly. The samples were determined by the spectrophotometer after a maximum of 1 h at the wave length of 595 nm, and using the standard curves, the samples were measured for protein concentration (mg/mL) of plant samples. The protein concentration (mg/mL) was calculated by multiplying the instrument value by the dilution factor.
Measurement of plant pigment contents
Using an acetone solution (80%), 0.2 g of leaf fresh sample was smashed in a mortar, followed by the extraction of a 20-mL solution, which was used to determine chlorophyll a, b, total and carotenoid by the spectrophotometer instrument (Sherwood model) and the following equations:
V is the volume of the filtered solution (the centrifuge supernatant), A is the light absorption at the wavelengths of 663, 645 and 470 nm, W is the sample fresh weight (g).
Measurement of heavy metal concentration
The concentrations of heavy metals in the plant samples were determined by the wet oxidation method using nitric acid (Bremner and Mulvaney 1982). One milligram of plant sample was treated with 10 mL of nitric acid 65% and 2-mL oxygen peroxide for 24 h and then using a heater (85–100 °C) the samples were heated until the samples became colorless and the brown steams were evaporated. The samples were cooled down and then filtered with Whatman filter paper (#42) and brought up to the volume of 50 mL and the concentration of heavy metals were determined using atomic absorption spectrophotometer (Perkin Elmer AAnalyst 800).
Plant proline, relative water content and sugar were measured according to Noein and Soleymani (2021), Arzanesh et al. (2011), and Askarnejad et al. (2021), respectively.
Statistical analysis
The results were subjected to analysis of variance to indicate the significant differences between the treatments using SAS. Means and their corresponding standard deviations were determined and compared using least significant difference (LSD) at P = 0.05. Graphs were plotted using SAS Proc Plot.
Results
Analysis of variance indicated the significant effects of heavy metals (Cd and Pb), growth stage, growth location, and their interactions on the heavy metal concentrations of plant aerial parts and roots. However, the single effect of growth location and its interaction with heavy metal treatment were not significant on the Cd concentration of aerial part (Table 2).
Cd concentration of plant aerial parts and roots
Increasing Cd concentration, increased plant concentrations of Cd, with significant differences between the aerial part (42.22 mg/kg) and the roots (92.76 mg/kg). The highest concentration of Cd in the aerial part was resulted by treatment Cd3StG (78.25 mg/kg) and the least one was resulted by Pb2TiG (0.32 mg/kg); however, in the case of root Cd concentration the corresponding values were related to Cd3StF (143.56 mg/kg) and Cd1TiG (0.31 mg/kg) treatments, respectively (Table 3).
Interestingly, although significantly higher concentration of Cd was observed in plant aerial part under greenhouse conditions, plant roots had significantly higher concentrations of Cd under field conditions, compared with the greenhouse conditions (Table 2). The stemming growth stage resulted in the highest Cd concentration of plant aerial part (14.14 mg/kg) and plant roots (30.57 mg/kg). There was not a significant difference between the field and greenhouse conditions in terms of Cd in plant aerial part (10.85 and 9.91 mg/kg), however roots contained significantly higher Cd concentration (26.85 mg/kg) than the aerial part (19.33 mg/kg) under field conditions (Fig. 1, Table 3).
Plant contents of Pb and Cd affected by the single effects of heavy metals and their interaction with plant growth stage. A Greenhouse, B field, Cd1, Cd2 and Cd3 stand for control, 10 and 40 mg/kg Cd, respectively. Pb1, Pb2, Pb3 stand for control, 60 and 180 mg/kg Cd, respectively. Fl, St and Ti stand for flowering, stemming and tillering, respectively. F and G stand for field and greenhouse, respectively
Pb concentration of plant aerial parts and roots
Higher concentrations of plant Pb were resulted by increasing the concentrations of Pb. Accordingly, the highest Pb concentration of aerial part (9.32 mg/kg) and roots (51.78 mg/kg) was resulted by Pb3. The stemming growth stage resulted in the highest and significantly different Pb accumulation (13.72 mg/kg) compared with the other growth stages (Table 3). However, in the case of plant roots, the highest and significantly different root concentration (48.49 mg/kg) was resulted by the tillering growth stage. Plant aerial part contained significantly higher concentration of Pb under field conditions (10.11 mg/kg), compared with the greenhouse (4.13 mg/kg), however plant roots had higher concentrations of Pb in the greenhouse (34.24 mg/kg) related to the field (23.97 mg/kg) (Fig. 1, Table 3).
Plant biochemical properties
Analysis of variance indicated the single and the combined experimental treatments significantly affected plant biochemical properties including chl a, chl b, chl T, carotenoid, proline, leaf relative water, sugar, lipid peroxidation, superoxide dismutase and catalase (Table 4).
Pigment contents
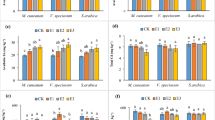
With increasing Cd and Pb concentration, plant pigment contents including chla, chlb, chlT, and carotenoid significantly decreased. There were significant differences between tillering and flowering growth stages in terms of chl a and chl T. Plant chl T was also significantly different at the flowering and stemming stages. Plant pigment contents were significantly higher in the greenhouse than the field (Fig. 2, Table 5).
Plant biochemical properties affected by the interaction effects of heavy metals and plant growth stage. A Greenhouse, B field, Cd1, Cd2 and Cd3 stand for control, 10 and 40 mg/kg Cd, respectively. Pb1, Pb2, Pb3 stand for control, 60 and 180 mg/kg Cd, respectively. Fl, St and Ti stand for flowering, stemming and tillering, respectively. F and G stand for field and greenhouse, respectively
Proline (Pro)
Increased concentration of Cd and Pb significantly enhanced plant Pro content and the highest one was resulted by Pb3 (913.46 mg/g fresh weight) significantly higher than the other treatments including Cd3 (595.34 mg/g fresh weight). Plant growth stages were significantly different in terms of proline content, which was significantly higher in the field (498.32 mg/g fresh weight) than the greenhouse (392.04 mg/g fresh weight) (Fig. 2, Table 5).
Leaf relative water
Plant relative water significantly increased with increasing Cd and Pb concentration as the highest one was resulted by Cd3 (101.44%) significantly different from the other treatments including Pb3 (86.24%). The highest LRW was resulted by the tillering growth stage significantly higher than the other growth stages. However, there were not significant differences between the field and greenhouse conditions in terms of LRW (Fig. 2, Table 5).
Sugar
Although with increasing Cd and Pb concentration, plant sugar content decreased, the differences were not significant. The tillering growth stage resulted in the highest and significantly different sugar content, compared with the other growth stages. The sugar content in the field and greenhouse were not significantly different (Fig. 2, Table 5).
Lipid peroxidation
The increased concentration of Cd and Pb significantly increased lipid peroxidation, and the highest oxidation was resulted by Pb3 (918.75 mg/g fresh weight) significantly higher than the other treatments. There were significant differences among different growth stages in terms of lipid peroxidation. Field conditions (615.17 mg/g fresh weight) resulted in significantly higher LX than the greenhouse conditions (523.34 mg/g fresh weight) (Fig. 2, Table 5).
Superoxide dismutase (SOD)
SOD level was significantly enhanced by increasing the concentration of Cd and Pb as the highest ones were related to Pb3 (350.27 µmole/mg protein) and Cd3 (335.26 µmole/mg protein) significantly higher than the other treatments. The highest SOD was resulted in the tillering growth stage significantly higher than the other growth stages. SOD in the field (252.56 µmole/mg protein) and in the greenhouse (206.06 µmole/mg protein) were not significantly different.
Catalase
Interestingly, increasing the level of Cd and Pb, did not alter plant CAT content. Catalase was also the highest at the tillering growth stage, significantly different from the other growth stages. The concentration of CAT in the field (1.30 µmole/mg protein) and in the greenhouse (1.54 µmole/mg protein) were not significantly different.
Discussion
The medicinal plant M. chamomilla L. was tested for its tolerance under heavy metal contamination of Cd and Pb. With increasing the concentration of Cd and Pb, plant accumulated higher rates of such heavy metals, especially by the roots. This can be of significance, because growing medicinal plants in heavy metal polluted soils is important from economic and environmental aspects. Research has indicated medicinal plants can tolerate heavy metal contamination up to some levels, however, the tolerance of some medicinal plants including M. chamomilla, under heavy metal stress, is higher compared with the other species of medicinal plants (Pandey et al. 2019).
According to our results, M. chamomilla was able to accumulate Cd and Pb to maximum levels of 78.25 (Cd3StG) and 143.56 mg/kg (Cd3StF), and 29.06 (Pb3StF) and 137.51 mg/kg (Pb3TiF) in plant aerial parts and roots, respectively. Research has indicated M. chamomilla., as a tolerant plant under heavy metal contamination, can accumulate high Cd concentration in the following order: roots > leaves > flowers, which is similar to our results. It has also been indicated that the mobility of Cd in plant is higher than Pb (Pandey et al. 2019; Zeng et al. 2020).
The effect of experimental place (growth conditions) was also significant on the accumulation of heavy metals in M. chamomilla as field results were significantly higher than the greenhouse. This might be due to the more suitable and natural conditions of the field for plant growth and physiological activities compared with the greenhouse. The results indicated less Pb was accumulated in the plant compared with Cd, which is similar to Pandey et al. (2019) and Huang et al. (2019).
Plant biochemical properties were also determined in this research to indicate how the accumulation of Cd and Pb may affect plant medicinal properties, which is of economic and environmental significance. The results indicated plant antioxidant activities improved M. chamomilla tolerance in a heavy metal polluted soil. Accordingly, under heavy metal stress of Cd and Pb, the production of the antioxidant enzyme superoxide dismutase (SOD) and lipid peroxidation significantly increased in M. chamomilla.
Plant growth stage was also a determining factor as the highest activities of the enzyme was resulted in the tillering growth stage. However, interestingly the activity of CAT was not increased by the stress of heavy metal. The peroxisomes are the main place for the production of CAT in cellular plants. The enzyme is able to catalyse dismutation reactions, without requiring any reductant (Anjum et al. 2016). The results are similar to the other research; for example, Abed et al. (2017) found at higher levels of Pb, the antioxidative response (CAT production) of Radish (Raphanus sativus) and cress (Lepidium sativus) were similar to that of the control treatment.
The stress of heavy metals significantly decreased M. chamomilla pigment contents including chla, chlb, chlt and carotenoid. Due to the production of reactive oxygen species by heavy metal stress, the integrity of cellular membrane, and the cellular organelles such as chloroplast (as the main site for the production of plant pigment contents), is damaged (Alyemeni et al. 2018; Huang et al. 2019). In another research by Rizvi and Khan (2019), it was indicated that the effects of cadmium were more toxic than chromium and nickel in corn. The heavy metal stress significantly decreased plant growth and enhanced the antioxidant activities of the plant including the production of proline (Sofy et al. 2020). The use and the increased production of proline under Pb stress in maize improved plant growth and alleviated the stress. Additionally, under the Pb stress the production of sugar also increased in corn plants.
Ahmad et al. (2016) found increased levels of Cd in mustard (Brassica juncea L.), significantly increased plant sugar and osmolyte contents including proline and glycine betaine. Although not investigated in the present research, it has been indicated that essentials oils of medicinal plants are not contaminated under heavy metals stress, showing the great potential of medicinal plants for the bioremediation of heavy metal polluted soils (Pandey et al. 2019). Leaf relative water increased under heavy metal stress, which can be due to the decreased growth of plant, suppressed activity of stomata, and reduced physiological activities of plant, which all collectively result in the less consumption and subsequent accumulation of water in plant.
Conclusion
The heavy metal tolerance of M. chamomilla was examined at different growth stages, in the greenhouse and in the field. The results indicated with increasing Cd and Pb concentrations, plant accumulated higher heavy metals, and the roots, and field conditions resulted in significantly higher accumulation of Cd and Pb. The most important results of the present research indicate it is possible to grow M. chamomilla in heavy metal polluted soils as: (1) the plant is able to accumulate high concentrations of Cd and Pb under the stress, and (2) the accumulation of Cd and Pb in M. chamomilla significantly affected plant biochemical properties including plant pigment contents (chlorophyll and carotenoid), proline, plant relative water, sugar, lipid peroxidation, and superoxide dismutase activity. The other important aspect of the research is to illustrate the role of plant growth stage affecting plant growth and physiology under stress as plant responses were significantly different during stemming, tillering and flowering. The results also indicated plant growth conditions (greenhouse and field) can also significantly affect plant response under the stress of Cd and Pb. The main conclusion is M. chamomilla can be used for the bioremediation of soils polluted with Cd and Pb affecting plant biochemical properties and hence plant nutritional, medicinal and pharmaceutical values. Such results are of economic significance because the enhanced production of such biochemical compounds can also affect the production of secondary metabolites in M. chamomilla. However, the important point, which determines the usability of M. chamomilla grown under heavy metals stress, is the level of accumulated Cd and Pb, as at levels higher than the permissible ones, the use of M. chamomilla may not be favorable for human health.
References
Abed EH, Jawad MM, Oudah HK (2017) Role of enzymes catalase, peroxidase and amino acid (proline) in Raphanus sativus and Lepidium sativus in exposure levels different water pollution of ion lead. Iraqi J Sci 58(2A):592–599
Ahmad P, Abd Allah EF, Hashem A, Sarwat M, Gucel S (2016) Exogenous application of selenium mitigates cadmium toxicity in Brassica juncea L. (Czern & Cross) by up-regulating antioxidative system and secondary metabolites. J Plant Growth Regul 35:936–950
Alyemeni MN, Ahanger MA, Wijaya L, Alam P, Bhardwaj R, Ahmad P (2018) Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 255:459–469
Anjum NA, Sharma P, Gill SS, Hasanuzzaman M, Khan EA, Kachhap K, Mohamed AA, Thangavel P, Devi GD, Vasudhevan P, Sofo A (2016) Catalase and ascorbate peroxidase—representative H2O2-detoxifying heme enzymes in plants. Environ Sci Pollut Res 23:19002–19029
Arzanesh MH, Alikhani HA, Khavazi K, Rahimian HA, Miransari M (2011) Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J Microbiol Biotechnol 27:197–205
Asghari BA, Ghorbanpour M, Nikabadi S (2017) Heavy metals in contaminated environment: destiny of secondary metabolite biosynthesis, oxidative status and phytoextraction in medicinal plants. Ecotoxicol Environ Saf 145:377–390
Askarnejad MR, Soleymani A, Javanmard HR (2021) Barley (Hordeum vulgare L.) physiology including nutrient uptake affected by plant growth regulators under field drought conditions. J Plant Nutr. https://doi.org/10.1080/01904167.2021.1889593
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Bremner JM, Mulvaney CS (1982) Nitrogen-total. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, Part 2. Agronomy, vol 9. American Society of Agronomy, Soil Science Society of America, Madison, pp 595–624
Doostikhah N, Panahpour E, Nadian H, Gholami A (2020) Tomato (Lycopersicon esculentum L.) nutrient and lead uptake affected by zeolite and DTPA in a lead-polluted soil. Plant Biol 22:317–322
Egendorf SP, Groffman P, Moore G, Cheng Z (2020) The limits of lead (Pb) phytoextraction and possibilities of phytostabilization in contaminated soil: a critical review. Int J Phytorem 22:916–930
Georgieva SK, Georgieva A, Peteva Z, Dimova D (2020) Trace elements in commonly used medicinal plants from Varna region. Environmental Science and Pollution Research in press, Bulgaria
Haque, M., Biswas, K., Sinha, S.N., 2020. Phytoremediation Strategies of Some Plants under Heavy Metal Stress. In: Plant Stress Physiology. IntechOpen.
Huang XH, Zhu F, Yan WD, Chen XY, Wang GJ, Wang RJ (2019) Effects of Pb and Zn toxicity on chlorophyll fluorescence and biomass production of Koelreuteria paniculata and Zelkova schneideriana young plants. Photosynthetica 57:688–697
Lindsay WL (1979) Chemical equilibria in soils. Wiley, New York
Maleki M, Ghorbanpour M, Kariman K (2017) Physiological and antioxidative responses of medicinal plants exposed to heavy metals stress. Plant Gene 11:247–254
Melkegna TH, Jonah SA (2020) Elemental analysis of medicinal plants used for the treatment of some gastrointestinal diseases in Ethiopia using INAA technique. Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02236-2
Miransari M (2011) Hyperaccumulators, arbuscular mycorrhizal fungi and stress of heavy metals. Biotechnol Adv 29:645–653
Miransari M, Bahrami HA, Rejali F, Malakouti MJ (2008) Using arbuscular mycorrhiza to alleviate the stress of soil compaction on wheat (Triticum aestivum L.) growth. Soil Biol Biochem 40:1197–1206
Nabaei M, Amooaghaie R (2020) Melatonin and nitric oxide enhance cadmium tolerance and phytoremediation efficiency in Catharanthus roseus (L.) G. Don. Environ Sci Pollut Res 27:6981–6994
Noein B, Soleymani A (2021) Corn (Zea mays L.) physiology and yield affected by plant growth regulators under drought stress. J Plant Growth Regul. https://doi.org/10.1007/s00344-021-10332-3
Pandey J, Verma RK, Singh S (2019) Suitability of aromatic plants for phytoremediation of heavy metal contaminated areas: a review. Int J Phytorem 21:405–418
Pirzad A, Shakiba MR, Zehtab-Salmasi S, Mohammadi SA, Darvishzadeh R, Samadi A (2011) Effect of water stress on leaf relative water content, chlorophyll, proline and soluble carbohydrates in Matricaria chamomilla L. J Med Plants Res 5:2483–2488
Rizvi A, Khan MS (2019) Heavy metal-mediated toxicity to maize: oxidative damage, antioxidant defence response and metal distribution in plant organs. Int J Environ Sci Technol 16:4873–4886
Sajedi NA, Ardakani MR, Rejali F, Mohabbati F, Miransari M (2010) Yield and yield components of hybrid corn (Zea mays L.) as affected by mycorrhizal symbiosis and zinc sulfate under drought stress. Physiol Mol Biol Plants 16:343–351
Sajedi NA, Ardakani MR, Madani H, Naderi A, Miransari M (2011) The effects of selenium and other micronutrients on the antioxidant activities and yield of corn (Zea mays L.) under drought stress. Physiol Mol Biol Plants 17:215–222
Sofy MR, Seleiman MF, Alhammad BA, Alharbi BM, Mohamed HI (2020) Minimizing adverse effects of pb on maize plants by combined treatment with jasmonic, salicylic acids and proline. Agronomy 10:699
Zeng J, Li X, Wang X, Zhang K, Wang Y, Kang H, Chen G, Lan T, Zhang Z, Yuan S, Wang C (2020) Cadmium and lead mixtures are less toxic to the Chinese medicinal plant Ligusticum chuanxiong Hort. than either metal alone. Ecotoxicol Environ Saf 193:110342
Acknowledgements
The authors would like to thank very much the international publisher, AbtinBerkeh Scientific Ltd. Company (https://AbtinBerkeh.com), Isfahan, Iran, for editing the manuscript, and revising it according to the Journal format.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bagheri, M., Javanmard, H.R. & Naderi, M.R. Soil cadmium and lead affecting biochemical properties of Matricaria chamomilla L. at different growth stages in the greenhouse and field. Biometals 34, 881–893 (2021). https://doi.org/10.1007/s10534-021-00314-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-021-00314-z