Abstract
Increased levels of trace metals are an important problem of environmental pollution. Ni is one of the metals essential for normal plant development, but elevated levels usually cause deleterious effects on plant growth. The aim of the study was to evaluate the effects sulphur nutrition on growth, oxidative status, and Ni bioaccumulation of Ni-treated rape (Brassica napus L.). Two different oilseed rape cultivars (Hammer and Compass) were grown under sulphur deficiency and under optimal S availability (0 and 1 mM sulphate, respectively) and exposed to 0.1, 0.3, and 0.5 mM Ni concentrations for 3 weeks. Exposure of plants to elevated Ni concentrations resulted in a decrease in the shoot and root biomass and chlorophyll content. The enhancement of Ni caused increased lipid peroxidation. The sulphur nutrition had an effect on the level of oxidative stress of Ni-treated plants—under the deficiency of sulphur the concentration of TBARS was significantly higher than under the optimal level of S. The beneficial effect of optimal sulphur nutrition was lower Ni accumulation in exposed plants but translocation of Ni was dependent on the cultivar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pollution of trace elements is one of the serious problems of environmental degradation (Li et al. 2019). Increased concentration in trace elements could induce negative effects on the environment and assimilation of them by plants could lead to metal accumulation in the trophic network and cause a threat to human health (Kumar et al. 2019; Mishra et al. 2019).
Mining, smelting, refining, alloy processing, scrap metal reprocessing, fossil fuel combustion, and waste incineration are the primary sources of atmospheric nickel, contributing to nickel loadings in terrestrial and aquatic ecosystems with high concentrations that are potentially toxic to wildlife (Cempel and Nikel 2006). Releases of Ni are of concern due to environmental pollution because of anthropogenic activities—the burning of coal and fuel oil, mining, and waste incineration. In small quantities, nickel, an essential plant nutrient, has been observed to improve plant growth and yield quality (Khoshgoftarmanesh and Bahmanziari 2012; Kumar et al. 2018). It plays an important role in plant metabolic processes and is a component of metalloenzymes (Boer et al. 2014). Like other micronutrients, Ni becomes toxic to plants at higher concentrations and has detrimental effects on plant growth and metabolism. Exposure of plants to elevated Ni concentrations results in inhibition of seed germination (Ahmad et al. 2011). Excess Ni retards shoot and root growth, decreases biomass production, induces leaf spotting, and produces Fe deficiency leading to chlorosis and necrosis (Ahmad and Ashraf 2012). In addition, a high concentration of Ni induces the potentially damaging effects of metal-induced reactive oxygen species (Baccouch et al. 2001). Nickel also has the function to reduce the activity of enzymes for nitrogen fixation in legume plants (Zobiole et al. 2010).
Sulphur is a key element that plays a pivotal role in plant growth and development as it is a component of amino acids, co‐factors, and several secondary metabolites. S‐containing defense compounds are involved in plant survival during abiotic stresses such as metals toxicity (Nawaz et al. 2019). Management of sulphur in crop plant nutrition is essential due to its crucial role in fundamental processes such as homeostasis, electron transport, catalysis, and regulation. S compound’s protective function of against excessive amounts of trace metals is related to the functional sulphydryl groups (–SH) of ligands (glutathione and phytochelatins), which can form complexes with trace metals. The former is essential for metal tolerance (Hossain et al. 2012; Zagorchev et al. 2013).
Some plant species can accumulate large amounts of trace elements, including rapeseed which belongs to the Brassicaceae family. Hence, we selected two cultivars of rapeseed as hyperaccumulating plants and as important crops for edible oil and biodiesel production (Carré and Pouzet 2014). Alternatively, oilseed rape has a high requirement for S in comparison to other species (Randall et al. 1997). Considering that an optimal level of S can improve the growth of plants under trace element stress since S has an important protective function against trace element stress, the study aims to assess the effect of S nutrition on growth and induced oxidative stress of Ni-treated rape (Brassica napus L.) and to compare the sensitivity of different cultivars.
Materials and methods
Seeds of two hybrid oilseed rape cultivars—Hammer and Compass of (Brassica napus L.) were chosen for the experiment. Compass cultivar is early emerging and steady with a dense root system and high resistance to drought. Hammer cultivar is very resistant to frost. The 3-month experiment was conducted under controlled environmental conditions (photon flux density 180–200 mol m−2 s−1; photoperiod: 12 h; day/night temperature: 22/15 °C; humidity: 65/75%). Three seeds per pot were sown in plastic pots filled with 500 g of the substrate [coarse sand (1.0–1.5 mm) as a medium] and germinated. The treatments were run in seven replicates (7 pots per treatment). For the optimal nutrients availability Blake–Kalff nutrient solution containing 1 mM MgSO4, 3 mM KNO3, 2 mM Ca(NO3)2, 1 mM NH4H2PO4, 50 µM KCl, 25 µM H3BO3, 2 µM MnCl2, 2 µM ZnCl2, 0,5 µM CuCl2, 0,5 µM (NH4)6Mo7O24,and 20 µM NaFeEDTA (Blake-Kalff et al. 1998) was applied daily to compensate for water losses and supply nutrients. The pH of the solution was adjusted to 5.5. To ensure uniform application, the substrate was sprayed with 75–80 mL of solution in each application.
After 3 weeks, plants were exposed to different Ni concentrations—Ni (as nickel chloride) was tested at four levels of contamination—0 (no addition, control), 0.1, 0.3, and 0.5 mM. As before the treatment, the plant watering was on the same schedule as the nutrient solution. To imitate sulphur deficiency conditions, sulphur from the nutrient solution was eliminated—MgSO4 was changed with MgCl2. The sulphur level was treated as deficient when S was excluded from the nutrient solution and considered sulphur deficient (0 mM sulphate). Therefore, the S-containing solution has an adequate level of S for plant nutrition, and it was suggested as the optimal level (1 mM sulphate). Seven replications were included in each treatment.
After the experiment, the plant’s dry weight was determined after the plant material was dried at 80 °C until constant weight. The content of photosynthetic pigments was analyzed according to Buschmann et al. (1984). Oxidative stress was determined by measuring lipid peroxidation products—the concentration of thiobarbituric acid reactive substances (TBARS) according to Hodges et al. (1999).
Ni concentration in plants was measured in shoot and root. Tissues were oven-dried at 60 °C until a constant weight was achieved. The powdered tissues were digested with concentrated nitric acid and filtered. The content of Ni was determined using an induced emission plasma spectrometer (iCAP 6000, Thermo Scientific). The translocation coefficient was calculated by dividing the concentration of metal in shoots by the concentration in roots.
To determine if Ni and S have any impact on measured parameters, two-Way ANOVA was used. Tukey’s multiple comparison test was used for the statistically significant difference assessment between means (p < 0.05).
Results and discussion
Effects of nickel on plant growth, pigment content, and oxidative stress under the different sulphur availability
Nickel had a significant negative effect on the shoot and root biomass of B. napus cultivars (p < 0.001; Fig. 1a, b). Even the lowest Ni concentration (0.1 mM) has a significantly negative effect on plant growth—the shoot biomass decreased by about 20% in this case (p < 0.05; Fig. 1a). The decrease in the shoot biomass of plants treated with the highest Ni concentration (0.5 mM) was 44–50% compared to Ni-untreated plants (p < 0.05). A similar tendency was obtained by analyzing the impact of Ni on the root biomass of both cultivars (Fig. 1b). The decrease in weight of the treated plants was more prominent in roots than in shoots of Ni-treated plants. The decrease in root biomass was significantly lower at the highest concentration of Ni comparing with the control without respect to sulphur nutrition (p < 0.05). The changes in plant biomass were also dependent on the cultivar (p < 0.001). The effect of Ni on Compass cultivar’s root biomass was more pronounced—the biomass was halved even at the lowest Ni concentration used (p < 0.05). The results are in accordance with other experiments where Ni negatively affected plants’ growth, such as wheat (Shevyakova et al. 2006), barley (Rahman et al. 2005), cucumber (Khoshgoftarmanesh and Bahmanziari 2012), chamomile (Kováčik et al. 2009), cowpea (Kopittke et al. 2007), and spinach (Mishra and Agrawal 2006). Ghasemi et al. (2009) suggested that the disruption of root‐to‐shoot Fe translocation is a major cause of nickel toxicity symptoms in Alyssum inflatum of Brassicaceae. The decrease in root growth at high Ni concentrations may result from carbohydrate accumulation in shoots (Baccouch et al. 1998).
Effect of nickel treatments (0 (control), 0.1, 0.3, 0.5 mM NiCl2) on dry weight (DW) shoots (a) and roots (b) of Brassica napus cultivars (Hammer and Compass) under deficiency (no sulphate) and optimal concentration of sulphur (1 mM sulphate) in the nutrient solution. Bars show means ± SE (n = 7). The different letters above the bars indicate a significant difference between the treatments (p < 0.05)
The deficiency of sulphur enhanced the negative effect of Ni on plant growth. Under S deficiency, Ni-treated plants’ shoot and root biomass was significantly reduced compared to the optimal S supply (p < 0.05). Treatment with two similar stressors—chromium toxicity and sulphur deficiency—indicated a slightly negative effect on the growth of Brassica juncea plants (Schiavon et al. 2008). It is important to note that S deficiency negatively affected shoot weight and root length of B.juncea in the absence of Cr as an essential nutrient but not in its presence.
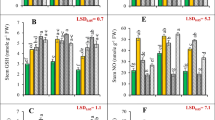
Increasing the concentration of Ni significantly negatively affected the concentration of the chlorophyll (p < 0.05, Fig. 2a). The content of chlorophyll significantly decreased in N-treated plants at 0.3 mM Ni, especially for Compass cultivar (27% decrease compared to Hammer). The lowest chlorophyll content was observed under the highest Ni concentration used, and there was no difference with respect to S nutrition (p > 0.05; Fig. 2a). This implies that Ni is the primary factor affecting the photosynthesis process through changes in chlorophyll concentrations (Ghasemi et al. 2009). Similarly, the effect of chromium induced no variation in chlorophyll regardless of S availability (Schiavon et al. 2008). It is suggested that substitution of Fe and Mg by Ni from the chlorophyll structure results in disruption of the normal production of chlorophyll and impair the plant metabolism decreasing photosynthesis rate (Piccini and Malavolta 1992; Ewais 1997). Higher sugar concentration in biomass could lead to a decrease in photosynthesis due to inhibition of dark reactions (Langford and Wainwright 1987; Rahman et al. 2005).
Effect of nickel treatments (0 (control), 0.1, 0.3, 0.5 mM NiCl2) on total chlorophyll content (a) and TBARS (b) of Brassica napus cultivars (Hammer and Compass) under deficiency (no sulphate) and optimal concentration of sulphur (1 mM sulphate) in the nutrient solution. Bars show means ± SE (n = 7). The different letters above the bars indicate a significant difference between the treatments (p < 0.05)
TBARS is one of the most frequently studied products of polyunsaturated fatty acid and it is considered a marker of lipid peroxidation (Zhang et al. 2007). The study results showed that with increasing Ni concentration TBARS concentration in both cultivars also increased (p < 0.05, Fig. 2b). The increased levels of TBARS in nickel treated plants confirmed enhanced lipid peroxidation (Gajewska and Skłodowska, 2007; Maheshwari and Dubey, 2009). Since Ni is a redox inactive metal, it cannot directly generate reactive oxygen species. It is affecting the activity and content of oxidants differently and the exact mechanism is unknown (Gajewska et al. 2006). The data obtained confirmed that deficiency of S results in higher levels of oxidative stress—under the deficiency of sulphur, the concentration of TBARS was significantly higher than under the standard level of S (p < 0.05). This was confirmed by the study results showing that the extra S supply induced an antioxidative response—reduced glutathione accumulated in the plants treated with Ni (Matraszek-Gawron and Hawrylak-Nowak 2019).
Metal accumulation and translocation
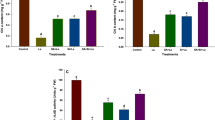
High nickel mobility from soil to plant is associated with its capacity to form complexes. Ni could be transported to plant with nickel-peptide or nickel-histidine complexes, and this may increase its mobility (Dan et al. 2002). Ni concentrations in shoots and roots of both cultivars increased with Ni level in growing medium despite S availability (Fig. 3). The exposure of the wheat seedlings to Ni also resulted in a rapid increase in Ni concentration in the shoots, and a significant reduction in these organs’ fresh biomass (Gajewska and Skłodowska 2009). The results suggest that Ni can pass the endodermic barrier and enter the stele and quickly move from roots to shoots (Gajewska and Skłodowska, 2007; Seregin and Kozhevnikova, 2006).
Effect of nickel treatments (0 (control), 0.1, 0.3, 0.5 mM NiCl2) on concentrations of nickel in shoots (a) and roots (b) in Brassica napus cultivars (Hammer and Compass) under deficiency (no sulphate) and optimal concentration of sulphur (1 mM sulphate) in the nutrient solution. Values are means ± SE (n = 7). The different letters above the bars indicate a significant difference between the treatments (p < 0.05)
Nickel accumulation was significantly higher in roots than in shoots (p < 0.05; Fig. 3b). Other studies also confirmed that Ni was mainly accumulated in the roots of wheat (Wang et al. 2015). Under the sulphur deficiency, Ni accumulation was higher in roots of Hammer cultivar treated with 0.5 mM Ni than in Compass while there was no statistically significant difference under optimal sulphur availability (p > 0.05).
Ni translocation from roots to shoots depends on its concentration in roots but not on its concentration in the cytoplasm of parenchyma. It confirmed that translocation to shoots is controlled by roots and is associated with the protective functions of roots (Cataldo et al. 1978). Translocation coefficients under 0.3 mM Ni were lower than 1, meaning that Ni accumulated in the roots of the Ni-treated plants. The more intense translocation from roots to shoots was noticed under the higher Ni concentration, indicating higher metal accumulation in Compass shoots (Fig. 4). Our study results indicate that Ni exposure could have different effects on Ni accumulation depending on the cultivar. It is important to choose a cultivar for cultivation in S-deficient soil; the deficiency of this nutrient could result in Ni accumulation. Under deficiency of S nutrition, the majority of Ni (45.7%) was accumulated in roots of Hammer, while higher Ni content was detected in shoots of Compass. In general, higher accumulation of Ni without S nutrition was observed indicating that the concentration of S in growth substratum could influence the ability of plants to accumulate elements, including Ni (Schiavon et al. 2008). This is in accordance with the results of Matraszek et al. (2016) where the intense S nutrition significantly increases Ni accumulation in roots of Ni-treated wheat. The beneficial effects of sufficient S nutrition were also noticed on Ni-treated plants’ micronutrient (Cu, Mo, B, Fe) balance, which could be explained by the changes in root surface properties—cation exchange capacity (Matraszek et al. 2016; Matraszek-Gawron and Hawrylak-Nowak 2019).
Effect of nickel treatments (0 (control), 0.1, 0.3, 0.5 mM NiCl2) on translocation coefficient of nickel in Brassica napus cultivars (Hammer and Compass) under different sulphur availability—deficiency (no sulphate) and optimal concentration (1 mM sulphate). Bars show means ± SE (n = 7). The different letters above the bars indicate a significant difference between the treatments (p < 0.05)
Conclusions
In the present study increasing levels of Ni reduced the growth of rapeseed cultivars. Both shoot and root biomass of Ni-treated plants were reduced, and S significantly influenced the growth parameters. Lipid peroxidation was statistically significantly enhanced even at the lowest Ni concentration with enhanced negative impact in sulphur starved plants. Ni accumulation was significantly higher in rape roots than in shoots. Rapeseed has a weak ability to translocate Ni from roots to shoots (TC < 1). Significantly higher Ni concentrations were determined under sulphur deficiency conditions, but the location depended on the cultivar.
References
Ahmad MSA, Ashraf M (2012) Essential roles and hazardous effects of nickel in plants. Reviews of environmental contamination and toxicology, vol 214. Springer, New York, pp 125–167. https://doi.org/10.1007/978-1-4614-0668-6_6
Ahmad MSA, Ashraf M, Hussain M (2011) Phytotoxic effects of nickel on yield and concentration of macro- and micro-nutrients in sunflower (Helianthus annuus L.) achenes. J Hazard Mater 185:1295–1303. https://doi.org/10.1016/j.jhazmat.2010.10.045
Baccouch S, Chaoui A, El Ferjani E (1998) Nickel toxicity: effects on growth and metabolism of maize. J Plant Nutr 21:577–588. https://doi.org/10.1080/01904169809365425
Baccouch S, Chaoui A, El Ferjani E (2001) Nickel toxicity induces oxidative damage in Zea mays roots. J Plant Nutr 24:1085–1097. https://doi.org/10.1081/PLN-100103805
Blake-Kalff MMA, Harrison KR, Hawkesford MJ et al (1998) Distribution of sulfur within oilseed rape leaves in response to sulfur deficiency during vegetative growth. Plant Physiol 118:1337–1344. https://doi.org/10.1104/pp.118.4.1337
Boer JL, Mulrooney SB, Hausinger RP (2014) Nickel-dependent metalloenzymes. Arch Biochem Biophys 544:142–152. https://doi.org/10.1016/j.abb.2013.09.002
Buschmann C, Prehn H, Lichtenthaler H (1984) Photoacoustic spectroscopy (PAS) and its application in photosynthesis research. Photosynth Res 5:29–46. https://doi.org/10.1007/BF00018373
Carré P, Pouzet A (2014) Rapeseed market, worldwide and in Europe. OCL 21:D102. https://doi.org/10.1051/ocl/2013054
Cataldo DA, Garland TR, Wildung RE (1978) Nickel in plants. Plant Physiol 62:563–565. https://doi.org/10.1104/pp.62.4.563
Cempel M, Nikel G (2006) Nickel: a review of its sources and environmental toxicology. Pol J Environ Stud 15(3):375–382
Dan TV, Krishnaraj S, Saxena PK (2002) Cadmium and nickel uptake and accumulation in scented geranium (Pelargonium sp. ’Frensham’). Water Air Soil Pollut 137:355–364. https://doi.org/10.1023/A:1015590007901
Ewais EA (1997) Effects of cadmium, nickel and lead on growth, chlorophyll content and proteins of weeds. Biol Plant 39:403–410. https://doi.org/10.1023/A:1001084327343
Gajewska E, Skłodowska M (2007) Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. Biometals 20:27–36. https://doi.org/10.1007/s10534-006-9011-5
Gajewska E, Skłodowska M (2009) Nickel-induced changes in nitrogen metabolism in wheat shoots. J Plant Physiol 166:1034–1044. https://doi.org/10.1016/j.jplph.2008.12.004
Gajewska E, Skłodowska M, Słaba M, Mazur J (2006) Effect of nickel on antioxidative enzyme activities, proline and chlorophyll contents in wheat shoots. Biol Plant 50:653–659. https://doi.org/10.1007/s10535-006-0102-5
Ghasemi R, Ghaderian SM, Krämer U (2009) Interference of nickel with copper and iron homeostasis contributes to metal toxicity symptoms in the nickel hyperaccumulator plant Alyssum inflatum. New Phytol 184:566–580. https://doi.org/10.1111/j.1469-8137.2009.02993.x
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611. https://doi.org/10.1007/s004250050524
Hossain MA, Piyatida P, da Silva JAT, Fujita M (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot 2012:1–37. https://doi.org/10.1155/2012/872875
Khoshgoftarmanesh AH, Bahmanziari H (2012) Stimulating and toxicity effects of nickel on growth, yield, and fruit quality of cucumber supplied with different nitrogen sources. J Plant Nutr Soil Sci 175:474–481. https://doi.org/10.1002/jpln.201100241
Kopittke PM, Asher CJ, Menzies NW (2007) Toxic effects of Ni2+ on growth of cowpea (Vigna unguiculata). Plant Soil 292:283–289. https://doi.org/10.1007/s11104-007-9226-4
Kováčik J, Klejdus B, Kaduková J, Bačkor M (2009) Physiology of Matricaria chamomilla exposed to nickel excess. Ecotoxicol Environ Saf 72:603–609. https://doi.org/10.1016/j.ecoenv.2007.12.013
Kumar O, Singh SK, Singh AP et al (2018) Effect of soil application of nickel on growth, micronutrient concentration and uptake in barley (Hordeum vulgare L.) grown in Inceptisols of Varanasi. J Plant Nutr 41:50–66. https://doi.org/10.1080/01904167.2017.1381724
Kumar V, Singh J, Kumar P (2019) Heavy metals accumulation in crop plants: sources, response mechanisms, stress tolerance and their effects. Contaminants in agriculture and environment: health risks and remediation. Agro Environ Media - Agriculture and Ennvironmental Science Academy, Haridwar, India, pp 38–57
Langford PJ, Wainwright H (1987) Effects of sucrose concentration on the photosynthetic ability of rose shoots in vitro. Ann Bot 60:633–640. https://doi.org/10.1093/oxfordjournals.aob.a087493
Li C, Zhou K, Qin W et al (2019) A review on heavy metals contamination in soil: effects, sources, and remediation techniques. Soil Sediment Contam 28:380–394. https://doi.org/10.1080/15320383.2019.1592108
Maheshwari R, Dubey RS (2009) Nickel-induced oxidative stress and the role of antioxidant defence in rice seedlings. Plant Growth Regul 59:37–49. https://doi.org/10.1007/s10725-009-9386-8
Matraszek-Gawron R, Hawrylak-Nowak B (2019) Micronutrient status and selected physiological parameters of roots in nickel-exposed Sinapis alba L. affected by different sulphur levels. Plants. https://doi.org/10.3390/plants8110440
Matraszek R, Hawrylak-Nowak B, Chwil S, Chwil M (2016) Macronutrient composition of nickel-treated wheat under different sulfur concentrations in the nutrient solution. Environ Sci Pollut Res 23:5902–5914. https://doi.org/10.1007/s11356-015-5823-6
Mishra S, Agrawal SB (2006) Interactive effects between supplemental ultraviolet-B radiation and heavy metals on the growth and biochemical characteristics of Spinacia oleracea L. Braz J Plant Physiol 18:307–314. https://doi.org/10.1590/S1677-04202006000200007
Mishra S, Bharagava RN, More N et al (2019) Heavy metal contamination: an alarming threat to environment and human health. Environmental biotechnology: for sustainable future. Springer, Singapore, pp 103–125
Nawaz F, Majeed S, Ahmad KS et al (2019) Reactive sulfur species - key regulators of abiotic stress tolerance in plants. Reactive oxygen, nitrogen and sulfur species in plants: production, metabolism, signaling and defense mechanisms, vol 2. John Wiley & Sons, pp 685–713. https://doi.org/10.1002/9781119468677.ch30
Piccini DF, Malavolta E (1992) Effect of nickel on two common bean cultivars. J Plant Nutr 15:2343–2350. https://doi.org/10.1080/01904169209364478
Rahman H, Sabreen S, Alam S, Kawai S (2005) Effects of nickel on growth and composition of metal micronutrients in barley plants grown in nutrient solution. J Plant Nutr 28:393–404. https://doi.org/10.1081/PLN-200049149
Randall PJ, Wang Q, Hocking PJ, Pinkerton A (1997) Critical values for sulfur in young plants of oilseed rape Brassica napus L. determined with reference to dry weight, leaf area and specific leaf weight. Plant nutrition for sustainable food production and environment. Springer, Netherlands, Dordrecht, pp 335–339
Schiavon M, Pilon-Smits EAH, Wirtz M et al (2008) Interactions between chromium and sulfur metabolism in Brassica juncea. J Environ Qual 37:1536–1545. https://doi.org/10.2134/jeq2007.0032
Seregin IV, Kozhevnikova AD (2006) Physiological role of nickel and its toxic effects on higher plants. Russ J Plant Physiol 53:257–277. https://doi.org/10.1134/S1021443706020178
Shevyakova NI, Rakitin VY, Stetsenko LA et al (2006) Oxidative stress and fluctuations of free and conjugated polyamines in the halophyte Mesembryanthemum crystallinum L. under NaCl salinity. Plant Growth Regul 49:1–10. https://doi.org/10.1007/s10725-006-0018-2
Wang Y, Wang S, Nan Z et al (2015) Effects of Ni stress on the uptake and translocation of Ni and other mineral nutrition elements in mature wheat grown in sierozems from northwest of China. Environ Sci Pollut Res 22:19756–19763. https://doi.org/10.1007/s11356-015-5153-8
Zagorchev L, Seal C, Kranner I, Odjakova M (2013) A central role for thiols in plant tolerance to abiotic stress. Int J Mol Sci 14:7405–7432. https://doi.org/10.3390/ijms14047405
Zhang F-Q, Wang Y-S, Lou Z-P, Dong J-D (2007) Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 67:44–50. https://doi.org/10.1016/j.chemosphere.2006.10.007
Zobiole LHS, Oliveira RS, Kremer RJ et al (2010) Effect of glyphosate on symbiotic N2 fixation and nickel concentration in glyphosate-resistant soybeans. Appl Soil Ecol 44:176–180. https://doi.org/10.1016/j.apsoil.2009.12.003
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sujetovienė, G., Bučytė, J. Effects of nickel on morpho-physiological parameters and oxidative status in Brassica napus cultivars under different sulphur levels. Biometals 34, 415–421 (2021). https://doi.org/10.1007/s10534-021-00290-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-021-00290-4