Abstract
Copper is an essential element in all forms of life. It acts as a cofactor of some enzymes and is involved in forming proper protein conformations. However, excess copper ions in cells are detrimental as they can generate free radicals or disrupt protein structures. Therefore, all life forms have evolved conserved and exquisite copper metabolic systems to maintain copper homeostasis. The yeast Saccharomyces cerevisiae has been widely used to investigate copper metabolism as it is convenient for this purpose. In this review, we summarize the mechanism of copper metabolism in Saccharomyces cerevisiae according to the latest literature. In brief, bioavailable copper ions are incorporated into yeast cells mainly via the high-affinity transporters Ctr1 and Ctr3. Then, intracellular Cu+ ions are delivered to different organelles or cuproproteins by different chaperones, including Ccs1, Atx1, and Cox17. Excess copper ions bind to glutathione (GSH), metallothioneins, and copper complexes are sequestered into vacuoles to avoid toxicity. Copper-sensing transcription factors Ace1 and Mac1 regulate the expression of genes involved in copper detoxification and uptake/mobilization in response to changes in intracellular copper levels. Though numerous recent breakthroughs in understanding yeast’s copper metabolism have been achieved, some issues remain unresolved. Completely elucidating the mechanism of copper metabolism in yeast helps decode the corresponding system in humans and understand how copper-related diseases develop.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Copper (Cu) is an essential element in all forms of life. There are two oxidation states of copper, Cu(I)/Cu+ (cuprous ion) and Cu(II)/Cu2+ (cupric ion). Cu+ prefers to bind to the thiol group in cysteine or the thioether group in methionine, while Cu2+ exhibits a high affinity for the secondary carboxyl group in aspartic/glutamic acid or the imidazole nitrogen group in histidine (Festa and Thiele 2011). Thus, copper ions readily form complexes with these amino acids or the peptides containing them. On the one hand, Cu acts as a cofactor of some enzymes due to its potential to either accept or donate an electron during the switch between Cu(I) and Cu(II). On the other hand, Cu can stabilize the conformations of proteins by binding to them (Festa and Thiele 2011). However, excess copper can be harmful to cells. Copper may generate reactive oxygen species (ROS), such as superoxide anions (O2−), nitric oxide (NO−), hydroxyl radicals (OH−), and hydrogen peroxide (H2O2), which can damage various molecules in cells. High levels of copper ions may also disrupt the normal conformations and functions of proteins by binding to them. Owing to the dual roles of this metal, all life forms evolved different mechanisms to maintain copper homeostasis, such as copper chelation, transport, and efflux (Festa and Thiele 2011; Xiao et al. 2004).
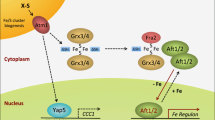
The primary understanding of copper metabolism comes from the study of baker’s yeast, Saccharomyces cerevisiae (Festa and Thiele 2011). Copper metabolic pathways that have been characterized in yeast share a high degree of conservation with those of mammalian systems (Zhou and Gitschier 1997). As a model organism, this microbe has advantages in multiple aspects: (a) it is the first eukaryote whose genome has been completely sequenced and well understood; (b) without long-stretch of non-coding DNA fragments, the yeast genome is relatively easy to manipulate; (c) this single-celled organism is the simplest eukaryote that shares many characteristics with multi-cellular creatures in terms of the cellular homeostasis of most transition metals; (d) it is convenient to manipulate yeast’s nutritional environment to study the biochemical pathways of the metabolism of copper and other metals. Due to these advantages, this eukaryote is widely employed to investigate the mechanism of copper metabolism and numerous breakthroughs have been made in this field (De Freitas et al. 2003). The key genes involved in copper metabolism have been identified (see Table 1). In this review, we will describe how copper is metabolized in Saccharomyces cerevisiae, including the processes of copper influx, utilization, detoxification and homeostasis (as shown in Fig. 1).
Schematic representation of copper metabolism in a yeast cell. Extracellular cupric ions (Cu2+) are reduced into cuprous ions (Cu+) by Fre1 or Fre2, prior to Ctr1-mediated incorporation. Cu2+ can be incorporated by Fet4. After incorporation by Ctr1, Cu+ ions bind to GSH in the cytosol. Then, GSH dispenses Cu+ to a series of chaperones, which deliver Cu+ to different utilization pathways: (1) Ccs1 escorts Cu+ to Sod1 in cytoplasm; (2) Atx1 passes Cu+ to Ccc2, which loads copper onto diverse secretory cuproproteins (CuPrs) at the Golgi body; (3) Pic2 or Mrs3 imports cuprous ions from cytosol into mitochondria, where they are loaded to CcO directly or via several chaperones including Cox17, Cox11, and Sco1/2. Excessive copper ions are sequestered by metallothioneins (Cup1 and Crs5) or separated in vacuoles. Vacuolar copper can be exported and mobilized by Ctr2. How Cu+ ions are transported into the vacuole and nucleus remains obscure. Genes involved in copper homeostasis are regulated by the copper sensing transcription factors Ace1 and Mac1: Cu+ promotes Ace1-mediated transactivation of copper scavenger genes (Cup1, Crs5, Sod1, etc.) and inhibits Mac1-mediated transactivation of genes responsible for copper uptake (Ctr1, Fre1, Fre2, etc.)
Influx of copper ions
Most extracellular copper ions are in the form of Cu(II). These cupric ions (Cu2+) are reduced into cuprous ions (Cu+) by reductase Fre1 or Fre2 on the cell surface before entering the yeast cell (Dancis et al. 1990; Georgatsou and Alexandraki 1999; Yun et al. 2001). It was recently reported that Fre1 overexpression is sufficient to increase copper internalization and increase stress tolerance to H2O2 exposure (Berterame et al. 2018), showing a key role of this reductase in copper influx. Once being reduced, the univalent ions are transported by copper transporters (CTRs) located on plasma membranes. Under physiological conditions, transmembrane transportation of copper is mediated by two high-affinity CTRs, Ctr1 and Ctr3 (Dancis et al. 1994a, b; Knight et al. 1996; Luk et al. 2003; Pena et al. 2000; Yonkovich et al. 2002), which both form a homo-trimeric Cu+-selective ion-channel-like architecture to import the metal (Pena et al. 2000; Ren et al. 2019). However, in most laboratory-bred yeast strains, such as BY4742, the Ctr3 gene is disabled by the insertion of a Ty2 transposon (Knight et al. 1996). Thus, copper-dependent enzymes in yeast cell lines lacking the components of the Ctr1 transport system generally exhibit copper deficiency (Dancis et al. 1994a; Pena et al. 2000). Yeast strains without functional Ctr1 and Ctr3 genes demonstrate growth defects on non-fermentable media due to a lack of Cu+ for cytochrome c oxidase (CcO). Using this characteristic, Zhou et al. cloned the human Ctr1 gene, whose expression perfectly complements the growth defect of Ctr1∆ yeast and significantly increases the level of cellular copper (Zhou and Gitschier 1997). These mutant yeast strains defective in high-affinity copper transport have also been used to identify candidate copper importers in a wide range of organisms, including algae and land plants (Page et al. 2009; Senovilla et al. 2018). Besides Cu+, platinum is also imported by Ctr1 (Bodiga et al. 2018). It has been reported that reduced glutathione (GSH) is the first acceptor of copper after it enters into the cell, following which Cu+ can be delivered to the chaperones or metallothioneins (MTs) (Freedman et al. 1989).
In addition to the high-affinity copper transport systems, yeast has a “backup” mechanism for obtaining copper (Lee et al. 2002). When the environmental concentration of copper is high, low-affinity copper proteins on cell surfaces, such as iron transporter 4 (Fet4), can mediate the influx of cupric ions (Dix et al. 1994; Hassett et al. 2000; Li and Kaplan 1998). In general, these low-affinity transporters incorporate not only copper but also a variety of divalent metal ions. Saccharomyces cerevisiae Fet4, for example, can nonspecifically transport Cu2+, Fe2+ and other divalent metal ions into cells (Hassett et al. 2000; Portnoy et al. 2001).
Detoxification of copper ions
After incorporation, a large proportion of the copper ions go through a detoxification pathway to prevent the accumulation of toxic free copper ions in the cell. It has been reported that the concentration of free copper ions within the cytoplasm is as low as 10–18 M (about one molecule of free Cu+ per cell) (Rae et al. 1999). The excess cytosolic copper ions are sequestered by scavengers such as metallothioneins (MTs) (Hamer 1986; Krezel and Maret 2017; Niederwanger et al. 2017). MTs are a family of low molecular weight proteins rich in cysteine residues, which can bind excess ions of copper and other heavy metals in the cell (Fogel and Welch 1982; Winge et al. 1985). MTs are encoded by multiple genes, including CRS5, CUP1-1 and CUP1-2 (Culotta et al. 1994; Karin et al. 1984). Expression of these genes is responsible for mediating copper resistance in yeast (Butt et al. 1984; Katju et al. 2009). This is because these MTs bear multiple thiol groups organized as clusters and can store high amounts of nonexchangeable copper (Calderone et al. 2005; Jensen et al. 1996). GSH is another natural chelator of transition metals. It is composed of three amino acids—glutamate, cysteine, and glycine—among which cysteine can directly bind to copper via its thiol group. GSH is not encoded by a gene but is synthesized in two steps by γ-glutamylcysteine synthetase (GSH1) and GSH synthetase (GSH2) in the cell (Grant et al. 1996). GSH can also be assimilated from the extracellular environment by specific transporters (Bourbouloux et al. 2000; Miyake et al. 1998). Besides its function in dispensing Cu+ from Ctr1 to other cuproprotiens, GSH can also act as a copper reservoir by forming complexes with Cu(I) (Freedman et al. 1989). Due to its copper buffering function, GSH can lower copper sensitivity and increase yeast vitality (Zimdars et al. 2019). Cu(I) complexes of GSH have been found in organelles such as the nucleus (Carroll et al. 2004). These GSH-bound Cu+ can directly metalize MTs and SOD1 (Carroll et al. 2004; Ferreira et al. 1993). Surplus copper ions can also be separated in vacuoles by an unknown mechanism (Miner et al. 2019). When necessary, vacuolar copper can be mobilized by Ctr2, which is a low-affinity copper transporter located at the vacuolar membrane and provides copper for cytosolic metallochaperones from vacuoles (Rees et al. 2004).
Copper utilization pathway
Another portion of copper ions is targeted to different destinations by multiple copper chaperones for the following uses (Festa and Thiele 2011): (a) Ccs1 mediates the assembly of Cu+ to Sod1 in cytosome; (b) Atx1 targets Cu+ to the Golgi body for various cuproproteins; (c) Cox17 passes Cu+ to mitochondrial cytochrome c oxidase (CcO) (Luk et al. 2003); and (d) Cu+ can also be delivered to the nucleus via an unknown mechanism.
Cytosolic pathway
Superoxide dismutase 1 (Sod1 or Cu/Zn SOD) is the most important copper protein in cytoplasm (Slekar et al. 1996). Each molecule of Sod1 also combines with a zinc atom, but the way that it obtains the zinc ion remains unknown. In contrast, it is well known that copper is targeted to Sod1 by its specific copper chaperone Ccs1 (copper chaperon for Sod1, also named Lys7) (Culotta et al. 1997). Ccs1 can bind to the cell membrane and directly interact with Ctr1 to obtain Cu+ (Pope et al. 2013). Then, Ccs1 can associate with Sod1 to deliver Cu+ to the latter molecule (Lamb et al. 2000). On the other hand, it has been reported that a small fraction of Sod1 molecules can acquire free Cu+ directly from the cytoplasm to maintain Sod1 activity in the absence of Ccs1 (Rae et al. 1999). Our previous data are consistent with this concept since we found that supplementation with copper endows yeast that is lacking Ccs1 with Sod1 activity (Li et al. 2010). This Ccs1-independent activation of Sod1 likely involves GSH, as it does in mammals (Carroll et al. 2004). Recently, Winkler’s group discovered a multifunctional chaperoning role for Ccs1 during Sod1 activation: Ccs1 delivers Cu+ to an entry site at the Sod1·Ccs1 interface upon binding, and then to the Sod1 active site by a thermodynamically-driven affinity gradient. The Sod1·Ccs1 interaction also promotes high-affinity Zn2+ binding in Sod1; as a result, Sod1 is fully activated (Boyd et al. 2019; Fetherolf et al. 2017). It has been reported that a fraction of active Sod1, along with Ccs1, localizes at the mitochondrial intermembrane space (IMS) and nucleus, where it protects yeast cells against oxidative damage (Sturtz et al. 2001), mediates copper-sensing activation of Mac1 (Wood and Thiele 2009), and functions as a transcription factor to regulate the expression of oxidative stress-responsive genes (Tsang et al. 2014).
Golgi pathway
All copper-dependent enzymes from the secretory pathway are loaded with copper ions on the Golgi apparatus and thus activated. The copper-transporting P-type ATPases translocate cuprous ions from cytoplasm to the lumen of the Golgi body, making them available for enzyme assembly (Yuan et al. 1995). In yeast cells, this P-type ATPase is called Ccc2, which cannot directly obtain free cuprous ions from cytoplasm, but accepts them through a copper chaperone, Atx1 (antioxidant 1) (Banci et al. 2001; Huffman and O'Halloran 2000). Atx1 was originally identified as a multi-copy suppressor of oxidative damage in yeast lacking SOD (Lin et al. 1997). The crystal structures demonstrate that Atx1 proteins dimerize to bind Cu+ (Lee et al. 2017). Though it was conventionally assumed that Ctr1 passes Cu+ to Atx1 via direct interaction between both proteins (Xiao et al. 2004), it has been reported that in the presence of copper, GSH induces dimerization of Atx1 and formation of a complex containing two Atx1, two Cu(I), and two GSH (Miras et al. 2008). This indicates that Cu+ incorporated by Ctr1 associates with GSH, which further delivers the metal ion to chaperon Atx1. In Golgi compartments, the secretory cuproproteins are loaded with Cu+ before they are targeted to specific organelles or secreted out of the cell (Fu et al. 1995; Lin et al. 1997; Pufahl et al. 1997; Yuan et al. 1995). The multicopper oxidase Fet3, which is required for high-affinity iron uptake, is metalized via this route (Askwith et al. 1994; Yuan et al. 1995). Thus, this Golgi pathway of copper delivery is required for iron uptake (Lin et al. 1997).
Mitochondrial pathway
Cytochrome c oxidase (CcO) is the terminal oxidase in the mitochondrial respiratory chain, catalyzing the transfer of electrons to oxygen to form H2O (Kloeckener-Gruissem et al. 1987). According to our previous study as well as data from other groups, copper deficiency leads to an impairment of CcO enzymatic activity (Li et al. 2010). Two copper binding sites were housed in the core subunits of CcO: Cox1 and Cox2 (Marechal et al. 2012). Incorporation of Cu+ into both sites requires multiple chaperones. Sco1 or Sco2 locates on the inner membrane of mitochondria and passes copper atoms to the copper A (CuA) site of Cox2 (Beers et al. 1997; Glerum et al. 1996a, b). It was traditionally assumed that Cox17 shuttles between cytoplasm and mitochondrial lumen, delivering cytosolic cuprous ions to Sco1 or Sco2 (Balatri et al. 2003; Lode et al. 2002; Nittis et al. 2001; Rentzsch et al. 1999; Schulze and Rodel 1988, 1989). However, recent reports suggest that Cox17 is imported unfolded into mitochondrial IMS and receives Cu from the mitochondrial lumen, not from the cytosol (Banci et al. 2009; Cobine et al. 2006). Cox17 also delivers copper to Cox11, which acts as a copper donor to the copper B (CuB) site of Cox1 (Hensgens et al. 1984; Hiser et al. 2000; Horng et al. 2004; Tzagoloff et al. 1990). In yeast, Cox17 is of great importance for normal growth and metabolism, since the deletion of Cox17 leads to defects in the respiratory chain. However, it seems that copper can partially bypass Cox17 and be loaded to CcO, given that supplementation with copper in the culture medium can make Cox17 mutant cells viable (Giaever et al. 2002).
The finding of a labile copper pool within the mitochondrial matrix may explain the Cox17-independent metallation of CcO (Cobine et al. 2004, 2006; Dodani et al. 2011; Vest et al. 2016). These IMS copper ions can be used to metalize CcO and mitochondrial SOD1 (Cobine et al. 2006). Cobine’s group also found that the mitochondrial carrier proteins Pic2 and Mrs3 are implicated in copper importation into mitochondria: Pic2- or Mrs3-deficient yeast strains exhibit defects in mitochondrial copper uptake and copper-dependent growth phenotypes owing to impaired CcO activity. Deletion of both genes (pic2Δmrs3Δ) leads to a more severe respiratory growth defect (Vest et al. 2013, 2016). However, it is still unclear where Pic2 and Mrs3 get copper from, since there is virtually no free copper in the cell, and it has been demonstrated that copper is associated almost exclusively with chaperones or the copper-binding proteins/peptides (Rae et al. 1999). In addition, the identity of the matrix copper ligand is unknown, since GSH was eliminated by Cobine et al. Organic acids such as citrate and oxaloacetate may act as ligands of these Cu+ ions (Cobine et al. 2004).
Nuclear pathway
As discussed below and shown in Fig. 1, fluctuation of the intracellular Cu+ concentration regulates copper-sensing transcription factors Ace1 and Mac1. It has been reported that metallation of Ace1 and Mac1 occurs independently within the nucleus (Keller et al. 2005). This indicates the presence of a labile nuclear Cu+ pool (Carroll et al. 2004). However, it is unclear how copper ions are transported into the nucleus. On the other hand, over-expression of the Crs5 metallothionein in this subcellular compartment does not compromise Mac1 Cu-responsive regulation (Keller et al. 2005), which is indicative of the inability of this labile nuclear Cu+ pool to promote Mac1 metallation. Wood et al. reported that Sod1 and Ccs1 partially localize to the yeast nucleus, and both molecules are required for the activation of Mac1 (Wood and Thiele 2009). This indicates that copper chaperone Ccs1 may deliver copper into the nucleus and activate Sod1, whose activity is involved in copper deficiency-induced Mac1 DNA binding. Furthermore, they found that C. elegans Sod1, which is copper-metalized and activated independently of Ccs1, can also rescue Mac1 activation and metallation in a Ccs1-independent manner in Saccharomyces cerevisiae (Wood and Thiele 2009). Currently, the ways that Mac1 and Ace1 are metalized remain unclear. In addition, the nature or mechanism of Cu transport into the nucleus remains obscure, in contrast to the existing body of knowledge on the routing of Cu by cytosolic chaperones and transporters between the secretory compartment, vacuole and mitochondria.
Copper homeostasis in yeast
Due to the dual effects of copper ions in cells, it is crucial to maintain a relatively constant concentration of cellular copper. On the one hand, there should be enough copper ions in the cell to execute normal physiological metabolism; on the other hand, over-intake of copper ions should be prevented to avoid toxicity. Organisms from yeast to humans maintain the cellular homeostasis of copper mainly by regulating the gene expressions of the transport and detoxification systems (Winge et al. 1998).
Genes involved in copper homeostasis in yeast are regulated mostly through two transcription factors, Ace1 and Mac1, which are positively and negatively regulated by copper ions, respectively. When a cell is abundant in copper, Ace1 (also called Cup2) is loaded with cuprous ions and subsequently activated (Buchman et al. 1989; Thiele 1988). As a consequence, proteins responsible for copper detoxification, such as metallothioneins (encoded by CUP1-1, CUP1-2 and CRS5) and Sod1 are up-regulated to neutralize the excess metal ions and remove harmful free radicals (Mehta et al. 2018; Rae et al. 1999). Mac1 contains an amino-terminal DNA binding domain and a carboxyl-terminal activation domain and is localized to the nucleus (Serpe et al. 1999). A high concentration of copper ions can inactivate Mac1, whose activity is essential to the expression of copper transporter genes including Ctr1, Ctr3, Fre1, Fre2, and Fre7. Without Cu+ binding to Mac1, this transcription factor occupies copper responsive elements (CuREs) in the promoters of its target genes and promotes their expression, facilitating the uptake of copper ions (Labbe et al. 1997; Martins et al. 1998). As mentioned above, Sod1 activity is essential to Mac1-mediated copper sensing: Mac1 is transcriptionally inactive in mutants that lack Sod1 or its copper chaperone Ccs1 (Wood and Thiele 2009). In response to mild DNA damage, Mac1 is activated via reduction of cysteine residues in its trans-activation domain, while severe DNA damage induces reversible oxidation of these residues and a consequent repression of Mac1 trans-activity. Both Sod1 and checkpoint kinase Rad53 are required for an unknown aspect of Mac1-mediated Ctr1 expression in response to mild DNA damage. Increased Ctr1 proteins can enhance copper influx, which can ensure Sod1 activity and Rad53 signaling in response to DNA damage in yeast (Dong et al. 2013). Recently, Alexandraki’s group found that another checkpoint protein, Rad9, directly binds with Mac1 under non-DNA-damaging conditions, suppressing its DNA binding and trans-activation functions. On the other hand, Rad53 also localizes to the Ctr1 coding region in a Rad9-dependent manner (Gkouskou et al. 2020). These data suggest a connection between copper-responsive transcription and the DNA repair pathway. The underlying mechanism remains obscure.
Mobilization of stored cuprous ions is another aspect of copper homeostasis (Rees et al. 2004; Rees and Thiele 2007). According to our investigation, the metal-sensing transcription factors Mac1 and Aft1 cooperatively activate the expression of vacuolar copper transporter Ctr2 upon deficiency of cytosolic Cu+, making yeast resistant to copper starvation (Liu et al. 2012; Qi et al. 2012).
Regulation of the key factors in copper homeostasis can also occur at a post-translational level. It has been reported that Ctr1 proteins are stable under a low level of intracellular copper and unstable at a high concentration of copper ions (Ooi et al. 1996). The copper sensing function of Mac1 is required in the degradation of Ctr1 at an elevated copper level (Yonkovich et al. 2002). Recently, Bodiga’s group found that the protein level of Ctr1 may be positively regulated by mitochondrial cuprochaperone Sco1. In Sco1-deficient yeast strains, copper restriction failed to increase the Ctr1 protein level, though Ctr1 mRNA was upregulated (Bodiga et al. 2018). This indicates that mitochondrial utilization of copper, which is mediated by Sco1, may up-regulate Ctr1 at the protein level via an as-yet-unknown mechanism. More importantly, this Sco1-dependent Ctr1 expression and consequential copper influx have also been found in humans (Hlynialuk et al. 2015; Leary et al. 2007). On the other hand, the stability of Mac1 itself is also regulated by the concentration of copper ions; when the intracellular copper concentration goes up, Mac1 protein is prone to degradation (Zhu et al. 1998).
Taken together, yeast cells evolve into a delicate system to maintain copper homeostasis (Fig. 1). In the presence of a high level of copper ions, genes involved in copper detoxification are switched on, while proteins responsible for copper incorporation are down-regulated at either the transcriptional or post-translational level. (Jungmann et al. 1993). On the contrary, upon deficiency of this metal, copper sensing transcription factors will down-regulate genes for copper detoxification and enhance the expression of proteins involved in copper intake or mobilization (Georgatsou et al. 1997; Hassett and Kosman 1995; Labbe et al. 1997; Yamaguchi-Iwai et al. 1997).
Conclusions
In summary, Saccharomyces cerevisiae has evolved delicate mechanisms for reaching copper homeostasis. Membrane proteins Fre1 and Fre2 mediate the reduction of extracellular Cu2+ into Cu+, which can be incorporated by the high-affinity transporters Ctr1 or Ctr3. Cu2+ can be directly transported by Fet4 in a low-affinity manner. Recent studies suggest that glutathione (GSH) is the first acceptor of Cu+ after it enters into the cell. Due to the sequestration effect of GSH, MTs, and vacuoles, there are almost no free copper ions present in the cell. Cu+ incorporated by Ctr1/3 can be targeted to different pathways. In cytosol, superoxide dismutase Sod1 is metalized and activated by Cu+ via its chaperone Ccs1. This process has recently been elucidated by studies revealing that Sod1 and Ccs1 also reside in mitochondria and nuclei to scavenge free radicals and that both proteins are involved in Mac1-dependent transcription. Atx1 is a chaperone targeting Cu+ into the Golgi body. GSH induces dimerization of Atx1 and passes Cu+ to it via direct interaction. Then, P-type ATPase Ccc2 obtains Cu+ from Atx1 and pumps Cu+ into the Golgi body, where various cuproproteins, including Fet3, are loaded with Cu+. Copper chaperone Cox17 shuttles between cytoplasm and mitochondrial lumen, delivering Cu+ to Sco1/2 or Cox11 on the inner mitochondrial membrane. Cox11 and Sco1/2 present Cu+ to different subunits of cytochrome c oxidase (CcO) to activate this essential enzyme in the respiratory chain. It was recently reported that Pic2 and Mrs3 mediate copper importation into mitochondria to generate a labile copper pool within the mitochondrial matrix, which may account for Cox17-independent metallation of CcO. However, the details remain obscure. It is unclear how copper ions are imported into the nucleus and how they are sensed by the copper-responsive transcription factors Mac1 and Ace1. Ace1 can mediate the transcription of MT-encoding genes and Sod1 in response to increased cellular Cu+. Copper deficiency induces Mac1-dependent transcription of copper transport components including Fre1/2 and Ctr1/3. A high concentration of Cu+ inactivates Mac1 or induces degradation of Mac1 and Ctr1. Mac1 is also involved in the transactivation of Ctr2 to mobilize vacuolar copper upon copper deficiency. On the other hand, several new pieces of data reveal that Mac1-dependent transcription is not only involved in copper uptake but also DNA damage and checkpoints. In future, these unresolved issues should be explored to thoroughly unravel the mechanism of copper metabolism in yeast. This will undoubtedly be helpful in elucidating the corresponding mechanism in mammals and unveiling the mechanisms of copper-related human disease.
References
Askwith C et al (1994) The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell 76:403–410. https://doi.org/10.1016/0092-8674(94)90346-8
Balatri E, Banci L, Bertini I, Cantini F, Ciofi-Baffoni S (2003) Solution structure of Sco1: a thioredoxin-like protein Involved in cytochrome c oxidase assembly. Structure 11:1431–1443. https://doi.org/10.1016/j.str.2003.10.004
Banci L, Bertini I, Ciofi-Baffoni S, Huffman DL, O'Halloran TV (2001) Solution structure of the yeast copper transporter domain Ccc2a in the apo and Cu(I)-loaded states J Biol Chem 276:8415–8426 doi:https://doi.org/10.1074/jbc.M008389200
Banci L et al (2009) MIA40 is an oxidoreductase that catalyzes oxidative protein folding in mitochondria. Nat Struct Mol Biol 16:198–206. https://doi.org/10.1038/nsmb.1553
Beers J, Glerum DM, Tzagoloff A (1997) Purification, characterization, and localization of yeast Cox17p, a mitochondrial copper shuttle. J Biol Chem 272:33191–33196
Berterame NM, Martani F, Porro D, Branduardi P (2018) Copper homeostasis as a target to improve Saccharomyces cerevisiae tolerance to oxidative stress. Metab Eng 46:43–50. https://doi.org/10.1016/j.ymben.2018.02.010
Bodiga S, Vemuri PK, Bodiga VL (2018) Low Ctr1p, due to lack of Sco1p results in lowered cisplatin uptake and mediates insensitivity of rho0 yeast to cisplatin. J Inorg Biochem 187:14–24. https://doi.org/10.1016/j.jinorgbio.2018.07.003
Bourbouloux A, Shahi P, Chakladar A, Delrot S, Bachhawat AK (2000) Hgt1p, a high affinity glutathione transporter from the yeast Saccharomyces cerevisiae. J Biol Chem 275:13259–13265. https://doi.org/10.1074/jbc.275.18.13259
Boyd SD, Calvo JS, Liu L, Ullrich MS, Skopp A, Meloni G, Winkler DD (2019) The yeast copper chaperone for copper-zinc superoxide dismutase (CCS1) is a multifunctional chaperone promoting all levels of SOD1 maturation. J Biol Chem 294:1956–1966. https://doi.org/10.1074/jbc.RA118.005283
Buchman C, Skroch P, Welch J, Fogel S, Karin M (1989) The CUP2 gene product, regulator of yeast metallothionein expression, is a copper-activated DNA-binding protein. Mol Cell Biol 9:4091–4095. https://doi.org/10.1128/mcb.9.9.4091
Butt TR, Sternberg E, Herd J, Crooke ST (1984) Cloning and expression of a yeast copper metallothionein gene. Gene 27:23–33. https://doi.org/10.1016/0378-1119(84)90235-x
Calderone V et al (2005) The crystal structure of yeast copper thionein: the solution of a long-lasting enigma. Proc Natl Acad Sci U S A 102:51–56. https://doi.org/10.1073/pnas.0408254101
Carroll MC, Girouard JB, Ulloa JL, Subramaniam JR, Wong PC, Valentine JS, Culotta VC (2004) Mechanisms for activating Cu- and Zn-containing superoxide dismutase in the absence of the CCS Cu chaperone. Proc Natl Acad Sci U S A 101:5964–5969. https://doi.org/10.1073/pnas.0308298101
Cobine PA, Ojeda LD, Rigby KM, Winge DR (2004) Yeast contain a non-proteinaceous pool of copper in the mitochondrial matrix. J Biol Chem 279:14447–14455. https://doi.org/10.1074/jbc.M312693200
Cobine PA, Pierrel F, Bestwick ML, Winge DR (2006) Mitochondrial matrix copper complex used in metallation of cytochrome oxidase and superoxide dismutase. J Biol Chem 281:36552–36559. https://doi.org/10.1074/jbc.M606839200
Culotta VC, Howard WR, Liu XF (1994) CRS5 encodes a metallothionein-like protein in Saccharomyces cerevisiae. J Biol Chem 269:25295–25302
Culotta VC, Klomp LW, Strain J, Casareno RL, Krems B, Gitlin JD (1997) The copper chaperone for superoxide dismutase. J Biol Chem 272:23469–23472
Dancis A, Klausner RD, Hinnebusch AG, Barriocanal JG (1990) Genetic evidence that ferric reductase is required for iron uptake in Saccharomyces cerevisiae. Mol Cell Biol 10:2294–2301. https://doi.org/10.1128/mcb.10.5.2294
Dancis A, Haile D, Yuan DS, Klausner RD (1994a) The Saccharomyces cerevisiae copper transport protein (Ctr1p). Biochemical characterization, regulation by copper, and physiologic role in copper uptake. J Biol Chem 269:25660–25667
Dancis A et al (1994b) Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell 76:393–402
De Freitas J, Wintz H, Kim JH, Poynton H, Fox T, Vulpe C (2003) Yeast, a model organism for iron and copper metabolism studies. Biometals 16:185–197
Dix DR, Bridgham JT, Broderius MA, Byersdorfer CA, Eide DJ (1994) The FET4 gene encodes the low affinity Fe(II) transport protein of Saccharomyces cerevisiae. J Biol Chem 269:26092–26099
Dodani SC, Leary SC, Cobine PA, Winge DR, Chang CJ (2011) A targetable fluorescent sensor reveals that copper-deficient SCO1 and SCO2 patient cells prioritize mitochondrial copper homeostasis. J Am Chem Soc 133:8606–8616. https://doi.org/10.1021/ja2004158
Dong K, Addinall SG, Lydall D, Rutherford JC (2013) The yeast copper response is regulated by DNA damage. Mol Cell Biol 33:4041–4050. https://doi.org/10.1128/MCB.00116-13
Ferreira AM, Ciriolo MR, Marcocci L, Rotilio G (1993) Copper(I) transfer into metallothionein mediated by glutathione. Biochem J 292(Pt 3):673–676. https://doi.org/10.1042/bj2920673
Festa RA, Thiele DJ (2011) Copper: an essential metal in biology. Curr Biol 21:R877–R883
Fetherolf MM et al (2017) Copper-zinc superoxide dismutase is activated through a sulfenic acid intermediate at a copper ion entry site. J Biol Chem 292:12025–12040. https://doi.org/10.1074/jbc.M117.775981
Fogel S, Welch JW (1982) Tandem gene amplification mediates copper resistance in yeast. Proc Natl Acad Sci U S A 79:5342–5346. https://doi.org/10.1073/pnas.79.17.5342
Freedman JH, Ciriolo MR, Peisach J (1989) The role of glutathione in copper metabolism and toxicity. J Biol Chem 264:5598–5605
Fu D, Beeler TJ, Dunn TM (1995) Sequence, mapping and disruption of CCC2, a gene that cross-complements the Ca(2+)-sensitive phenotype of csg1 mutants and encodes a P-type ATPase belonging to the Cu(2+)-ATPase subfamily. Yeast 11:283–292
Georgatsou E, Alexandraki D (1999) Regulated expression of the Saccharomyces cerevisiae Fre1p/Fre2p Fe/Cu reductase related genes. Yeast 15:573–584. doi:https://doi.org/10.1002/(SICI)1097-0061(199905)15:7<573::AID-YEA404>3.0.CO;2-7
Georgatsou E, Mavrogiannis LA, Fragiadakis GS, Alexandraki D (1997) The yeast Fre1p/Fre2p cupric reductases facilitate copper uptake and are regulated by the copper-modulated Mac1p activator. J Biol Chem 272:13786–13792
Giaever G et al (2002) Functional profiling of the Saccharomyces cerevisiae genome. Nature 418:387–391
Gkouskou K, Fragiadakis GS, Voutsina A, Alexandraki D (2020) Distinct associations of the Saccharomyces cerevisiae Rad9 protein link Mac1-regulated transcription to DNA repair. Curr Genet 66:531–548. https://doi.org/10.1007/s00294-019-01047-w
Glerum DM, Shtanko A, Tzagoloff A (1996a) Characterization of COX17, a yeast gene involved in copper metabolism and assembly of cytochrome oxidase. J Biol Chem 271:14504–14509
Glerum DM, Shtanko A, Tzagoloff A (1996b) SCO1 and SCO2 act as high copy suppressors of a mitochondrial copper recruitment defect in Saccharomyces cerevisiae. J Biol Chem 271:20531–20535
Grant CM, MacIver FH, Dawes IW (1996) Glutathione is an essential metabolite required for resistance to oxidative stress in the yeast Saccharomyces cerevisiae. Curr Genet 29:511–515. https://doi.org/10.1007/BF02426954
Hamer DH (1986) Metallothionein. Annu Rev Biochem 55:913–951
Hassett R, Kosman DJ (1995) Evidence for Cu(II) reduction as a component of copper uptake by Saccharomyces cerevisiae. J Biol Chem 270:128–134
Hassett R, Dix DR, Eide DJ, Kosman DJ (2000) The Fe(II) permease Fet4p functions as a low affinity copper transporter and supports normal copper trafficking in Saccharomyces cerevisiae. Biochem J 351(Pt 2):477–484
Hensgens LA, van der Horst G, Vos HL, Grivell LA (1984) RNA processing in yeast mitochondria: characterization of mit(-) mutants disturbed in the synthesis of subunit I of cytochrome c oxidase. Curr Genet 8:457–465. https://doi.org/10.1007/BF00433912
Hiser L, Di Valentin M, Hamer AG, Hosler JP (2000) Cox11p is required for stable formation of the Cu(B) and magnesium centers of cytochrome c oxidase. J Biol Chem 275:619–623. https://doi.org/10.1074/jbc.275.1.619
Hlynialuk CJ, Ling B, Baker ZN, Cobine PA, Lisa DY, Boulet A, Wai T, Hossain A, El Zawily AM, McFie PJ, Stone SJ (2015) The Mitochondrial Metallochaperone SCO1 Is Required to Sustain Expression of the High-Affinity Copper Transporter CTR1 and Preserve Copper Homeostasis. Cell Rep 10(6):933–43
Horng YC, Cobine PA, Maxfield AB, Carr HS, Winge DR (2004) Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome C oxidase. J Biol Chem 279:35334–35340
Huffman DL, O’Halloran TV (2000) Energetics of copper trafficking between the Atx1 metallochaperone and the intracellular copper transporter, Ccc2. J Biol Chem 275:18611–18614
Jensen LT, Howard WR, Strain JJ, Winge DR, Culotta VC (1996) Enhanced effectiveness of copper ion buffering by CUP1 metallothionein compared with CRS5 metallothionein in Saccharomyces cerevisiae. J Biol Chem 271:18514–18519. https://doi.org/10.1074/jbc.271.31.18514
Jungmann J, Reins HA, Lee J, Romeo A, Hassett R, Kosman D, Jentsch S (1993) MAC1, a nuclear regulatory protein related to Cu-dependent transcription factors is involved in Cu/Fe utilization and stress resistance in yeast. EMBO J 12:5051–5056
Karin M, Najarian R, Haslinger A, Valenzuela P, Welch J, Fogel S (1984) Primary structure and transcription of an amplified genetic locus: the CUP1 locus of yeast. Proc Natl Acad Sci U S A 81:337–341. https://doi.org/10.1073/pnas.81.2.337
Katju V, Farslow JC, Bergthorsson U (2009) Variation in gene duplicates with low synonymous divergence in Saccharomyces cerevisiae relative to Caenorhabditis elegans. Genome Biol 10:R75. https://doi.org/10.1186/gb-2009-10-7-r75
Keller G, Bird A, Winge DR (2005) Independent metalloregulation of Ace1 and Mac1 in Saccharomyces cerevisiae. Eukaryot Cell 4:1863–1871. https://doi.org/10.1128/EC.4.11.1863-1871.2005
Kloeckener-Gruissem B, McEwen JE, Poyton RO (1987) Nuclear functions required for cytochrome c oxidase biogenesis in Saccharomyces cerevisiae: multiple trans-acting nuclear genes exert specific effects on expression of each of the cytochrome c oxidase subunits encoded on mitochondrial DNA. Curr Genet 12:311–322. https://doi.org/10.1007/BF00405753
Knight SA, Labbe S, Kwon LF, Kosman DJ, Thiele DJ (1996) A widespread transposable element masks expression of a yeast copper transport gene. Genes Dev 10:1917–1929
Krezel, A., & Maret, W. (2017). The functions of metamorphic metallothioneins in zinc and copper metabolism. Int J Mol Sci, 18(6), 1237.
Labbe S, Zhu Z, Thiele DJ (1997) Copper-specific transcriptional repression of yeast genes encoding critical components in the copper transport pathway. J Biol Chem 272:15951–15958
Lamb AL, Torres AS, O’Halloran TV, Rosenzweig AC (2000) Heterodimer formation between superoxide dismutase and its copper chaperone. Biochemistry 39:14720–14727
Leary SC et al (2007) The human cytochrome c oxidase assembly factors SCO1 and SCO2 have regulatory roles in the maintenance of cellular copper homeostasis. Cell Metab 5:9–20. https://doi.org/10.1016/j.cmet.2006.12.001
Lee J, Petris MJ, Thiele DJ (2002) Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter. Identification of a Ctr1-independent copper transport system. J Biol Chem 277:40253–40259
Lee M, Cooray NDG, Maher MJ (2017) The crystal structures of a copper-bound metallochaperone from Saccharomyces cerevisiae. J Inorg Biochem 177:368–374. https://doi.org/10.1016/j.jinorgbio.2017.08.009
Li L, Kaplan J (1998) Defects in the yeast high affinity iron transport system result in increased metal sensitivity because of the increased expression of transporters with a broad transition metal specificity. J Biol Chem 273:22181–22187. https://doi.org/10.1074/jbc.273.35.22181
Li C, Wang J, Zhou B (2010) The metal chelating and chaperoning effects of clioquinol: insights from yeast studies. J Alzheimers Dis 21:1249–1262
Lin SJ, Pufahl RA, Dancis A, O’Halloran TV, Culotta VC (1997) A role for the Saccharomyces cerevisiae ATX1 gene in copper trafficking and iron transport. J Biol Chem 272:9215–9220
Liu L, Qi J, Yang Z, Peng L, Li C (2012) Low-affinity copper transporter CTR2 is regulated by copper-sensing transcription factor Mac1p in Saccharomyces cerevisiae. Biochem Biophys Res Commun 420:600–604
Lode A, Paret C, Rodel G (2002) Molecular characterization of Saccharomyces cerevisiae Sco2p reveals a high degree of redundancy with Sco1p. Yeast 19:909–922. https://doi.org/10.1002/yea.883
Luk E, Jensen LT, Culotta VC (2003) The many highways for intracellular trafficking of metals. J Biol Inorg Chem 8:803–809
Marechal A, Meunier B, Lee D, Orengo C, Rich PR (2012) Yeast cytochrome c oxidase: a model system to study mitochondrial forms of the haem-copper oxidase superfamily. Biochim Biophys Acta 1817:620–628
Martins LJ, Jensen LT, Simon JR, Keller GL, Winge DR (1998) Metalloregulation of FRE1 and FRE2 homologs in Saccharomyces cerevisiae. J Biol Chem 273:23716–23721
Mehta GD, Ball DA, Eriksson PR, Chereji RV, Clark DJ, McNally JG, Karpova TS (2018) Single-molecule analysis reveals linked cycles of RSC chromatin remodeling and Ace1p transcription factor binding in yeast. Mol Cell 72(875–887):e879
Miner GE et al (2019) Copper blocks V-ATPase activity and SNARE complex formation to inhibit yeast vacuole fusion. Traffic 20:841–850
Miras R, Morin I, Jacquin O, Cuillel M, Guillain F, Mintz E (2008) Interplay between glutathione, Atx1 and copper. 1. Copper(I) glutathionate induced dimerization of Atx1. J Biol Inorg Chem 13:195–205. https://doi.org/10.1007/s00775-007-0310-2
Miyake T, Hazu T, Yoshida S, Kanayama M, Tomochika K, Shinoda S, Ono B (1998) Glutathione transport systems of the budding yeast Saccharomyces cerevisiae. Biosci Biotechnol Biochem 62:1858–1864. https://doi.org/10.1271/bbb.62.1858
Niederwanger M, Calatayud S, Zerbe O, Atrian S, Albalat R, Capdevila M, Palacios Ò, Dallinger R (2017) Biomphalaria glabrata metallothionein: lacking metal specificity of the protein and missing gene upregulation suggest metal sequestration by exchange instead of through selective binding. Int J Mol Sci 18(7):1457
Nittis T, George GN, Winge DR (2001) Yeast Sco1, a protein essential for cytochrome c oxidase function is a Cu(I)-binding protein. J Biol Chem 276:42520–42526. https://doi.org/10.1074/jbc.M107077200
Ooi CE, Rabinovich E, Dancis A, Bonifacino JS, Klausner RD (1996) Copper-dependent degradation of the Saccharomyces cerevisiae plasma membrane copper transporter Ctr1p in the apparent absence of endocytosis. Embo J 15:3515–3523
Page MD, Kropat J, Hamel PP, Merchant SS (2009) Two Chlamydomonas CTR copper transporters with a novel cys-met motif are localized to the plasma membrane and function in copper assimilation. Plant Cell 21:928–943. https://doi.org/10.1105/tpc.108.064907
Pena MM, Puig S, Thiele DJ (2000) Characterization of the Saccharomyces cerevisiae high affinity copper transporter Ctr3. J Biol Chem 275:33244–33251
Pope CR, De Feo CJ, Unger VM (2013) Cellular distribution of copper to superoxide dismutase involves scaffolding by membranes. Proc Natl Acad Sci U S A 110:20491–20496
Portnoy ME, Schmidt PJ, Rogers RS, Culotta VC (2001) Metal transporters that contribute copper to metallochaperones in Saccharomyces cerevisiae. Mol Genet Genom 265:873–882
Pufahl RA et al (1997) Metal ion chaperone function of the soluble Cu(I) receptor Atx1. Science 278:853–856
Qi J, Han A, Yang Z, Li C (2012) Metal-sensing transcription factors Mac1p and Aft1p coordinately regulate vacuolar copper transporter CTR2 in Saccharomyces cerevisiae. Biochem Biophys Res Commun 423:424–428
Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV (1999) Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284:805–808
Rees EM, Thiele DJ (2007) Identification of a vacuole-associated metalloreductase and its role in Ctr2-mediated intracellular copper mobilization. J Biol Chem 282:21629–21638. https://doi.org/10.1074/jbc.M703397200
Rees EM, Lee J, Thiele DJ (2004) Mobilization of intracellular copper stores by the ctr2 vacuolar copper transporter. J Biol Chem 279:54221–54229. https://doi.org/10.1074/jbc.M411669200
Ren F, Logeman BL, Zhang X, Liu Y, Thiele DJ, Yuan P (2019) X-ray structures of the high-affinity copper transporter Ctr1. Nat Commun 10:1386
Rentzsch A, Krummeck-Weiss G, Hofer A, Bartuschka A, Ostermann K, Rodel G (1999) Mitochondrial copper metabolism in yeast: mutational analysis of Sco1p involved in the biogenesis of cytochrome c oxidase. Curr Genet 35:103–108. https://doi.org/10.1007/s002940050438
Schulze M, Rodel G (1988) SCO1, a yeast nuclear gene essential for accumulation of mitochondrial cytochrome c oxidase subunit II. Mol Gen Genet 211:492–498. https://doi.org/10.1007/BF00425706
Schulze M, Rodel G (1989) Accumulation of the cytochrome c oxidase subunits I and II in yeast requires a mitochondrial membrane-associated protein, encoded by the nuclear SCO1 gene. Mol Gen Genet 216:37–43. https://doi.org/10.1007/BF00332228
Senovilla M et al (2018) Medicago truncatula copper transporter 1 (MtCOPT1) delivers copper for symbiotic nitrogen fixation. New Phytol 218:696–709. https://doi.org/10.1111/nph.14992
Serpe M, Joshi A, Kosman DJ (1999) Structure-function analysis of the protein-binding domains of Mac1p, a copper-dependent transcriptional activator of copper uptake in Saccharomyces cerevisiae. J Biol Chem 274:29211–29219
Slekar KH, Kosman DJ, Culotta VC (1996) The yeast copper/zinc superoxide dismutase and the pentose phosphate pathway play overlapping roles in oxidative stress protection. J Biol Chem 271:28831–28836. https://doi.org/10.1074/jbc.271.46.28831
Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta VC (2001) A fraction of yeast Cu, Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J Biol Chem 276:38084–38089. https://doi.org/10.1074/jbc.M105296200
Thiele DJ (1988) ACE1 regulates expression of the Saccharomyces cerevisiae metallothionein gene. Mol Cell Biol 8:2745–2752
Tsang CK, Liu Y, Thomas J, Zhang Y, Zheng XF (2014) Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat Commun 5:3446. https://doi.org/10.1038/ncomms4446
Tzagoloff A, Capitanio N, Nobrega MP, Gatti D (1990) Cytochrome oxidase assembly in yeast requires the product of COX11, a homolog of the P. denitrificans protein encoded by ORF3. EMBO J 9:2759–2764
Vest KE, Leary SC, Winge DR, Cobine PA (2013) Copper import into the mitochondrial matrix in Saccharomyces cerevisiae is mediated by Pic2, a mitochondrial carrier family protein. J Biol Chem 288:23884–23892. https://doi.org/10.1074/jbc.M113.470674
Vest KE et al (2016) Overlap of copper and iron uptake systems in mitochondria in Saccharomyces cerevisiae. Open Biol 6:150223. https://doi.org/10.1098/rsob.150223
Winge DR, Nielson KB, Gray WR, Hamer DH (1985) Yeast metallothionein sequence and metal-binding properties. J Biol Chem 260:14464–14470
Winge DR, Jensen LT, Srinivasan C (1998) Metal-ion regulation of gene expression in yeast. Curr Opin Chem Biol 2:216–221
Wood LK, Thiele DJ (2009) Transcriptional activation in yeast in response to copper deficiency involves copper-zinc superoxide dismutase. J Biol Chem 284:404–413
Xiao Z, Loughlin F, George GN, Howlett GJ, Wedd AG (2004) C-terminal domain of the membrane copper transporter Ctr1 from Saccharomyces cerevisiae binds four Cu(I) ions as a cuprous-thiolate polynuclear cluster: sub-femtomolar Cu(I) affinity of three proteins involved in copper trafficking. J Am Chem Soc 126:3081–3090. https://doi.org/10.1021/ja0390350
Yamaguchi-Iwai Y, Serpe M, Haile D, Yang W, Kosman DJ, Klausner RD, Dancis A (1997) Homeostatic regulation of copper uptake in yeast via direct binding of MAC1 protein to upstream regulatory sequences of FRE1 and CTR1. J Biol Chem 272:17711–17718
Yonkovich J, McKenndry R, Shi X, Zhu Z (2002) Copper ion-sensing transcription factor Mac1p post-translationally controls the degradation of its target gene product Ctr1p. J Biol Chem 277:23981–23984
Yuan DS, Stearman R, Dancis A, Dunn T, Beeler T, Klausner RD (1995) The Menkes/Wilson disease gene homologue in yeast provides copper to a ceruloplasmin-like oxidase required for iron uptake. Proc Natl Acad Sci U S A 92:2632–2636
Yun CW, Bauler M, Moore RE, Klebba PE, Philpott CC (2001) The role of the FRE family of plasma membrane reductases in the uptake of siderophore-iron in Saccharomyces cerevisiae. J Biol Chem 276:10218–10223. https://doi.org/10.1074/jbc.M010065200
Zhou B, Gitschier J (1997) hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci USA 94:7481–7486
Zhu Z, Labbe S, Pena MM, Thiele DJ (1998) Copper differentially regulates the activity and degradation of yeast Mac1 transcription factor. J Biol Chem 273:1277–1280
Zimdars S, Schrage L, Sommer S, Schieber A, Weber F (2019) Influence of glutathione on yeast fermentation efficiency under copper stress. J Agric Food Chem 67:10913–10920. https://doi.org/10.1021/acs.jafc.9b03519
Funding
This work was supported by the National Natural Science Foundation of China (91749121 to Li), the Fundamental Research Funds for Central Universities (SCU2019D013 to Li and SCU2019C4005 to Shi), and the Outstanding Young and Middle-aged Scientific and Technological Innovation Team Program of the Colleges and Universities in Hubei Province (T201819 to Jiang).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shi, H., Jiang, Y., Yang, Y. et al. Copper metabolism in Saccharomyces cerevisiae: an update. Biometals 34, 3–14 (2021). https://doi.org/10.1007/s10534-020-00264-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-020-00264-y