Abstract
Objective Evaluate the safety and efficacy of bovine lactoferrin (bLf) versus the ferrous sulphate standard intervention in curing iron deficiency (ID) and ID anaemia (IDA) in pregnant women affected by hereditary thrombophilia (HT). Design Interventional study. Setting Secondary-level hospital for complicated pregnancies in Rome, Italy. Population 295 HT pregnant women (≥18 years) suffering from ID/IDA. Methods Women were enrolled in Arm A or B in accordance with their personal choice. In Arm A, 156 women received oral administration of 100 mg of bLf twice a day; in Arm B, 139 women received 520 mg of ferrous sulphate once a day. Therapies lasted until delivery. Main outcome measures Red blood cells, haemoglobin, total serum iron, serum ferritin (haematological parameters) were assayed before and every 30 days during therapy until delivery. Serum IL-6, key factor in inflammatory and iron homeostasis disorders, was detected at enrolment and after therapy at delivery. Possible maternal, foetal, and neonatal adverse effects were assessed. Results Haematological parameters were significantly higher in Arm A than in Arm B pregnant women (P ≤ 0.0001). Serum IL-6 significantly decreased in bLf-treated women and increased in ferrous sulphate-treated women. BLf did not exert any adverse effect. Adverse effects in 16.5 % of ferrous sulphate-treated women were recorded. Arm A women experienced no miscarriage compared to five miscarriages in Arm B women. Conclusions Differently from ferrous sulphate, bLf is safe and effective in curing ID/IDA associated with a consistent decrease of serum IL-6. The absence of miscarriage among bLf-treated women provided an unexpected benefit. Trial registration: ClinicalTrials.gov Identifier NCT01221844.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
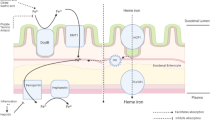
The iron homeostasis disorders like iron deficiency (ID) and ID anaemia (IDA) affect pregnant women due to the significant increase of iron requirement during the second and third trimester of gestation (from 1–2 to 4 and 8 mg/day, respectively) (Bothwell 2000) as well as for the enhanced blood volume and development of the foetal-placental unit (Umbreit 2005; Scholl 2005). Pregnancy-associated ID/IDA increase health maternal risks, preterm delivery, retardation of foetal growth, low birth weight, and inferior neonatal health. Fortunately, the degree of foetal ID is not always as severe as that in the mother since iron transfer from mother to foetus is regulated through transferrin receptors, located in the apical side of the placental syncytiotrophoblasts, which increase in iron deficiency (Bradley et al. 2004; Bastin et al. 2006).
Maternal iron absorption occurs in the proximal duodenum, where it is sequestered into the enterocyte’s cytoplasm by ferritin and is exported by ferroportin (Fpn), the only known iron exporter from cells into blood (Abboud and Haile 2000). Fpn has been found also in hepatocytes (Ganz 2005), erythroblasts (Zhang et al. 2011), placental cells (Bastin et al. 2006) and macrophages which daily recycle 20 mg of iron from lysed erythrocytes (Donovan et al. 2005).
Another component of iron homeostasis is hepcidin, a peptide synthesized by hepatocytes, excreted in urine and blood which regulates the entry of iron into plasma (Park et al. 2001; Nicolas et al. 2001, 2002). Hepcidin binding to Fpn induces Fpn internalization and degradation, thus blocking iron efflux from cells into blood (Nemeth et al. 2004; Qiao et al. 2012).
Maternal hepcidin and Fpn synthesis is regulated by changes in iron status and iron requirements, and by inflammatory cytokines which also modulate foetal Fpn synthesis (Collard 2009). In particular, Interleukin-6 (IL-6) up-regulates the transcription of the hepcidin gene in hepatocytes and down-regulates the Fpn gene in enterocytes and macrophages (Nemeth and Ganz 2006; Ganz 2013), as well as in placental syncytiotrofoblasts (Collard 2009) and in erythroblasts (Zhang et al. 2011). When iron flow into plasma is hindered due to hepcidin and/or Fpn dysregulation, both iron saturation of serum transferrin and erythropoiesis decrease concomitantly with an increase of intracellular iron stores and a decrease of iron in blood. Therefore, anaemia of inflammation is characterized by ID despite adequate iron stores (Nemeth and Ganz 2006).
Even though the Fpn-hepcidin role in iron homeostasis is known, maternal ID/IDA is still treated with administration of inorganic iron as ferrous sulphate. Oral ferrous sulphate, often ineffective in curing ID/IDA, induces several adverse effects and increases serum IL-6 levels resulting in a persistence of iron and inflammatory homeostasis disorders (Kadiiska et al. 1995; Oldenburg et al. 2000; Reifen et al. 2000; Paesano et al. 2009, 2010a, b, 2012a). Similarly, neonatal ID is frequently not reversed by iron supplementation (Shafir et al. 2008).
Any iron therapy unable to regulate hepcidin and Fpn, the main proteins of iron homeostasis, must be revised extensively in order to take into account the dangerous iron overload inside cells in ID/IDA worsened by the iron supplementation therapy (Paesano et al. 2012b).
A promising approach, alternative to iron supplementation, is the oral administration of bovine milk derivative lactoferrin (bLf), a cationic iron-binding glycoprotein showing high similarity with human Lf in structure (Baker and Baker 2005) and functions (Valenti and Antonini 2005; Baker and Baker 2012). BLf is an important regulator of iron and inflammatory homeostasis useful in treating pregnancy-associated ID/IDA since it restores the physiological export of iron from cells to circulation (Paesano et al. 2009, 2010a, b) and prevents preterm delivery (Paesano et al. 2012a). In bLf-treated uncomplicated pregnancies, a significant improvement of the haematological parameters was observed, that was associated with a consistent decrease of serum IL-6 levels in contrast with the outcome of the oral ferrous sulphate therapy (Paesano et al. 2009, 2010b).
On the basis of these results, we hypothesized that bLf could be likewise useful in treating ID/IDA in complicated pregnancies associated to hereditary thrombophilia (HT).
HT, a genetic predisposition to the formation of venous thrombus due to coagulation abnormalities (Rosendaal 1999; Khan and Dickerman 2006), represents an additional significant risk for both maternal and infant health as it causes recurrent miscarriages, intrauterine foetal death, growth retardation, preeclampsia and placental abruption (Stella and Sibai 2006; Patnaik et al. 2007). In view of the well known correlation between adverse pregnancy outcomes and pro-inflammatory cytokines in HT pregnant women (Fox and Kahn 2005; Zhao et al. 2008 ), ferrous sulphate oral administration could enhance inflammation, thus representing an additional maternal and infant risk during gestation.
Here, the safety and efficacy of innovative bLf intervention versus the Italian standard ferrous sulphate therapy is evaluated in pregnant women affected by HT to verify whether bLf could provide a promising approach to restore iron and inflammatory homeostasis in these complicated pregnancies.
Materials and methods
Study design
To compare the safety and the efficacy of the innovative bLf therapy versus the standard ferrous sulphate therapy in curing ID and IDA in HT pregnant women, a monocentric interventional clinical study was designed. This study has been conducted at Clinica Fabia Mater, Via Olevano Romano, 25 Rome, Italy, a secondary-level hospital for complicated pregnancies, in accordance with the ethical principles of the Declaration of Helsinki and the Good Clinical Practice. Approval was granted by the Ethics Committee of Clinica Fabia Mater, Via Olevano Romano, 25 Rome, Italy (FM MOD 26/02/2010). All pregnant women gave written informed consent before undergoing any study procedure.
Patients
Pregnant women from 18 to 40 years, between the 6th and 8th week of gestation, with a history of adverse outcomes including previous recurrent miscarriages, preterm birth, intrauterine growth restriction, were screened for the following HT markers before the enrolment: protein C, protein S, activated protein C resistance, antithrombin deficiencies, elevated coagulation factors, hyperhomocysteinemia, F5 R506Q (factor V Leiden) and F2 G20210A (prothrombin G20210A) mutations. When at least one of these HT markers was found, pregnant women were considered affected by HT and further screened for haematological parameters before starting the therapy against ID and IDA. The HT pregnant women were enrolled when one of the haematological parameters corresponded to the following values: red blood cells ≤4.000.000/ml, haemoglobin ≤11 g/dl, total serum iron ≤30 mg/dl, and serum ferritin ≤12 ng/ml. Pregnant women were excluded if they had uncomplicated pregnancy, anti-phospholipid syndrome, other concomitant diseases, previous iron supplementation therapy, recent blood transfusion(s), and allergy to milk proteins.
Laboratory analyses
HT markers were detected at Institute Regina Elena, Department of Medical Pathology, Rome, Italy according to Jackson et al. (2008).
The haematological parameters, i.e.: number of red blood cells and haemoglobin, total serum iron, serum ferritin concentrations from venous blood samples, were assessed as previously described (Meier et al. 2003). The haematological analyses were performed in clinical laboratories freely chosen by the pregnant women as well as other chemical analysis routinely requested during gestation.
Serum IL-6 levels were determined by standard ELISA Quantitative kits (R&D Systems, Wiesbaden, Germany) at Department of Public Health and Infectious Diseases, Sapienza University of Rome, Italy, on the samples collected before the therapy and after therapy at delivery.
Interventions
HT pregnant women affected by ID and IDA were assigned to Arm A or Arm B on the basis of a personal choice. The random allocation in the Arms as well as the administration of placebo was considered unethical. Women included in Arm A received the innovative treatment based on oral administration of one capsule containing 100 mg of bLf plus excipients (Lattoglobina® or Isoferine®) two times a day before meals. BLf iron saturation in both Lattoglobina® and Isoferine® capsules ranged from 20 to 30 %, as determined by optical spectroscopy. The total amount of iron supplied by two Lattoglobina® or Isoferine® capsules corresponded to about 70–84 μg/day. Women included in Arm B received the standard therapy based on the oral administration of a tablet containing 520 mg of ferrous sulphate (Ferro-Grad, Abbot Laboratories, USA) once a day during meals, according to the Italian Standard of Care. Iron supplied by one ferrous sulphate tablet was 156 mg/day.
All enrolled HT pregnant women received low-molecular-weight heparin (0.3 U/day of Seleparina, Italfarmaco SpA, Milano-Italy) and low dose aspirin (100 mg every two days of Cardioaspirin®100, Bayer SpA, Milano-Italy) to prevent and reduce the risk of venous thromboembolism and miscarriage associated to hypercoagulability (Kaandorp et al. 2009).
Outcomes
Enrolled HT pregnant women were examined at the time of enrolment and every 30 days at the scheduled visits until delivery.
The main outcomes for the evaluation of the efficacy of bLf versus the ferrous sulphate oral administration were based on the assessment of the haematological parameters and of serum IL-6 levels. In addition, the values of haematocrit, glycaemia, uricaemia, bilirubin, glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, cholesterol, triglyceride acid, and electrolytes were evaluated at each visit.
The main outcomes for the safety evaluation of bLf versus the ferrous sulphate oral administration were based on the estimation of maternal compliance or adverse effects, foetal vital signs, new-born weight and APGAR score (Apgar 1953).
The maternal adverse effects were detected by evaluating gastrointestinal discomfort, nausea, vomiting, diarrhoea, and constipation. Foetal vital sign assessments were monitored by ultrasonographic measurements of intrauterine growth and through the amount of amniotic fluid, expressed as amniotic fluid index (AFI), an index for the foetal well-being (Petrozella et al. 2011). New-born weight and the APGAR score were also registered. The APGAR score is a practical method of evaluating the physical condition of a new-born shortly after delivery. An APGAR score of 0–3 at 5–10 min of age is predictive of high morbidity and mortality, while a value of 9–10 indicates that the infant is in the best possible condition.
Statistical analysis
Statistical analysis was carried out using the ANOVA test. P values ≤0.0001 were considered significant.
Results
Demographics
A total of 295 HT pregnant women suffering from ID and IDA was enrolled in the interventional clinical trial within the first trimester of pregnancy. On the basis of their personal choice, 156 HT pregnant women chose to be included in Arm A (bLf innovative intervention) and 139 in Arm B (ferrous sulphate standard intervention). Demographics and haematological parameters of enrolled HT pregnant women are reported in Table 1.
Safety and efficacy of ID and IDA therapies
Among the 295 enrolled women, a total of 253 completed the study. Among the 156 pregnant women included in Arm A, 153 completed the study (98.0 %), while three were lost for incomplete analysis. No adverse effect was registered. Among the 139 pregnant women enrolled in Arm B, 100 completed the study (71.9 %) while 39 were lost: 23 for adverse effects, 8 for protocol violation, 5 for miscarriage within the 12th and 13th week of gestation, and 3 for incomplete analysis (Fig. 1).
Even though the haematological parameters were assessed before and every 30 days during therapy until delivery, in Fig. 2 only the haematological values before therapy and at delivery after therapy are reported. At delivery, the bLf treated-HT women showed significantly higher haematological values than those observed in ferrous sulphate-treated HT women.
Haematological values before and after therapy at delivery in HT pregnant women suffering from ID and IDA. Mean values±standard deviation of haematological parameters before and after therapy at delivery of HT pregnant women receiving bLf oral administration (Arm A, 153 women) or the ferrous sulphate oral administration (Arm B, 100 women). Statistical analysis was performed by ANOVA. *Significant differences (P ≤ 0.0001)
In a previous study on uncomplicated pregnancies, a significant improvement of haematological parameters associated with a consistent decrease of serum IL-6 levels was observed in bLf-treated women (Paesano et al. 2009, 2010b).
Here, we evaluated the serum IL-6 concentrations at the moment of enrolment and after therapy at delivery in randomly selected 20 HT pregnant women of each Arm (Fig. 3). Serum IL-6 levels significantly decreased from 94 ± 10 to 69.5 ± 6.0 in women included in Arm A, but increased from 93 ± 13 to 112.6 ± 6.7 in women included in Arm B even if not significantly.
Serum IL-6 levels before therapy and after therapy at delivery in HT pregnant women suffering from ID and IDA. Mean values±standard deviation of serum IL-6 levels before therapy and after therapy at delivery of HT pregnant women receiving bLf oral administration (Arm A, 20 women) (triangle) or the ferrous sulphate oral administration (Arm B, 20 women) (circle). Statistical analysis was performed by ANOVA. *Significant differences (P ≤ 0.0001) respect the values recorded before the therapy
Foetus and new-born outcomes
The intrauterine growth, AFI, new-born weight and APGAR score values showed the foetus and new-born well-being, irrespective of the iron sulphate or bLf therapy.
Discussion
Women affected from HT are subjected to significant risk factors in pregnancy since HT predisposes to hypertension, preeclampsia and placental intrauterine growth retardation. In these high risk pregnancies recurrent miscarriages are ninefold more frequent than in uncomplicated pregnancies (Patnaik et al. 2007). The iron disorders as ID and IDA can further complicate HT pregnancy management. These complications are not fully appreciated since the impact of ID and IDA in this population has not been thoroughly studied. Conversely, a high degree of correlation between adverse pregnancy outcomes and pro-inflammatory cytokines, including IL-6 in HT pregnant women has been observed (Fox and Kahn 2005; Hagberg et al. 2005; Zhao et al. 2008). Interestingly, IL-6 is a key molecule in the interplay between inflammatory and iron homeostasis by modulating hepcidin and Fpn synthesis, the most important regulators of iron homeostasis (Wessling-Resnick 2010). In this respect, it is important to recognize the tight relation among inflammatory and iron homeostasis disorders and adverse outcomes in HT pregnancy.
In the present study, bLf was significantly more effective than the ferrous sulphate therapy in curing ID and IDA (Fig. 2). The bLf efficacy was not due to the amount of iron supplied (70–84 μg/day) that is lower than that required daily in pregnancy, and that provided by ferrous sulphate (156 mg/day). Moreover, the bLf efficacy was not related to an increased iron absorption, since this is recognized to take place similarly well from both bLf and ferrous sulphate (Lönnerdal and Bryant 2006). Taken together, these data pointed a putative influence of bLf on the key factors of iron and inflammatory homeostasis including IL-6. The results here reported confirm this hypothesis. After bLf therapy, serum IL-6 levels significantly decreased (P ≤ 0.0001), even though they remained higher than those detected in uncomplicated pregnancy (Paesano et al. 2009, 2010a, b). Conversely, in HT pregnant women treated with ferrous sulphate, serum IL-6 levels increased even if not to a significant extent. Consequently, the effect of bLf in increasing haematological parameters is indirectly related to IL-6 decrease, even if a bLf direct effect on hepcidin and Fpn synthesis cannot be ruled out (Paesano et al. 2010b).
Concerning safety, similarly to the observations in uncomplicated pregnancies (Paesano et al. 2006, 2010b), 16.5 % of the pregnant women included in Arm B withdrew from the clinical trial due to adverse effects of ferrous sulphate. On the contrary, no women included in Arm A experienced adverse effects (Fig. 1).
Even though all enrolled pregnant HT women were treated with anticoagulant agents to decrease hypercoagulability and miscarriage risk, five miscarriages within the 12th–13th week of gestation were reported among the ferrous sulphate-treated women. Of note, haematological values of these five women indicated the persistence of an ID/IDA status (data not shown) and high levels of serum IL-6 (>90 pg/ml). Conversely, no miscarriage was observed in the women population receiving bLf (Arm A). The incidence of miscarriages as well as the increase of serum IL-6 levels and the persistence of an ID/IDA status in HT ferrous sulphate-treated pregnant women suggest a strong relationship among the failure of a therapy and the persistence of iron and inflammatory homeostasis disorders.
Since pregnancy-associated ID and IDA, as well as inflammation, are among the major risk factors for adverse outcomes in HT pregnancies, the administration of a compound endowed with the capacity to rescue iron homeostasis and attenuate inflammation, such as bLf, could represent an extremely important safe therapy against ID/IDA. The evidence that no women treated with bLf experienced a miscarriage differently from the five miscarriages observed in the ferrous sulphate treated ones encourages us to continue this study in order to confirm the bLf efficacy also in preventing miscarriage on HT pregnant women.
References
Abboud S, Haile DJ (2000) A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem 275:19906–19912. doi:10.1074/jbc.M000713200
Apgar V (1953) A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg 32:260–267
Baker EN, Baker HM (2005) Molecular structure, binding properties and dynamics of lactoferrin. Cell Mol Life Sci 62:2531–2539. doi:10.1007/s00018-005-5368-9
Baker HM, Baker EN (2012) A structural perspective on lactoferrin function. Biochem Cell Biol 90:320–328. doi:10.1139/o11-071
Bastin J, Drakesmith H, Rees M, Sargent I, Townsend A (2006) Localisation of proteins of iron metabolism in the human placenta and liver. Br J Haematol 134:532–543. doi:10.1111/j.1365-2141.2006.06216.x
Bothwell TH (2000) Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr 72:257–264
Bradley J, Leibold EA, Harris ZL, Wobken JD, Clarke S, Zumbrennen KB, Eisenstein RS, Georgieff MK (2004) Influence of gestational age and fetal iron status on IRP activity and iron transporter protein expression in third-trimester human placenta. Am J Physiol Regul Integr Comp Physiol 287:R01–R894. doi:10.1152/ajpregu.00525.2003
Collard KJ (2009) Iron homeostasis in the neonate. Pediatrics 123:1208–1221. doi:10.1542/peds.2008-1047
Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC (2005) The iron exporter ferroportin Slc40a1 is essential for iron homeostasis. Cell Metab 1:191–200. doi:10.1016/j.cmet.2005.01.003
Fox EA, Kahn SR (2005) The relationship between inflammation and venous thrombosis. A systematic review of clinical studies. Thromb Haemost 94:362–365. doi:10.1160/TH05-04-0266
Ganz T (2005) Cellular iron: ferroportin is the only way out. Cell Metab 1:155–157. doi:10.1016/j.cmet.2005.02.006
Ganz T (2013) Systemic iron homeostasis. Physiol Rev 93:1721–1741. doi:10.1152/physrev.00008.2013
Hagberg H, Mallard C, Jacobsson B (2005) Role of cytokines in preterm labour and brain injury. BJOG 112:16–18. doi:10.1111/j.1471-0528.2005.00578.x
Jackson R, Holmes K, Phansalkar A, Rodgers GM (2008) Testing for hereditary thrombophilia: a retrospective analysis of testing referred to a national laboratory. BMC Clin Pathol 8:1–7. doi:10.1186/1472-6890-8-3
Kaandorp S, Di Nisio M, Goddijn M, Middeldorp S (2009) Aspirin or anticoagulants for treating recurrent miscarriage in women without antiphospholipid syndrome. Cochrane Database Syst Rev 21:4734. doi:10.1002/14651858.CD004734.pub3
Kadiiska MB, Burkitt MJ, Xiang QH, Mason RP (1995) Iron supplementation generates hydroxyl radical in vivo. An ESR spin-trapping investigation. J Clin Invest 96:1653–1657. doi:10.1172/JCI118205
Khan S, Dickerman JD (2006) Hereditary thrombophilias. Thromb J 4:15. doi:10.1186/1477-9560-4-15
Lönnerdal B, Bryant A (2006) Absorption of iron from recombinant human lactoferrin in young US women. Am J Clin Nutr 83:305–309
Meier PR, Nickerson HJ, Olson KA, Berg RL, Meyer JA (2003) Prevention of iron deficiency anemia in adolescent and adult pregnancies. Clin Med Res 1:29–36. doi:10.3121/cmr.1.1.29
Nemeth E, Ganz T (2006) Regulation of iron metabolism by hepcidin. Annu Rev Nutr 26:323–342. doi:10.1146/annurev.nutr.26.061505.111303
Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093. doi:10.1126/science.1104742
Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S (2001) Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci USA 98:8780–8785. doi:10.1073/pnas.151179498
Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S (2002) The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest 110:1037–1044. doi:10.1172/JCI15686
Oldenburg B, Van Berge Henegouwen GP, Rennick D, Van Asbeck BS, Koningsberger JC (2000) Iron supplementation affects the production of proinflammatory cytokines in IL-10 deficient mice. Eur J Clin Invest 30:505–510. doi:10.1046/j.1365-2362.2000.00650.x
Paesano R, Torcia F, Berlutti F, Pacifici E, Ebano V, Moscarini M, Valenti P (2006) Oral administration of lactoferrin increases hemoglobin and total serum iron in pregnant women. Biochem Cell Biol 8:377–380
Paesano R, Pietropaoli M, Gessani S, Valenti P (2009) The influence of lactoferrin, orally administered, on sistemi iron homeostasis in pregnant women suffering of iron deficiency and iron deficiency anemia. Biochimie 91:44–51. doi:10.1016/j.biochi.2008.06.004
Paesano R, Berlutti F, Pietropaoli M, Pantanella F, Pacifici E, Goolsbee W, Valenti P (2010a) Lactoferrin efficacy versus ferrous sulfate in curing iron deficiency and iron deficiency anemia in pregnant women. Biometals 23:411–417. doi:10.1007/s10534-010-9335-z
Paesano R, Berlutti F, Pietropaoli M, Goolsbee W, Pacifici E, Valenti P (2010b) Lactoferrin efficacy versus ferrous sulfate in curing iron disorders in pregnant and non pregnant women. Int J Immunopathol Pharmacol 23:577–587
Paesano R, Pietropaoli M, Berlutti F, Valenti P (2012a) Bovine lactoferrin in preventing preterm delivery associated with sterile inflammation. Biochem Cell Biol 90:468–475. doi:10.1139/o11-060
Paesano R, Natalizi T, Berlutti F, Valenti P (2012b) Body iron delocalization: the serious drawback in iron disorders in both developing and developed countries. Pathog Glob Health 106:200–216. doi:10.1179/2047773212Y.0000000043
Park CH, Valore EV, Waring AJ, Ganz T (2001) Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276:7806–7810. doi:10.1074/jbc.M008922200
Patnaik MM, Haddad T, Morton CT (2007) Pregnancy and thrombophilia. Expert Rev Cardiovasc Ther 5:753–765. doi:10.1586/14779072.5.4.753
Petrozella LN, Dashe JS, McIntire DD, Leveno KJ (2011) Clinical significance of borderline amniotic fluid index and oligohydramnios in preterm pregnancy. Obstet Gynecol 117:338–342. doi:10.1097/AOG.0b013e3182056766
Qiao B, Sugianto P, Fung E, Del-Castillo-Rueda A, Moran-Jimenez MJ, Ganz T, Nemeth E (2012) Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metab 15:918–924. doi:10.1016/j.cmet.2012.03.018
Reifen R, Matas Z, Zeidel L, Berkovitch Z, Bujanover Y (2000) Iron supplementation may aggravate inflammatory status of colitis in a rat model. Dig Dis Sci 45:394–397
Rosendaal FR (1999) Venous thrombosis: a multicausal disease. Lancet. doi:10.1016/S0140-6736(98)10266-0
Scholl TO (2005) Iron status during pregnancy: setting the stage for mother and infant. Am J Clin Nutr 81:1218S–1222S
Shafir T, Angulo-Barroso R, Jing Y, Angelilli ML, Jacobson SW, Lozoff B (2008) Iron deficiency and infant motor development. Early Hum Dev 84:479–485. doi:10.1016/j.earlhumdev.2007.12.009
Stella CL, Sibai BM (2006) Thrombophilia and adverse maternal perinatal outcome. Clin Obstet Gynecol 49:850–860
Umbreit J (2005) Iron deficiency: a concise review. Am J Hematol 78:225–231. doi:10.1002/ajh.20249
Valenti P, Antonini G (2005) Lactoferrin: an important host defense against microbial and viral attack. Cell Mol Life Sci 62:2576–2587. doi:10.1007/s00018-005-5372-0
Wessling-Resnick M (2010) Iron homeostasis and the inflammatory response. Annu Rev Nutr 30:105–122. doi:10.1146/annurev.nutr.012809.104804
Zhang DL, Senecal T, Ghosh MC, Ollivierre-Wilson H, Tu T, Rouault TA (2011) Hepcidin regulates ferroportin expression and intracellular iron homeostasis of erythroblasts. Blood 118:2868–2877. doi:10.1182/blood-2011-01-330241
Zhao S, Gua Y, Dong Q, Fan R, Wang Y (2008) Altered interleukin-6 receptor, IL-6R and gp130, production and expression and decreased SOCS-3 expression in placentas from women with pre-eclampsia. Placenta 29:1024–1028. doi:10.1016/j.placenta.2008.09.011
Acknowledgments
This project is supported by Microbo srl, Biotechnology Company, Rome-Italy Grant. IL-6 analyses are supported by Sapienza University of Rome, Italy Grant to PV.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paesano, R., Pacifici, E., Benedetti, S. et al. Safety and efficacy of lactoferrin versus ferrous sulphate in curing iron deficiency and iron deficiency anaemia in hereditary thrombophilia pregnant women: an interventional study. Biometals 27, 999–1006 (2014). https://doi.org/10.1007/s10534-014-9723-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-014-9723-x