Abstract
In the last 20 years, several new genes and proteins involved in iron metabolism in eukaryotes, particularly related to pathological states both in animal models and in humans have been identified, and we are now starting to unveil at the molecular level the mechanisms of iron absorption, the regulation of iron transport and the homeostatic balancing processes. In this review, we will briefly outline the general scheme of iron metabolism in humans and then focus our attention on the cellular iron export system formed by the permease ferroportin and the ferroxidase ceruloplasmin. We will finally summarize data on the role of the iron binding protein lactoferrin on the regulation of the ferroportin/ceruloplasmin couple and of other proteins involved in iron homeostasis in inflamed human macrophages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Little is fundamental for living organisms such as iron, as only members of the Lactobacillus and Bacillus families can sustain life without this metal. Many biological pathways exploit its ability to accept and donate electrons by redox-cycling between the Fe(II) and Fe(III) forms. In fact, iron is involved in (and drives) a number of pivotal processes, most notably including electron transport and DNA synthesis and, in multicellular eukaryotes, oxygen transport/storage and drug detoxification. However, iron is toxic when in excess.
Any defect impairing iron metabolism has severe consequences at both cellular and systemic levels. In humans, the loss of regulation of iron metabolism can lead to development of iron overload as seen in hereditary hemochromatosis, a common inherited disorder that often results in progressive organ dysfunction. Excess iron is mainly stored in macrophages and hepatocytes. When tissue storage capacity is saturated, free iron catalyzes the formation of reactive oxygen species (ROS), which are causative of tissue fibrosis and lead to clinical consequences of liver failure, cardiomyopathy, and endocrinopathies. On the other hand, when iron becomes chronically scarce different pathological states can develop, such as the anaemia of chronic disease or anaemia associated with inflammation. Anaemia is the most obvious outcome of iron deficiency in blood because of the enormous amount of iron required for hemoglobin production in developing erythroid cells.
The general scheme of iron absorption, transport and storage in humans
In mammals, and therefore in humans, no regulated excretory pathways for iron exist, thus iron absorption in normal adults has to match the rate of iron loss essentially due to bleeding or sloughing of skin and mucosal cells. Therefore, the first step of regulation of iron homeostasis in humans is at the level of intestinal absorption, which tightly depends on the body’s demand for the metal. However, the rate of iron absorption by the intestine is normally low (1–2 mg/day) relative to total body iron content (3–5 g). In this respect, macrophages play a major role as it can be estimated that up to 30 mg/day of iron is made available to cells through recycling of aged/damaged red blood cells by macrophages (see below). Several physio-pathological signals, including iron load, erythropoiesis, hypoxia and inflammation, can regulate the absorption of dietary iron by the intestine. Inorganic iron enters the duodenal enterocyte through the apical membrane as Fe(II) through the divalent metal transporter-1 (DMT1, also known as SLC11A2, natural resistance– associated macrophage protein 2, and divalent cation transporter 1). A ferrireductase (most likely Dcytb) is needed at this stage to make divalent iron available for DMT1. Although iron is the metal that is most efficiently transported by DMT1, this protein is indeed a nonspecific divalent metal symporter, able to promote transport of Mn(II) and Co(II), beside Fe(II), in a pH-dependent molecular mechanism requiring a number of protons per metal ion (Gunshin et al. 1997).
By studying DMT1-defective knockout mice, it has been shown that DMT1 may be the primary means for cellular iron import, but it is not the sole mechanism (Gunshin et al. 2005). Other, not well characterized yet, mechanisms for inorganic iron import are apparently present on the cell surface. In any case, iron can alternatively get into most cells, including enterocytes, as part of haem, coming from the digestion of hemoglobin and myoglobin from dietary meat. Two putative intestinal haem permeases have been so far identified, namely haem carrier protein 1 (HCP1) (Shayeghi et al. 2005) and haem responsive gene-1 (HRG-1) (Rajagopal et al. 2008). Regardless of the permease used, haem iron becomes metabolically available within the cell after haem degradation catalyzed by haem oxygenases. Interestingly, upregulation of the haem oxygenase 1 gene is accompanied by an increase in HCP1 expression, suggesting that uptake and degradation of haem are tightly connected (Latunde-Dada et al. 2006).
Iron taken up by enterocytes through apical DMT1 or as haem is not retained in the cytoplasm, but it is rather mostly delivered to the bloodstream through ferroportin (Fpn, initially also named Ireg-1 or MTP-1), a protein localized on the basal side of polarized enterocytes and that constitutes the only mammalian iron exporter known so far. Iron is extruded by Fpn as Fe(II), and a family of enzymes that convert Fe(II) to Fe(III) is needed for iron to be correctly loaded onto transferrin (Tf), which can only bind the oxidized form of the metal. These enzymes are multicopper oxidases endowed with ferroxidase activity and include ceruloplasmin (Cp) and hephaestin, a membrane-bound paralog of Cp (Vulpe et al. 1999). A number of studies have indicated that hephaestin and Fpn may interact (Collins and Anderson 2012). In fact, this multicopper oxidase is believed to be the principal partner of Fpn in duodenal iron uptake: in the sex-linked anaemia mouse, where hephaestin is mutated, iron accumulation in intestinal enterocytes and systemic anaemia occur (Vulpe et al. 1999).
In serum, iron is mostly bound to transferrin (Tf), a powerful chelator, able to bind iron tightly but reversibly. A molecule of Tf can bind two atoms of ferric iron with an extremely high affinity (Kd = 10− 23 M) (Aisen et al. 1978), which decreases when the protein is internalized in the cell and enters the acidified endosomes, allowing the dissociation of Fe(III). Tf belongs to a family of homologous iron-binding glycoproteins comprising also lactoferrin (found in secretions, including milk), delta-lactoferrin (localized intracellularly) melanotrasferrin (in melanoma cells) and ovotransferrin (in egg white) (Lambert 2012). Iron chelation by transferrin not only maintains iron in a soluble oxidized form under physiologic conditions, but also prevents the generation of ROS by keeping the metal in a redox-inert state and, last but not least, facilitates regulated iron transport and cellular uptake. Finally, diferric holo-Tf exerts a key regulatory function in the expression of hepcidin, the main regulator of iron homeostasis (see below).
Tf donates iron to cells (notably those that are rapidly dividing, in particular erythroid precursor cells) by a receptor-mediated endocytosis, following interaction of iron-loaded Tf with the cell surface transferrin receptor 1 (TfR1). This transmembrane glycoprotein is a homodimer stabilized by disulfide bonds, which can bind one Tf molecule at each of its subunits (Aisen 2004). The iron status of Tf determines its affinity for TfR1, with diferric Tf binding to TfR1 30- and 500-fold better compared to monoferric and apo-Tf, respectively (Young et al. 1984).
Following the binding of Tf to its receptor, the Tf-TfR1 complex undergoes endocytosis via clathrin-coated pits. Acidification of the endosome to pH 5.5 by a proton pump ATPase triggers a conformational change in Tf resulting in a drop of the affinity for iron and in Fe(III) release (Klausner et al. 1983). For Fe(III) to pass across the endosomal membrane via DMT1, it has to be reduced to Fe(II). The ferric reductase STEAP3 performs this function in immature erythroid cells (Ohgami et al. 2005). Finally, the apo-Tf/TfR1 complex returns to the cell membrane, where apo-Tf is recycled back to the bloodstream, available to rebind iron.
Going into regulatory aspects, since both iron overload and iron deficiency are detrimental for cells, the levels of biologically available iron must be tightly controlled by complex mechanisms of iron acquisition, trafficking and storage. As discussed later, systemic iron homeostasis is mainly controlled by the hormone hepcidin, which effectively buffers circulating iron levels by inducing Fpn degradation, thus blocking intestinal iron absorption and iron release from macrophages (Nemeth et al. 2004). At the cellular level, transcriptional and post-transcriptional regulatory mechanisms are active, which finely modulate the expression of iron homeostasis-related genes. Among these mechanisms, it should be mentioned the transactivation of genes in enterocytes by the hypoxia-inducible factor HIF2α (Mastrogiannaki et al. 2009) and the well-known post-transcriptional control of mRNA via interaction of iron-responsive elements or IREs with cytosolic iron-sensing proteins collectively named iron-regulatory proteins or IRPs. In low iron conditions, binding of IRPs to IREs hampers mRNA translation when the RNA elements are located in 5′UTR (e.g., ferritin) and stabilizes mRNAs bearing the elements in the 3′UTR (e.g., TfR1). On the other hand, when iron is in excess, IRPs do not bind anymore to IREs, ferritin synthesis is allowed and TfR1 is downregulated (Torti and Torti 2002). Fpn expression too is regulated by iron-responsive sequences at both the 5′UTR and 3′UTR ends of its mRNA. While repression of Fpn mRNA translation in conditions of iron deficiency is due to the canonical mechanism involving an IRE sequence at the 5′UTR, the 3′UTR of Fpn plays a role through a recently discovered miRNA-dependent mechanism. In fact, it has been demonstrated that miR-485-3p is induced during iron deficiency and it targets the 3′UTR of Fpn to reduce iron export in several cell lines and primary macrophages (Sangokoya et al. 2013).
The intracellular content of iron thus comes from the balance between import and export rates. On the other hand, the amount of bioactive iron inside a cell is also modulated by the ferritin complex, which sequesters excess iron and neutralizes the ability of the redox metal to trigger and amplify the production of ROS. Ferritins constitute a large superfamily of proteins, with most members consisting of 24 subunits arranged to form an approximately spherical protein shell into which inorganic iron is deposited. A single ferritin molecule of this type can hold up to 4300 iron ions in its central cavity. Despite ferritin has been identified several decades ago, and a huge amount of experimental work has been done on this topic, the mechanism by which ferritins accumulate and release iron has not yet been fully elucidated.
The iron exporter ferroportin
Fpn is the sole iron exporter so far identified in vertebrates. It belongs to one of the largest secondary transporter families, the major facilitator superfamily (MFS). The amino acid sequence of Fpn is well conserved throughout evolutionarily distant organisms, with over 60% identity between distantly related proteins, such as human and zebrafish Fpn, and Fpn-like sequences identified in Arabidopsis thaliana and Caenorhabditis elegans, indicating a wide distribution and suggesting a critical role for Fpn. Further evidence comes from the observation that inactivation of the Fpn gene in mice is embryonically lethal (Donovan et al. 2005).
The human protein is constituted by 571 amino acids, with a number of transmembrane (TM) segments. The topology of Fpn was initially predicted to bear between 9 and 12 TM regions with the N-terminus positioned inside and the C-terminus on the intracellular or extracellular side, depending on the number of TM segments. It is now generally acknowledged that both N- and C-terminal extremities of Fpn are intracellular, indicating that the protein has an even number of TM segments. The exact positioning of some loops connecting the helices has been established through proper epitope tagging and insertion of cysteine residues in internal regions of the protein and analysis of accessibility to anti-tag antibodies or cysteine-specific labelling with cell-impermeant reagents (Liu et al. 2005). The data corroborate a topological model of Fpn with 12 TM helices. Structural features relevant for Fpn function are a large intracellular loop between TM6 and TM7, which contains key residues important for internalization and degradation of Fpn (De Domenico et al. 2007a), and an extracellular loop between TM7 and TM8, where the binding site for the regulatory hormone hepcidin is located (Preza et al. 2011).
Like all polytopic membrane proteins, Fpn is problematic to study from a structural point of view and its exact three-dimensional structure remains unknown. Based on the known structures of other members of the MFS family, it has been proposed that Fpn operates through an alternating-access mechanism, where the two halves of the protein cyclically move after local structural rearrangements. The rotation of the two domains composing the protein would lead to three different conformational states: outward-facing, occluded, and inward-facing (Tortosa et al. 2016). Because of the lack of a crystal structure, information has been gained through homology modeling, and different models of human Fpn have been proposed. The first available Fpn model (Wallace et al. 2010) was indeed constructed without homology, as the transmembrane helices were predicted and built de novo and then manually fitted over the three-dimensional structure of the Escherichia coli glycerol-3-phosphate transporter in the inward-facing conformation. The model displayed an inward- open conformation and clustering of “gain-of-function” variants (see below) in a solvent accessible channel, with “loss-of-function” mutations conversely located at the membrane/cytoplasm interface. Le Gac et al. (2013) then described a novel model based on the MFS transporter EmrD in the occluded conformation, which proved the role of residue Trp42 in both iron transport and hepcidin binding. However, loops outside of the transmembrane helices were not modeled and some critical structural details of this model were later shown to be incorrect. The first structural models of human Fpn in both the inward- and outward-facing conformations were later obtained by some of us (Bonaccorsi di Patti et al. 2014).
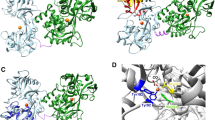
Recently, a bacterial homologue of human Fpn has been identified in the predatory bacterium Bdellovibrio bacteriovorus (Taniguchi et al. 2015, Bonaccorsi di Patti et al. 2015) and the three-dimensional crystallographic structure of this protein has been determined in both the inward- and outward-facing conformational states (Taniguchi et al. 2015). Taking advantage of the availability of these structures, the only ones available for a member of the Fpn family, we then built updated models of human Fpn in the inward- and outward-facing conformations (Tortosa et al. 2016) (Fig. 1a) and carried out a comprehensive structure–function analysis of known Fpn pathogenic mutations (Tortosa et al. 2017). A putative iron binding site, centered around aspartates 39 and 181, was identified, whose relevance was experimentally confirmed through mutational studies and measurement of iron export ability of wild type and mutant protein (Bonaccorsi di Patti et al. 2014). According to our models, the binding site is in place in the inward-open conformation, while in the outward-open state the putative iron ligands move several angstroms away from each other, consistent with release of the metal upon the inward-outward conformational change. A possible iron-transport mechanism has been also presented on the basis of the models in all three mechanistically relevant conformations (Tortosa et al. 2016). In particular, a role for the Asp325/Arg466 couple as an electrostatic switch modulating the conformational change during iron translocation has been envisaged. The proposed translocation mechanism can be summarized as follows. In the inward-open conformation, iron would bind to the Asp39-Asp181 couple and possibly to His32 and Asn185. Iron binding would attract Asp325 toward the metal coordination sphere, driving the transition to the occluded state. At the same time, rupture of the Asp325-Arg466 salt bridge would generate a high-energy state due to the partially desolvated arginine charge. This high-energy state would be relaxed upon transition to the outward-open state, where iron ligands would move apart from each other and the metal would be released in the extracellular space. Residues involved in the proposed mechanism are shown in Fig. 1b for the inward-open conformation.
In humans, mutations in the gene coding for Fpn cause type 4 hemochromatosis (Montosi et al. 2001), an autosomal dominant iron overload condition. Mutations are generally classified into two subclasses: “loss-of-function” and “gain-of-function”. The majority of pathogenic variants are defined as “loss-of-function” as they lead to impaired functionality of the protein and to decreased iron export from cells. This pathological condition is known as type 4A hemochromatosis, or “ferroportin disease” (Pietrangelo 2017). On the other hand, “gain-of-function” mutations, responsible for type 4B hemochromatosis, confer resistance to the action of hepcidin. Since hepcidin binding to Fpn leads to the protein internalization, ubiquitination and degradation (Nemeth et al. 2004), gain-of-function mutations are so because they inhibit this mechanism. Resistance of Fpn mutants to downregulation by hepcidin is therefore an essential component of type 4B hemochromatosis, and results in continued cellular iron export even in the presence of hepcidin concentrations that induce Fpn degradation and iron retention in cells expressing wild type Fpn.
Table 1 shows a list of all human Fpn mutations so far known to cause either loss- or gain-of-function, together with the corresponding biochemical phenotype. The majority of the mutations are located on transmembrane helices and, as can be seen from Fig. 2a, especially in the inward-facing model of human Fpn, most of the loss-of-function mutations are found on the N-terminal half of the protein where three residues essential for iron binding and transport (Asp39, His43 and Asp181) are located (Bonaccorsi di Patti et al. 2014; Tortosa et al. 2017). This region also hosts the residues building up the Motif-A, a structural motif largely conserved in members of the MFS family, that is essential for stabilizing the outward-facing conformation of Fpn and for the release of iron. Indeed, loss-of-function mutations affect residues Gly80, Asp84 and Arg88 belonging to motif-A (Table 1). As far as gain-of-function mutations are concerned, the outward-facing model (Fig. 2b) provides a ready explanation of their effect. In fact, almost all of these mutations are clustered around an area where the hepcidin “docking” site is located. These mutations involve residues Tyr64, Ala69, Ser71, Val72, Asn144, Cys326, Tyr501, Asp504 and His507 (Table 1) which define an almost continuous patch of the cavity of human Fpn in the outward-facing conformation, in line with homology models based on the bacterial Fpn in outward open conformation (Taniguchi et al. 2015; Tortosa et al. 2017). Mutation of each of these residues likely modifies the hepcidin interaction surface on human Fpn resulting in a decreased or impaired binding of this hormone and failure to initiate Fpn internalization and degradation.
Hepcidin can be fully considered the main physiological regulatory ligand of Fpn. It is a peptide of 25 amino acids, structured as a bent β-hairpin stabilized by four disulfide bonds. The peptide is produced by hepatocytes in the liver and inflammatory states and/or increased iron stores trigger the hepatic synthesis. Hepcidin-induced degradation of Fpn is a well-established process, however, the exact molecular mechanism is still matter of debate. The hepcidin binding site has been identified on the extracellular loop of Fpn containing cysteine in position 326 (Fig. 2b). Cells expressing the C326S mutant Fpn export iron normally but do not bind the peptide and can export iron even in the presence of hepcidin. Hepcidin binding to Fpn likely involves a disulfide-thiol interaction between hepcidin and Fpn C326 residue (Fernandes et al. 2009). Modeling of the hepcidin–Fpn interaction suggested that Cys326 is involved in a thiol-dependent interaction with hepcidin, perhaps involving the disulfide framework of hepcidin, while Phe324 and Tyr333 may form crucial contacts with two phenylalanine residues on the hepcidin moiety (Preza et al. 2011).
It has been reported that hepcidin treatment inhibited iron efflux in Caco-2 cells, but failed to decrease Fpn protein levels, suggesting that hepcidin can directly block iron export through Fpn in these cells (Chung et al. 2009). A similar proposal, i.e., the possibility that Fpn is occluded by hepcidin binding without necessarily entering the degradation pathway, has been recently made through analysis of the structure of the prokaryotic homologue from B. bacteriovorus (Taniguchi et al. 2015). Very recently, analysis of a number of natural Fpn mutations leading to gain-of-function in type 4B hemochromatosis has revealed that these mutants display hepcidin resistance because of either impaired hepcidin binding or altered hepcidin-induced conformational change of Fpn with consequently decreased hepcidin-induced ubiquitination and occlusion of the central cavity of the protein (Aschemeyer et al. 2017).
The ferroxidase ceruloplasmin
Over half a century ago it was reported that the blue protein Cp had ferroxidase activity and was required for efficient mobilization of iron (Osaki et al. 1966). Yet, several decades had to pass before the role of Cp in iron homeostasis began to be unveiled at the molecular level. Cp belongs to the family of the multicopper oxidases, enzymes which couple the one-electron oxidation of substrate(s) to full reduction of dioxygen to water by way of three types of copper units with specific spectroscopic and functional properties. Type 1 “blue” copper is the primary electron acceptor from the substrate; type 2 copper and binuclear type 3 copper form a trinuclear cluster where oxygen binds and is reduced (Solomon et al. 1996). The crystallographic structure of Cp has been solved over 20 years ago (Zaitseva et al. 1996) and is reported in Fig. 3. Cp is a large multi-domain protein made up of six plastocyanin-like domains arranged with a ternary pseudosymmetry (Ryden 1982). Domains 1 and 2, 3 and 4, and 5 and 6 interact with each other through extensive, highly packed hydrophobic interfaces, while polar interactions and loosely packed interfaces are observed between domains 2 and 3 and 4 and 5. The interface between domains 6 and 1 hosts the catalytically essential trinuclear copper cluster and domains 2, 4 and 6 each harbour a type 1 copper site. Despite the knowledge of the three-dimensional structure, the biological function of Cp has been under debate for many years and is still in part uncertain. In fact, beside its established role in iron oxidation, several functions have been attributed to Cp, ranging from copper transport to biological amines oxidation, to antioxidant activity exerted through a number of different mechanisms (Plonka et al. 1980; Stoj and Kosman 2003; Kim and Park 1998; Shiva et al. 2006; Floris et al. 2000). A particularly intriguing role as a modulator of the release of nitric oxide, the endothelial-derived relaxing factor, has also been suggested (Cappelli-Bigazzi et al. 1997, Bianchini et al. 1999). However, among various oxidizable substrates, the enzyme definitely displays the highest affinity for ferrous ions and it is now accepted that the main function of Cp is related to its ferroxidase activity. Reduced Fe(II) readily oxidizes, at physiological pH, even in the absence of a protein catalyst. However, spontaneous oxidation of Fe(II) is potentially dangerous as it triggers the formation of ROS via Fenton chemistry. Thus, ferroxidation by Cp prevents iron-induced oxidative stress. The largest amount of Cp is synthesized as a soluble isoform by hepatocytes, where the P-type ATPase ATP7B incorporates copper into apo-Cp during transit through the trans-Golgi network (Lutsenko et al. 2007), and secreted into the plasma where it is found at micromolar concentrations. Cp has been recognized to be an acute phase protein many years ago, and, under specific experimental conditions, can be induced in response to pro-inflammatory stimuli, such as IL-1β (Barber and Cousins 1988; Kuhlow et al. 2003; Bonaccorsi di Patti et al. 2004; Persichini et al. 2010) and INF-γ (Mazumder et al. 1997).
A GPI-anchored form of Cp has been initially identified on the plasma membrane of astrocytes (Patel and David 1997) and then on a number of other cell types, including macrophages (Marques et al. 2012). Synthesis of this isoform is via alternative splicing with the last five aminoacids replaced by 30 alternative residues leading to addition of the GPI anchor (Patel et al. 2000). Most of the cells expressing Cp-GPI are separated from systemic circulation, suggesting a critical function in iron management for the membrane-anchored form of this protein.
Individuals carrying a defective Cp gene suffer of aceruloplasminemia, a rare late-onset autosomal disease characterized by partial or total Cp protein deficiency. They have normal copper homeostasis (thus apparently ruling out a major role of Cp in copper transport) but present a severely impaired iron metabolism (Miyajima et al. 2003; Kono 2013). In particular, homozygotes have iron overload mainly in the brain, but also in liver, pancreas and retina and develop retinal degeneration, diabetes mellitus and neurological symptoms, which include ataxia, involuntary movements and dementia. Beside absent serum Cp ferroxidase activity, the signatures of the disease include low transferrin saturation, high serum ferritin and moderate anaemia; magnetic resonance imaging of the brain shows iron deposits in the basal ganglia, striatum, thalamus and dentate nucleus. These features place aceruloplasminemia in the group of disorders known as NBIA (neurodegeneration with brain iron accumulation), distinct from hereditary hemochromatosis where serum iron is high and the brain is usually not affected, and from disorders of copper metabolism like Menkes and Wilson disease.
The essential role of the ferroxidase activity of Cp in iron release from cells was attributed to facilitation of loading of Fe(III) onto Tf. However, a new regulatory connection between Cp and Fpn was disclosed 10 years ago by the finding that ferroxidase activity is required to stabilize Fpn at the cell surface in rat glioma cells expressing Cp-GPI (De Domenico et al. 2007b). Loss of endogenous Cp-GPI by gene silencing induced the rapid internalization and degradation of Fpn. Any exogenously added ferroxidase activity, including transfection with heterologous Cp or with the yeast ferroxidase Fet3, or even addition of soluble purified Cp, was able to restore Fpn (and iron transport) at the cell surface in cells silenced for endogenous Cp-GPI. We found that the activity of the ferroxidase was essential to lower the concentration of extracellular Fe(II), thus establishing an iron gradient that could promote quick removal of the metal from Fpn. Instead, in the absence of Cp-GPI, iron remained associated with Fpn and under these conditions the protein underwent ubiquitination and was rapidly degraded. The requirement of a ferroxidase to maintain iron transport is specific to cells endogenously expressing Cp-GPI, because transfected Fpn is stable in many cell lines that do not express this isoform of Cp. This regulatory function of Cp is particularly relevant for brain iron metabolism because any factor affecting the ferroxidase activity of Cp-GPI cannot be compensated by circulating plasma Cp, which is unable to cross the blood–brain barrier. Very recent data demonstrate that in neurons the ferroxidase hephaestin is required for efficient iron export by Fpn (Ji et al. 2017), confirming the critical role of ferroxidases for correct iron homeostasis in the brain.
The finding that addition of a ferroxidase activity could rescue Fpn from degradation induced by the lack of an active Cp was exploited to study the functionality of several Cp missense mutants associated with aceruloplasminemia, that could be characterized and classified in different groups according to their ability to stabilize Fpn on the plasma membrane of cells silenced for endogenous Cp-GPI (Bonaccorsi di Patti et al. 2009). Non-functional Cp mutants are inactive (i.e., do not rescue Fpn) due to retention in the endoplasmic reticulum or secretion as apo-Cp lacking copper, while partially or fully functional mutants are enzymatically active and correctly localize to the plasma membrane.
Mutant Cp R701W displayed a surprisingly anomalous behavior. This mutation has been found in a very young heterozygous patient with severe extrapyramidal movement coordination deficit (Kuhn et al. 2005). Both isoforms of Cp R701W (secreted and GPI-anchored) were inactive due to lack of copper (i.e., they were apo-Cp), and dominant over wild type Cp in glioma cells (Bonaccorsi di Patti et al. 2009). In other words, this mutation apparently destroys the Fpn/Cp system even in heterozygosity. Moreover, in cells transfected with Cp R701W the Golgi apparatus appeared dispersed and the copper pump ATP7B, which is needed for proper holo-Cp synthesis, was inactive. Mutation R701W apparently preserved the overall structure of the protein as Cp R701W was found to be intrinsically able to load copper in appropriate conditions, in particular when Ccc2p, the yeast homologue of ATP7B, was co-expressed. As expected, the resulting holo-Cp R701W was fully functional with respect to stabilization of Fpn. Further investigations showed that Cp R701W caused massive production of ROS in the cell and that inhibition of ROS production restored Golgi morphology and rescued Fpn on the cell membrane (Persichini et al. 2012). Residue Arg701 is found in the surface-exposed loop connecting domains 4 and 5 of Cp and it is difficult to understand why replacement with tryptophan should cause such a dramatic phenotype.
The multifunctional iron-binding lactoferrin
As discussed above, mammals have developed sophisticated strategies to chelate iron in a nontoxic form, and lactoferrin (Lf) shows a main role in this respect. Human lactoferrin (hLf), a glycoprotein of 691 amino acids possessing a high similarity to other mammalian Lfs, is constitutively expressed and secreted by glandular epithelial cells and by neutrophils in infection and inflammation sites. The iron binding capacity of Lf ensures that free available iron in the body fluids does not overcome the concentration of 10−18 M, thus hindering iron precipitation as insoluble hydroxides as well as the formation of ROS, responsible for tissue, cell, DNA, protein and lipid damage. Several crystallographic studies have point out that hLf, like all members of its family, is constituted by two homologous lobes (N-lobe residues 1–333 and C-lobe residues 345–691) connected by a 3-turn α-helix peptide (residues 334–344) (Anderson et al. 1987, 1989). Each lobe binds one ferric ion with high affinity (Kd ~ 10−20 M) through highly conserved residues: an aspartic acid, two tyrosines and a histidine. Of note, iron binding is stabilized and made reversible by the presence of a CO32− anion (MacGillivray et al. 1998). Depending on its iron-binding status, Lf can assume an open, metal-free (apo-Lf) conformation or a closed, metal-bound (holo-Lf) one. Holo-Lf is highly stable and more resistant to both thermal denaturation and digestion by proteases compared to the apo form (Baker and Baker 2012).
As expected on the basis of the highly conserved three-dimensional structures among Lfs, human and bovine Lf (bLf) show equal functions, spanning from antibacterial, antifungal, antiviral and antiparasitic to anti-inflammatory and immunomodulatory activities (Puddu et al. 2009, 2011). Therefore, the majority of the in vitro and in vivo studies are carried out using bLf, a substance generally recognized as a safe (GRAS) by the Food and Drug Administration (USA) and commercially available in large quantities.
Besides its antimicrobial activity, described by several studies and reviews (i.e., Valenti and Antonini 2005), bLf has been demonstrated to possess a potent anti-inflammatory activity able to both modulate the inflammatory response in epithelial cells infected by facultative and obligate intracellular bacteria (Valenti et al. 2011; Frioni et al. 2014; Sessa et al. 2017) and revert/attenuate the inflammatory response triggered by Toll-like receptor engagement in antigen-presenting cells (Puddu et al. 2009, 2011). Recently, it has been reported that exogenous bLf can localize in the cell nucleus and proposed that bLf exerts its anti-inflammatory activity through a direct transcriptional regulation (Ashida et al. 2004). In particular, bLf has been shown to be able to reach the nucleus in both intestinal cells (Paesano et al. 2012) and freshly-isolated monocytes (Puddu et al. 2011), suggesting that this molecule may act as a transcriptional modulator of the inflammatory process through the inhibition of pro-inflammatory cytokines (Puddu et al. 2011; Paesano et al. 2012; Kim et al. 2012). Despite these emerging evidences, the molecular mechanisms by which bLf exerts its immunomodulatory activity are still largely unknown.
The regulation of the Fpn/Cp by Lf
There were several good reasons to test whether Lf had an effect on the expression of Cp and Fpn. As already highlighted, several evidences indicate that the bLf modulates inflammation by affecting expression of cytokines, chemokines, and other effector molecules (Rosa et al. 2017). In this respect, 30 days of oral administration of bLf (20–30% iron saturated, 100 mg twice a day) to pregnant women suffering from iron deficiency anaemia (IDA) or anaemia of inflammation (AI) has been demonstrated to downregulate the expression of IL-6 and to rebalance the haematological parameters, thus counteracting the iron and inflammatory homeostasis disorders (Paesano et al. 2009, 2014). Worth of note, despite the quantity of the supplemented iron (about 80 µg/day) was far from that daily required (1–2 mg), a significant increase in the red blood cell number, concentration of haemoglobin, total serum iron, serum ferritin and percentage of hematocrit was observed. Therefore, it was presumable that bLf efficacy in curing IDA and AI was not directly linked to the iron supplementation, but to a more complex mechanism involving this protein in iron homeostasis.
As far as Cp is concerned, interactions of this ferroxidase with other proteins in a number of unrelated processes has been reported, including incorporation of Fe(III) into ferritin after formation of a Cp-ferritin complex (Van Eden and Aust 2000), regulation of clotting through competition with blood coagulation factors FV and FVIII for protein C binding (Walker and Fay 1990), interaction with myeloperoxidase (Sokolov et al. 2007, 2008, 2010) finalized to inhibit its pro-oxidant properties (Segelmark et al. 1997; Sokolov et al. 2015), involvement in neuroregulation processes after interaction with neuropeptide PACAP 38 (Tams et al. 1999). Formation of a complex between Cp-GPI and Fpn in astrocytes has also been reported (Patel et al. 2002), although we were never able to reproduce this very reasonable and somewhat expected finding. What matters here is that a complex of Cp with Lf has been described and characterized (Pulina et al. 2002; Sabatucci et al. 2007; Samygina et al. 2013). The ferroxidase activity of Cp increases in the presence of Lf (Sokolov et al. 2009), so it can be speculated that the complex plays a role in iron metabolism.
As already said, expression of both Cp and Fpn is related to infection and inflammation and Lf has been shown to possess potent antibacterial and anti-inflammatory properties. In fact, it is known that Fpn expression is down-regulated at the transcriptional level by pro-inflammatory cytokines in reticuloendothelial cells, as demonstrated by the finding that treatment with IFN-γ and LPS reduced Fpn mRNA and iron release from monocytes (Ludwiczek et al. 2003; Yang et al. 2002). Fpn mRNA and protein was also found to decrease significantly in astrocytes treated with LPS but not with IL-6 or TNF-α (Urrutia et al. 2013). Our first approach was to choose cellular model relevant for iron supply to circulating plasma, namely the macrophagic THP-1 line and the Caco-2 colonic epithelial cells (Cutone et al. 2014; Frioni et al. 2014). Infection or inflammation was induced with LPS on THP-1, and with IFN-γ and E. coli strain LF82 in Caco-2 cells. In both cases, we found that expression of Fpn was downregulated upon treatment with the proinflammatory agent and that bovine Lf (bLf) significantly counteracted this effect. In THP-1 cells this effect was found to be related to reduction of IL-6 secretion (Cutone et al. 2014). This latter observation is interesting as we had previously found that in rat C6 glioma cells Fpn is up-regulated by IL-1β (Bonaccorsi di Patti et al. 2004; Persichini et al. 2010), suggesting that the response of Fpn to cytokines might be tissue-specific. IL-6 produced upon LPS stimulation leads to enhanced expression of hepcidin mRNA in human monocytes and THP-1 cells and, in turn, to increased hepcidin-dependent degradation of Fpn with consequent intracellular iron retention (Theurl et al. 2008). On this ground, it was assumed that bLf prevents the decrease of Fpn levels in THP-1 cells stimulated with LPS by reducing the LPS- and/or IL- 6-dependent induction of hepcidin.
There was another good reason to investigate the behavior of Fpn and Cp in inflamed macrophages and the possible effects of Lf. These phagocytic cells are in fact crucial components of the innate immune system, which is obviously turned on when pathogens invade the host and attempt to take up iron from it. Moreover, macrophages are a heterogeneous population of immune cells resulting from cytokine stimulation and pathogen or its products sensing, able to polarize into active subpopulations, with a continuum of macrophage subsets ranging from pro-inflammatory M1 to regulatory/anti-inflammatory M2 phenotypes. We have therefore extended our studies, and the effect of bLf on the expression not only of Fpn but also of other pivotal proteins involved in mammalian iron and inflammatory homeostasis (membrane-bound Cp-GPI, cytosolic ferritin, transferrin receptor 1, and various cytokines) was investigated (Cutone et al. 2017). The rationale was that macrophages should be able to adapt their phenotype in response to different environmental stimuli and that the iron system proteins could be differently expressed in specific macrophagic phenotypes. To this end, THP-1 cells were stimulated with either a classical mixture of low concentrations of LPS and IFN-γ, known to induce a pure M1 polarization, or with high concentrations of LPS, known to induce a mixed inflammatory M1/tolerogenic M2 phenotypic population (Genin et al. 2015).
Results of this study are summarized in Figs. 4 and 5. It is evident at a glance that each protein of the iron homeostasis set changes its expression upon treatment in a reasonably independent way on the treatment itself, i.e., both the high dose LPS and the LPS/IFN-γ mix essentially produce the same effects. In particular, Fpn, Cp and TfR1 are invariably downregulated upon the inflammatory stimulus, while the two interleukins and ferritin are upregulated. As a whole, data clearly show that the whole set of proteins involved in iron homeostasis is coordinately regulated upon stimulation of macrophages with inflammatory triggers. These changes are consistent with the production of an intracellular iron overload phenotype. The decrease of the Fpn/Cp couple weakens iron export, and higher ferritin levels are pathognomonic of iron accumulation, a potentially dangerous condition in vivo, prodromic to higher host susceptibility to infections and to the development of anaemias. The data also clearly demonstrate that the anti-inflammatory effect of bovine lactoferrin is exerted on all examined components of the iron homeostasis machinery.
While it has been reported that macrophages can express different “iron-retention” or “iron-release” phenotypes (Recalcati et al. 2010; Corna et al. 2010), we have shown that under conditions where an “iron retention” phenotype is achieved, bLf affects the expression of Fpn, TfR1, Ftn, and Cp-GPI, counteracting the effect of LPS and IFN-γ and restoring Fpn, Cp-GPI, Ftn, and TfR1 levels to those of unstimulated, uninflamed cells. Under our experimental conditions, high doses of LPS seem to induce a strongly inflamed macrophagic phenotype, as evidenced by much higher levels of cytokines IL-6 and IL-1β compared to those measured after low-dose LPS/IFN-γ. On the other hand, the two treatments produced a very different outcome when the anti-inflammatory cytokine IL-10 was assessed (Fig. 5). While IL-10 was downregulated upon LPS/IFN-γ treatment, its levels significantly increased upon stimulation with high doses of LPS, in line with other reports (Genin et al. 2015). In any case, the effects were counteracted by bLf, although only partially in the case of cell challenged with high doses of LPS, suggesting that, at variance with stimulation with the LPS/IFN-γ mix, treatment with LPS alone triggers an endogenous anti-inflammatory response in THP-1 mediated by IL-10 and finalized to counteract the massive increase of IL-6 and IL-1β and possibly of other pro-inflammatory factors. It is interesting to note that bLf exerts its effect both under conditions mimicking the initial stages of infection (i.e., high doses of LPS) and when an inflammatory outcome has been set up (i.e., LPS/IFN-γ mix), suggesting that bLf may work as an anti-inflammatory agent able to both prevent the onset of inflammation and to relieve it once it has been established.
The decrease of Cp-GPI in inflamed macrophages is apparently at odd with Cp being an acute-phase protein. A rationale could be that the soluble and the membrane-bound isoforms of Cp are subject to differential regulation, and this could be related to the multifunctional nature of this protein (see above). In plasma, the soluble isoform would play a major antioxidant role, scavenging ROS produced in inflammation; in specialized cells, such as macrophages, membrane-bound Cp-GPI would predominantly act as a ferroxidase in combination with Fpn. Thus, it would make sense that in this latter case, bLf restores the synthesis of both Cp-GPI and Fpn that is impaired upon inflammation. Overall, the ability of bLf to reduce proinflammatory cytokine production in inflamed macrophages and to counteract the changes of the proteins involved in iron homeostasis, underlines the critical role of this iron-binding protein in the modulation of iron and inflammatory homeostasis.
References
Aisen P (2004) Transferrin receptor 1. Int J Biochem Cell Biol 36:2137–2143
Aisen P, Leibman A, Zweier J (1978) Stoichiometric and site characteristics of the binding of iron to human transferrin. J Biol Chem 253:1930–1937
Anderson BF, Baker HM, Dodson EJ, Norris GE, Rumball SV, Waters JM, Baker EN (1987) Structure of human lactoferrin at 3.2-A resolution. Proc Natl Acad Sci USA 84:1769–1773
Anderson BF, Baker HM, Norris GE, Rice DW, Baker EN (1989) Structure of human lactoferrin: crystallographic structure analysis and refinement at 2.8 A resolution. J Mol Biol 209:711–734
Aschemeyer S, Qiao B, Stefanova D, Valore EV, Sek AC, Ruwe TA, Vieth KR, Jung G, Casu C, Rivella S, Jormakka M, Mackenzie B, Ganz T, Nemeth E (2017) Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood. https://doi.org/10.1182/blood-2017-05-786590
Ashida K, Sasaki H, Suzuki YA, Lönnerdal B (2004) Cellular internalization of lactoferrin in intestinal epithelial cells. Biometals 17:311–315
Baker HM, Baker EN (2012) A structural perspective on lactoferrin function. Biochem Cell Biol 90:320–328
Barber EF, Cousins RJ (1988) Interleukin-1–stimulated induction of ceruloplasmin synthesis in normal and copper-deficient rats. J Nutr 118:375–381
Bianchini A, Musci G, Calabrese L (1999) Inhibition of endothelial nitric-oxide synthase by ceruloplasmin. J Biol Chem 274:20265–20270
Bonaccorsi di Patti MC, Persichini T, Mazzone V, Polticelli F, Colasanti M, Musci M (2004) Interleukin-1beta up-regulates iron efflux in rat C6 glioma cells through modulation of ceruloplasmin and ferroportin-1 synthesis. Neurosci Lett 363:182–186
Bonaccorsi di Patti MC, Maio N, Rizzo G, De Francesco G, Persichini T, Colasanti M, Polticelli F, Musci G (2009) Dominant mutants of ceruloplasmin impair the copper loading machinery in aceruloplasminemia. J Biol Chem 284:4545–4554
Bonaccorsi di Patti MC, Polticelli F, Cece G, Cutone A, Felici F, Persichini T, Musci G (2014) A structural model of human ferroportin and of its iron binding site. FEBS J 281:2851–2860
Bonaccorsi di Patti MC, Polticelli F, Tortosa V, Furbetta PA, Musci G (2015) A bacterial homologue of the human iron exporter ferroportin. FEBS Lett 21:3829–3835
Cappelli-Bigazzi M, Ambrosio G, Musci G, Battaglia C, Bonaccorsi di Patti MC, Golino P, Ragni M, Chiariello M, Calabrese L (1997) Ceruloplasmin impairs endothelium-dependent relaxation of rabbit aorta. Am J Physiol 273:H2843–H2849
Chung B, Chaston T, Marks J, Srai SK, Sharp PA (2009) Hepcidin decreases iron transporter expression in vivo in mouse duodenum and spleen and in vitro in THP-1 macrophages and intestinal Caco-2 cells. J Nutr 139:1457–1462
Collins JF, Anderson GI (2012) Intestinal iron absorption. In: Johnson LR, Ghishan FK, Kaunitz J, Merchant JL, Said HM, Wood JD (eds) Physiology of the gastrointestinal tract, 5th edn. Elsevier, New York, pp 1921–1947
Corna G, Campana L, Pignatti E, Castiglioni A, Tagliafico E, Bosurgi L, Campanella A, Brunelli S, Manfredi AA, Apostoli P, Silvestri L, Camaschella C, Rovere-Querini P (2010) Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica 95:1814–1822
Cutone A, Frioni A, Berlutti F, Valenti P, Musci G, Bonaccorsi di Patti MC (2014) Lactoferrin prevents LPS-induced decrease of the iron exporter ferroportin in human monocytes/macrophages. Biometals 27:807–813
Cutone A, Rosa L, Lepanto MS, Jinnett Scotti M, Berlutti F, Bonaccorsi di Patti MC, Musci G, Valenti P (2017) Lactoferrin efficiently counteracts the inflammation-induced changes of the iron homeostasis system in macrophages. Front Immunol 8:705
De Domenico I, McVey Ward D, Langelier C, Vaughn MB, Nemeth E, Sundquist WI, Ganz T, Musci G, Kaplan J (2007a) The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell 18:2569–2578
De Domenico I, Ward DM, Bonaccorsi di Patti MC, Jeong SY, David S, Musci G, Kaplan J (2007b) Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI ceruloplasmin. EMBO J 26:2823–2831
Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC (2005) The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 1:191–200
Fernandes A, Preza GC, Phung Y, De Domenico I, Kaplan J, Ganz T, Nemeth E (2009) The molecular basis of hepcidin-resistant hereditary hemochromatosis. Blood 114:437–443
Floris G, Medda R, Padiglia A, Musci G (2000) The physiopathological significance of ceruloplasmin. A possible therapeutic approach. Biochem Pharmacol 60:1735–1741
Frioni A, Conte MP, Cutone A, Longhi C, Musci G, Bonaccorsi di Patti MC, Natalizi T, Marazzato M, Lepanto MS, Puddu P, Paesano R, Valenti P, Berlutti F (2014) Lactoferrin differently modulates the inflammatory response in epithelial models mimicking human inflammatory and infectious diseases. Biometals 27:843–856
Genin M, Clement F, Fattaccioli A, Raes M, Michiels C (2015) M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 15:577
Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388:482–488
Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC (2005) Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest 115:1258–1266
Ji C, Steimle BL, Bailey DK, Kosman DJ (2017) The ferroxidase hephaestin but not amyloid precursor protein is required for ferroportin-supported iron efflux from primary hippocampal neurons. Cell Mol Neurobiol. https://doi.org/10.1007/s10571-017-0568-z
Kim IG, Park SY (1998) Requirement of intact human ceruloplasmin for the glutathione-linked peroxidase activity. FEBS Lett 437:293–296
Kim CW, Lee TH, Park KH, Choi SY, Kim J (2012) Human lactoferrin suppresses TNF-α -induced intercellular adhesion molecule-1 expression via competition with NF-kB in endothelial cells. FEBS Lett 586:229–234
Klausner RD, Ashwell G, van Renswoude J, Harford JB, Bridges KR (1983) Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci USA 80:2263–2266
Kono S (2013) Aceruloplasminemia: an update. Int Rev Neurobiol 110:125–151
Kuhlow CJ, Krady JK, Basu A, Levison SW (2003) Astrocytic ceruloplasmin expression, which is induced by IL-1beta and by traumatic brain injury, increases in the absence of the IL-1 type 1 receptor. Glia 44:76–84
Kuhn J, Miyajima H, Takahashi Y, Kunath B, Hartmann-Klosterkoetter U, Cooper-Mahkorn D, Schaefer M, Bewermeyer H (2005) Extrapyramidal and cerebellar movement disorder in association with heterozygous ceruloplasmin gene mutation. J Neurol 252:111–113
Lambert LA (2012) Molecular evolution of the transferrin family and associated receptors. Biochim Biophys Acta 1820:242–253
Latunde-Dada GO, Takeuchi K, Simpson RJ, McKie AT (2006) Haem carrier protein 1 (HCP1): expression and functional studies in cultured cells. FEBS Lett 580:6865–6870
Le Gac G, Ka C, Joubrel R, Gourlaouen I, Lehn P, Mornon JP, Férec C, Callebaut I (2013) Structure-function analysis of the human ferroportin iron exporter (SLC40A1): effect of hemochromatosis type 4 disease mutations and identification of critical residues. Hum Mutat 34:1371–1380
Liu XB, Yang F, Haile DJ (2005) Functional consequences of ferroportin 1 mutations. Blood Cells Mol Dis 35:33–46
Ludwiczek SL, Aigner E, Theurl I, Weiss G (2003) Cytokine mediated regulation of iron transport in human monocytic cells. Blood 101:4148–4154
Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY (2007) Function and regulation of human copper-transporting ATPases. Physiol Rev 87:1011–1046
MacGillivray RTA, Moore SA, Chen J, Anderson BF, Baker H, Luo Y, Bewley M, Smith CA, Murphy MEP, Wang Y, Mason AB, Woodworth RC, Brayer GD, Baker EN (1998) Two high-resolution crystal structures of the recombinant N-lobe of human transferrin reveal a structural change implicated in iron release. Biochemistry 37:7919–7928
Marques L, Auriac A, Willemetz A, Banha J, Silva B, Canonne-Hergaux F, Costa L (2012) Immune cells and hepatocytes express glycosylphosphatidylinositol-anchored ceruloplasmin at their cell surface. Blood Cells Mol Dis 48:110–120
Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C (2009) HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest 119:1159–1166
Mazumder B, Mukhopadhyay CK, Prok A, Cathcart MK, Fox PL (1997) Induction of ceruloplasmin synthesis by IFN-gamma in human monocytic cells. J Immunol 159:1938–1944
Miyajima H, Takahashi Y, Kono S (2003) Aceruloplasminemia, an inherited disorder of iron metabolism. Biometals 16:205–213
Montosi G, Donovan A, Totaro A, Garuti C, Pignatti E, Cassanelli S, Trenor CC, Gasparini P, Andrews NC, Pietrangelo A (2001) Autosomal-dominant hemochromatosis is associated with a mutation in the ferroportin (SLC11A3) gene. J Clin Invest 108:619–623
Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093
Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J, Sharp JJ, Fujiwara Y, Barker JE, Fleming MD (2005) Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet 37:1264–1269
Osaki S, Johnson DA, Frieden E (1966) The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem 241:2746–2751
Paesano R, Pietropaoli M, Gessani S, Valenti P (2009) The influence of lactoferrin, orally administered, on systemic iron homeostasis in pregnant women suffering of iron deficiency and iron deficiency anaemia. Biochimie 91:44–51
Paesano R, Natalizi T, Berlutti F, Valenti P (2012) Body iron delocalization: the serious drawback in iron disorders in both developing and developed countries. Pathog Glob Health 106:200–216
Paesano R, Pacifici E, Benedetti S, Berlutti F, Frioni A, Polimeni A, Valenti P (2014) Safety and efficacy of lactoferrin versus ferrous sulphate in curing iron deficiency and iron deficiency anaemia in hereditary thrombophilia pregnant women: an interventional study. Biometals 27:999–1006
Patel BN, David S (1997) A novel glycosylphosphatidylinositolanchored form of ceruloplasmin is expressed by mammalian astrocytes. J Biol Chem 272:20185–22019
Patel BN, Dunn RJ, David S (2000) Alternative RNA splicing generates a glycosylphosphatidylinositol-anchored form of ceruloplasmin in mammalian brain. J Biol Chem 275:4305–4310
Patel BN, Dunn RJ, Jeong SY, Zhu Q, Julien JP, David S (2002) Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J Neurosci 22:6578–6586
Persichini T, Maio N, Bonaccorsi di Patti MC, Rizzo G, Toscano S, Colasanti M, Musci G (2010) Interleukin-1β induces ceruloplasmin and ferroportin-1 gene expression via MAP kinases and C/EBPβ, AP-1, and NF-κB activation. Neurosci Lett 484:133–138
Persichini T, De Francesco G, Capone C, Cutone A, Bonaccorsi di Patti MC, Colasanti M, Musci G (2012) Reactive oxygen species are involved in ferroportin degradation induced by ceruloplasmin mutant Arg701Trp. Neurochem Int 60:360–364
Pietrangelo A (2017) The ferroportin disease: pathogenesis, diagnosis and treatment. Haematologica 102:1972–1984
Plonka A, Metodiewa D, Zgirski A, Hilewicz M, Leyko W (1980) ESR evidence of superoxide radical dismutation by human ceruloplasmin. Biochem Biophys Res Commun 95:978–984
Preza GC, Ruchala P, Pinon R, Ramos E, Qiao B, Peralta MA, Sharma S, Waring A, Ganz T, Nemeth E (2011) Minihepcidins are rationally designed small peptides that mimic hepcidin activity in mice and may be useful for the treatment of iron overload. J Clin Invest 121:4880–4888
Puddu P, Valenti P, Gessani S (2009) Immunomodulatory effects of lactoferrin on antigen presenting cells. Biochimie 91:11–18
Puddu P, Latorre D, Carollo M, Catizone A, Ricci G, Valenti P, Gessani S (2011) Bovine lactoferrin counteracts Toll-like receptor mediated activation signals in antigen presenting cells. PLoS ONE 6:e22504
Pulina MO, Zakharova ET, Sokolov AV, Shavlovski MM, Bass MG, Solovyov KV, Kokryakov VN, Vasilyev VB (2002) Studies of the ceruloplasmin-lactoferrin complex. Biochem Cell Biol 80:35–39
Rajagopal A, Rao AU, Amigo J, Tian M, Upadhyay SK, Hall C, Uhm S, Mathew MK, Fleming MD, Paw BH, Jrauze M, Hamza I (2008) Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature 453:1127–1131
Recalcati S, Locati M, Marini A, Santambrogio P, Zaninotto F, De Pizzol M, Zammataro L, Girelli D, Cairo G (2010) Differential regulation of iron homeostasis during human macro-phage polarized activation. Eur J Immunol 40:824–835
Rosa L, Cutone A, Lepanto MS, Paesano R, Valenti P (2017) Lactoferrin: a natural glycoprotein involved in iron and inflammatory homeostasis. Int J Mol Sci. https://doi.org/10.3390/ijms18091985
Ryden L (1982) Model of the active site in the blue oxidases based on the ceruloplasmin-plastocyanin homology. Proc Natl Acad Sci USA 79:6767–6771
Sabatucci A, Vachette P, Vasilyev VB, Beltramini M, Sokolov A, Pulina M, Salvato B, Angelucci CB, Maccarrone M, Cozzani I, Dainese E (2007) Structural characterization of the ceruloplasmin:lactoferrin complex in solution. J Mol Biol 371:1038–1046
Samygina VR, Sokolov AV, Bourenkov G, Petoukhov MV, Pulina MO, Zakharova ET, Vasilyev VB, Bartunik H, Svergun DI (2013) Ceruloplasmin: macromolecular assemblies with iron-containing acute phase proteins. PLoS ONE 8:e67145
Sangokoya C, Doss JF, Chi JT (2013) Iron-responsive miR-485-3p regulates cellular iron homeostasis by targeting ferroportin. PLoS Genet 9:e1003408
Segelmark M, Persson B, Hellmark T, Wieslander J (1997) Binding and inhibition of myeloperoxidase (MPO): a major function of ceruloplasmin? Clin Exp Immunol 108:167–174
Sessa R, Di Pietro M, Filardo S, Bressan A, Rosa L, Cutone A, Frioni A, Berlutti F, Paesano R, Valenti P (2017) Effect of bovine lactoferrin on Chlamydia trachomatis infection and inflammation. Biochem Cell Biol 95:34–40
Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, Frazer DM, Anderson GJ, Vulpe CD, Simpson RJ, McKie AT (2005) Identification of an intestinal haem transporter. Cell 122:789–801
Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT (2006) Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol 2:486–493
Sokolov AV, Pulina MO, Ageeva KV, Ayrapetov MI, Berlov MN, Volgin GN, Markov AG, Yablonsky PK, Kolodkin NI, Zakharova ET, Vasilyev VB (2007) Interaction of ceruloplasmin, lactoferrin, and myeloperoxidase. Biochemistry (Moscow) 72(409):415
Sokolov AV, Ageeva KV, Pulina MO, Cherkalina OS, Samygina VR, Vlasova II, Panasenko OM, Zakharova ET, Vasilyev VB (2008) Ceruloplasmin and myeloperoxidase in complex affect the enzymatic properties of each other. Free Rad Res 42:989–998
Sokolov AV, Ageeva KV, Pulina MO, Zakharova ET, Vasilyev VB (2009) Effect of lactoferrin on oxidative features of ceruloplasmin. Biometals 22:521–529
Sokolov AV, Ageeva KV, Cherkalina OS, Pulina MO, Zakharova ET, Prozorovskii VN, Aksenov DV, Vasilyev VB, Panasenko OM (2010) Identification and properties of complexes formed by myeloperoxidase with lipoproteins and ceruloplasmin. Chem Phys Lipids 163:347–355
Sokolov AV, Acquasaliente L, Kostevich VA, Frasson R, Zakharova ET, Pontarollo G, Vasilyev VB, De Filippis V (2015) Thrombin inhibits the anti-myeloperoxidase and ferroxidase functions of ceruloplasmin: relevance in rheumatoid arthritis. Free Rad Biol Med 86:279–294
Solomon EI, Sundaram UM, Machonkin TE (1996) Multicopper oxidases and oxygenases. Chem Rev 96:2563–2606
Stoj C, Kosman DJ (2003) Cuprous oxidase activity of yeast Fet3p and human ceruloplasmin: implication for function. FEBS Lett 554:422–426
Tams JW, Johnsen AH, Fahrenkrug J (1999) Identification of pituitary adenylate cyclase-activating polypeptide 1–38-binding factor in human plasma, as ceruloplasmin. Biochem J 341:271–276
Taniguchi R, Kato HE, Deshpande CN, Wada M, Ito K, Ishitani R, Jormakka M, Nureki O (2015) Outward- and inward-facing structures of a putative bacterial transition-metal transporter with homology to ferroportin. Nat Commun 6:8545
Theurl I, Theurl M, Seifert M, Mair S, Nairz M, Rumpold H, Zoller H, Bellmann-Weiler R, Niederegger H, Talasz H, Weiss G (2008) Autocrine formation of hepcidin induces iron retention in human monocytes. Blood 111:2392–2399
Torti FM, Torti SV (2002) Regulation of ferritin genes and protein. Blood 99:3505–3516
Tortosa V, Bonaccorsi di Patti MC, Musci G, Polticelli F (2016) The human iron exporter ferroportin Insight into the transport mechanism by molecular modeling. Bio-Algorithms Med Syst 12:1–7
Tortosa V, Bonaccorsi di Patti MC, Brandi V, Musci G, Polticelli F (2017) An improved structural model of the human iron exporter ferroportin Insight into the role of pathogenic mutations in hereditary hemochromatosis type 4. Bio-Algorithms Med Syst 13(4):215–222
Urrutia P, Aguirre P, Esparza A, Tapia V, Mena N, Arredondo M, Gonzalez-Billault C, Nunez MT (2013) Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J Neurochem 126:541–549
Valenti P, Antonini G (2005) Lactoferrin: an important host defence against microbial and viral attack. Cell Mol Life Sci 62:2576–2587
Valenti P, Catizone A, Pantanella F, Frioni A, Natalizi T, Tendini M, Berlutti F (2011) Lactoferrin decreases inflammatory response by cystic fibrosis bronchial cells invaded with Burkholderia cenocepacia iron-modulated biofilm. Int J Immunopathol Pharmacol 24:1057–1068
Van Eden ME, Aust SD (2000) Intact human ceruloplasmin is required for the incorporation of iron into human ferritin. Arch Biochem Biophys 381:119–126
Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ (1999) Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet 21:195–199
Walker FJ, Fay PJ (1990) Characterization of an interaction between protein C and ceruloplasmin. J Biol Chem 265:1834–1836
Wallace DF, Harris JM, Subramaniam VN (2010) Functional analysis and theoretical modeling of ferroportin reveals clustering of mutations according to phenotype. Am J Physiol Cell Physiol 298:C75–C84
Yang F, Liu XB, Quinones M, Melby PC, Ghio A, Haile DJ (2002) Regulation of reticuloendothelial iron transporter MTP1 (Slc11a3) by inflammation. J Biol Chem 277:39786–39791
Young SP, Bomford A, Williams R (1984) The effect of the iron saturation of transferrin on its binding and uptake by rabbit reticulocytes. Biochem J 219:505–510
Zaitseva I, Zaitsev V, Card G, Moshkov K, Bax B, Ralph A, Lindley P (1996) The nature of the copper centres in human ceruloplasmin. J Biol Inorg Chem 1:49–63
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonaccorsi di Patti, M.C., Cutone, A., Polticelli, F. et al. The ferroportin-ceruloplasmin system and the mammalian iron homeostasis machine: regulatory pathways and the role of lactoferrin. Biometals 31, 399–414 (2018). https://doi.org/10.1007/s10534-018-0087-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-018-0087-5