Abstract
Siderophores are structurally diverse, complex natural products that bind metals with extraordinary specificity and affinity. The acquisition of iron is critical for the survival and virulence of many pathogenic microbes and diverse strategies have evolved to synthesize, import and utilize iron. There has been a substantial increase of known siderophore scaffolds isolated and characterized in the past decade and the corresponding biosynthetic gene clusters have provided insight into the varied pathways involved in siderophore biosynthesis, delivery and utilization. Additionally, therapeutic applications of siderophores and related compounds are actively being developed. The study of biosynthetic pathways to natural siderophores augments the understanding of the complex mechanisms of bacterial iron acquisition, and enables a complimentary approach to address virulence through the interruption of siderophore biosynthesis or utilization by targeting the key enzymes to the siderophore pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron is an essential element for all microorganisms and an important cofactor required for key cellular, metabolic and biosynthetic processes (Sutak et al. 2008; Saha et al. 2012). The specific acquisition of elemental iron is critical for a microbe’s survival and frequently virulence (Miethke and Marahiel 2007; Weinberg 2009). Under common aerobic and neutral pH conditions, environmental ferric ion has a low bioavailability concentration (10−9–10−18 M), which is usually below the requirement for optimal growth (Braun et al. 1997). As a consequence, bacteria and fungi have evolved diverse strategies to import and utilize iron under iron-deficient conditions exemplified as: the direct extracellular reduction of ferric compounds of low solubility to soluble ferrous iron (Deneer et al. 1995); the acquisition of iron-bound heme or heme-containing proteins (Tong and Guo 2009); the removal of metal from host iron-bound transferrin, lactoferrin or ferritin via specific outer membrane receptors (Braun 2001); and the synthesis, secretion and utilization of iron specific chelators, ferric-binding siderophores (Hider and Kong 2010).
Siderophores are low molecular weight compounds (typically ~400–1200 Da) with extraordinary high affinity for ferric (less so for ferrous) ion, and tend to use negatively charged oxygen as coordinating, donor atoms (Hider and Kong 2010; Saha et al. 2012). Broadly, siderophores can be classified into three categories, depending on the ligand moiety for Fe3+ coordination: catecholate, hydroxamate/carboxylate, and mixed-type, exemplified by enterobactin (1, Escherichia coli), aerobactin (2, E. coli)/rhizoferrin (Rhizopus microsporus) and mycobactin (3, Mycobacterium tuberculosis) respectively (Fig. 1). The different subclasses typically contain multi-dentate ligands, and show strong affinity for the higher oxidation state of iron with association constants of ~1030 or greater. Siderophores help maintain the intracellular iron concentration between 10−7 and 10−5 M necessary for survival and replication (Braun et al. 1997). In addition to iron, siderophores also have the ability to seize and transport other metals (Johnstone and Nolan 2015).
General approaches of siderophore biosynthesis
Siderophores are commonly produced in the cytosol, or sometimes in peroxisomes (Miethke and Marahiel 2007; Gründlinger et al. 2013). A large variety of siderophores have been discovered in the past decades with two biosynthetic pathways resulting in structurally distinct siderophores. Most peptide-based siderophores are biosynthesized via well-characterized, non-ribosomal peptide synthetases (NRPSs). NRPSs are large multi-enzyme complexes responsible for the synthesis of diverse peptide-based secondary metabolites (Condurso and Bruner 2012). NRPSs consist of common domains responsible for peptide synthesis, adenylation (A), condensation (C), peptidyl carrier protein (PCP) and thioesterase (Te), along with other specific functional domains including epimerization (E), oxidation (Ox), methylation (Mt), and cyclization (Cy) (Lazos et al. 2010; Condurso and Bruner 2012). NRPS functional modules carry out steps of monomer selection (A-PCP), modification (E, Ox, Mt), peptidyl chain elongation (C), and cyclization/termination (Cy, Te). Siderophore NRPS assemblies frequently initiate the peptidyl chain with a non-canonical amino acid building block derived from an aryl acid, such as salicylic acid (SA) or 2,3-dihydroxybenzoic acid (DHB) functioning as catecholate chelating moiety. The presence of metal chelating thiazoline or oxazoline rings in siderophore pathways is also common, installed by NRPS embedded Cy/Ox domains acting on cysteine and serine residues. Post assembly-line modification of siderophore NPRs is less usual, a typical example is enterobactin C-glycosylation catalysed by the glycosyltransferase, IroB, in salmochelin (4) production (Fischbach et al. 2005). In addition, a suite of marine siderophores have a peptidic head group for ferric chelation, as well as fatty acid tailoring that varies in length and saturation to tune the overall molecular amphiphilicity (Kem and Butler 2015). Marinobactin (5) is produced by Marinobacter sp. largely using NRPS machinery and is further acylated with saturated or unsaturated C12-18 fatty acids (Gauglitz et al. 2014; Kem et al. 2015). Similarly, amphi-enterobactin (6) produced in Vibrio harveyi BAA-1116 also contains a fatty acid moiety. The amphi-enterobactin biosynthesis pathway contains a unique bi-functional C-domain, which can accept amino acyl-phosphopantothenyl-PCP, as well as acyl-CoAs as substrates (Zane et al. 2014). Other recently described novel NRPS siderophore scaffolds include mirubactin (7) from Actinosynnema mirum (Giessen et al. 2012; Kishimoto et al. 2015).
In addition to NRPS systems, a number of microbial siderophores are constructed by NRPS-independent synthetase (NIS) pathways (Barry and Challis 2009). NIS-based siderophores include aerobactin (2), achromobactin (8, Pseudomonas syringae) and desferrioxamine (9, Streptomyces griseus). These siderophores are usually assembled from alternating dicarboxylic acid and diamine/amino alcohol building blocks linked by amide or ester bonds. Recently described examples of NIS-siderophores include baumannoferrin, (10) isolated from an acinetobactin (the preliminary NRPS siderophore)-deficient Acinetobacter baumannii AYE strain under iron-limiting conditions (Penwell et al. 2015); and putrebactins (11), the first identified unsaturated macrocyclic dihydroxamic acid siderophores in Shewanella putrefaciens, synthesized by a precursor-directed mechanism (Soe and Codd 2014).
Siderophore secretion
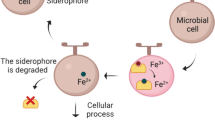
After biosynthesis, apo-siderophores are secreted into the media to scavenge iron (Fig. 2) (Miethke and Marahiel 2007). Siderophore secretion is one of the least understood steps of the iron acquisition process in microorganisms. Several different secretion systems have been identified as being implicated in the process, comprising transporters from the major facilitator superfamily (MFS) and the efflux pumps of the resistance, nodulation, and cell division superfamily (RND).
Schematic of typical siderophore pathways found in Gram-negative bacteria as discussed in the text. Figure was prepared with PyMol (https://www.pymol.org/) with PDB entries used listed: 3WDO, 2XMN, 4DK0, 4C48, 1FI1, 2W76, 2GSK, 2PFU, 5DH0, 2GZR, 4G1U and 4YHB
Many NRPS-based siderophore gene clusters contain an MFS transporter-encoding gene. MFS is one of the largest groups of transporters, conserved from bacteria to human, with a wide spectrum of substrates including ions, carbohydrates, lipids, peptides, nucleosides, and other primary and secondary metabolites (Reddy et al. 2012). The majority of MFS members contain 12-transmembrane α-helices (TMs), with some having 14-TMs or more (Reddy et al. 2012). Several MFS proteins from different subfamilies have been structurally elucidated, but none known to be associated with siderophore secretion (Yan 2015). In Gram-negative E. coli, apo-enterobactin is transported by EntS, a 43-kDa archetype MFS transporter encoded by the gene ybdA within the Fur-regulated ent-fep gene cluster (Furrer et al. 2002). EntS exports enterobactin across the cytoplasmic membrane. Mutant ΔentS (ΔybdA) shows significant reduction of enterobactin secretion with an increased release of biosynthetic byproducts. In the Gram-positive Bacillus. subtilis, the MFS protein YmfE was identified to participate in siderophore bacillibactin secretion from screening mutants (Miethke et al. 2008). The YmfE mutant strain is deficient in bacillibactin secretion and does not grow in iron deficient medium.
In addition to the MFS class, the RND superfamily is a ubiquitous group of proton antiporters, especially common among Gram-negative bacteria. The class promotes the active efflux of heavy metals, xenobiotics and siderophores (Nikaido and Pagès 2012). The iron-regulated MexAB-OprM system in Pseudomonas aeruginosa was is the first RND identified in siderophore (pyoverdin) secretion (Li et al. 1995). In E. coli, complementary with MFS transporters, TolC is responsible for the siderophore enterobactin efflux across the outer membrane (Bleuel et al. 2005; Pei et al. 2011). TolC is a trans-outer membrane protein and is an essential component of the RND pump AcrAB-TolC. The complex is a general transporter for bacteria antibiotics and imparts tolerance to toxic compounds, and the whole complex has been structurally characterized (Du et al. 2014). The AcrAB-TolC pump consists of AcrB:AcrA:TolC in a 3:6:3 ratio. AcrA interacts with TolC through a hairpin domain and connects with AcrB through β-barrel and membrane-proximal domains. The role of AcrAB in E. coli enterobactin secretion is not entirely clear, the triple mutant ΔacrB/ΔacrD/ΔmdtABC, but not any individual deletions, results in decreased enterobactin excretion (Horiyama and Nishino 2014). AcrAD and MdtABC are two additional TolC-dependent RND pumps. Additionally, the MmpS5-MmpL5 (or 4) RND efflux pump, a homolog of AcrAB, has been identified as an critical siderophore export system in M. tuberculosis (Wells et al. 2013). MmpS5-MmpL5 is regulated by Rv0678, an MarR-like transcriptional regulator (Radhakrishnan et al. 2014). Mutants lacking the mmpS4 and mmpS5 genes do not affect the uptake of external carboxymycobactin (12), but stops cell growth under low iron conditions (Jones et al. 2014). The MmpS5-MmpL5 efflux system also results in azole resistance in M. tuberculosis (Milano et al. 2009).
Outside of the MFS and RND subclasses, IroC, a type-I ATP-binding cassette (ABC) transporter, similar to eukaryotic multidrug resistance (MDR) proteins, was previously suggested as responsible for the uptake of ferric-salmochelin in E. coli, and is also proposed to be an active enterobactin/salmochelin exporter (Caza et al. 2008; Crouch et al. 2008). The double mutant ΔentS/ΔiroC has a severely compromised growth rate, while IroC does not impact the ferric-enterobactin/salmochelin utilization in the growth promotion (Crouch et al. 2008; Caza et al. 2011).
Holo-siderophore acquisition
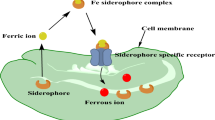
Holo-siderophore acquisition from the extracellular environment is one of the key steps in iron assimilation. Most siderophores have molecular weights in excess of 600 Da, and their ability to permeate the outer membrane, passively, is low (Hider and Kong 2010). Therefore, specific energy-dependent integral, outer membrane porins are essential to deliver an iron-siderophore complex from the extracellular into the periplasmic space. The ferric-ferricrocin receptor, FhuA, is a 22-stranded β-barrel that spans the outer membrane, with an N-terminus plug domain accessible to the barrel pore, facilitating ligand recognition and binding (Pawelek et al. 2006). FhuA also includes an N-terminus TonB box, which forms a protein/protein interaction through a four-stranded β-sheet with TonB, a cytoplasmic membrane sigma regulator with receptor specificity (Naikare et al. 2013). The ferric-citrate receptor FecA in E. coli is a member of the TonB-transducer family and contains a signaling domain upstream from the TonB box and is able to self-regulate their own synthesis as well as their cognate siderophores (Ferguson et al. 2002). TonB is part of the TonB/ExbB/ExbD energy transduction system. The complex is located in the cytoplasmic membrane, and provides the energy required for the active transport of holo-siderophores through the outer receptor (Krewulak and Vogel 2011). TonB undergoes energized motion in the bacterial cell envelope, interacts with the ExbB4-ExbD2 complex to generate an electrochemical gradient and initiate the energization (Jordan et al. 2013; Sverzhinsky et al. 2014). The interactions promote iron uptake through outer membrane transporters using a rotational mechanism (Ollis and Postle 2012a, b).
The ABC transporter superfamily constitutes a group of transmembrane proteins that perform ATP-coupled translocation on a wide range of substrates across cell membranes (Rees et al. 2009). ABC transporters have the general function of delivering environmental ferric-siderophores to the cytosol in Gram-positive bacteria, or from the periplasm to cytosol in Gram-negative. ABC transporters have a common architecture that consists of a pair of transmembrane domains (TMDs) embedded in the membrane lipid bilayer, and a pair of nucleotide-binding domains (NBDs) that are located in the cytoplasm (Rees et al. 2009). Most siderophore ABC transporters belong to the type-II ABC subclass, and a related homolog, the heme transporter HmuUV in Yersinia pestis has been structurally characterized and identified to employ an coupling mechanism distinct from that of other ABC transporter subclasses (Woo et al. 2012). Some additional siderophore-specific ABC transporters have been reported to be regulated by iron-dependent regulators (Rodriguez and Smith 2006; Granger et al. 2013). Additionally, siderophore ABC transporters commonly interact with type-III ABC-transporter periplasmic binding proteins (PBPs) to facilitate substrate delivery into the cytosol (Chu and Vogel 2011). Unlike type-I and type-II PBPs, which undergo significant domain transition during interaction with substrate, most siderophore type-III PBPs have a relatively rigid α-helical structure, serving as the hinge between the two domains and ensures the overall structural stability. The flexibility also allows domain movement between open/closed states upon specific substrate(s) recognition (Chu et al. 2014; Li and Bruner 2016). ABC transporter meditated siderophore delivery in Gram-positive bacteria, exemplified by the YxeB-dependent system in Bacillus cereus, shuttle metal using an iron-exchange mechanism, from ferric-siderophore to apo-siderophore, without utilizing iron reduction (Fukushima et al. 2013, 2014).
In contrast to ABC importers common in bacteria, the MFS transporter, MirB in the fungal pathogen Aspergillus fumigates, is a 14-TMs protein and reported to be responsible for the uptake of hydroxamate siderophore N,N′,N″-triacetylfusarinine-C (TAFC) (Raymond-Bouchard et al. 2012). No bacterial MFS, however, has so far been reported in holo-siderophore acquisition.
Siderophore utilization
Imported ferric iron tightly bound to its cognate siderophore must be released upon holo-siderophore delivery to make the metal available to cellular machinery. One strategy for iron release is through hydrolytic destruction of the holo-siderophore by esterases of the α,β-hydrolase family of enzymes, represented by the E. coli fes (iroE) gene product and is a common strategy for macrolactone-based siderophores (Larsen et al. 2006). IroE is a periplasmic trilactone hydrolase that cleaves one of the three esters of enterobactin producing linearized trimers, which still retain a considerable affinity to ferric ion. Thus, in addition to iron assimilation, IroE is likely involved in the production of triscatecholate siderophores (Lin et al. 2005). In contrast, the periplasmic trilactone esterase, Cee, in Campylobacter hydrolyses both apo- and holo- forms of enterobactin effectively, and further digests the linearized trimer into dimers and monomers, and this degradation significantly diminishes ferric binding affinity (Zeng et al. 2013). Genomic evidence further demonstrates that Cee is involved in enterobactin mediated iron uptaken (Zeng et al. 2013).
For cleavage-independent siderophore paths, iron release is commonly proposed to be facilitated by single-electron reduction of the ferric-siderophore to the ferrous oxidation state (Li et al. 2015). The relative weaker affinity of the reduced ferrous-siderophore interaction allows kinetic exchange with downstream iron-chelating sites found in cellular proteins or small molecules (Hider and Kong 2010). Two families of proteins have been identified so far involved in the process, termed siderophore-interacting protein (SIP), and ferric-siderophore reductase (FSR). FSR contains a unique C-terminal [2Fe-2S] cluster as C–C-x10-C-x2-C, and does not show significant similarities to other known [2Fe-2S] proteins. The E. coli FSR, FhuF is a part of the siderophore utilization system and has been shown to have a sufficient redox potential to reduce ferric-ferrioxamine (Matzanke et al. 2004). Additional biochemical evidence of function comes from FchR, a FhuF homolog found in Bacillus halodurans DSM497 that participates in a three-component electron donor system, along with NADPH and a ferredoxin to reduce various iron chelates with optimal demonstrated reduction activity against ferric-dicitrate (Miethke et al. 2011b). Meanwhile, members of the SIP family are flavoreductases. The most studied example is the E. coli YqjH which contains a covalently bound FAD cofactor and can reduce ferric-enterobactin in the presence of NADPH (Miethke et al. 2011a). The yqjH gene is regulated by YqjI, a Zn2+-dependent regulator (Wang et al. 2011, 2014). The YqjH homolog, FscN in T. fusca has been structurally characterized with a FAD-binding domain and an NAD(P)H-interaction domain (Li et al. 2015). FscN contains a non-covalently bound FAD and reduces ferric-fuscachelin-A (13) using NADH as the electron source. Interestingly, the M. tuberculosis iron-regulated ABC transporter, IrtA contains an extended N-terminal FAD/NAD(P)H binding domain similar to the SIP family (Ryndak et al. 2010). As a result, IrtA is predicted to have dual function, iron delivery and assimilation.
Siderophores as therapeutical drug leads
Bacterial siderophores have been shown to exhibit specific antifungal or antibiotic activities (Pramanik et al. 2007; Sulochana et al. 2014). Therapeutic iron chelators containing siderophore scaffolds have also been used in the treatments of blood-transfusion requiring diseases (Olivieri et al. 1995). In addition, in the past decade, siderophore-antibiotic conjugates have been developed as drug leads with reduced permeability-mediated drug resistance using “Trojan Horse”-type mechanisms (Górska et al. 2014). The strategy can impressively reduce permeability-mediated drug resistance with target selectivity, and especically advantageous in multidrug resistance pathogen control (Wencewicz et al. 2009).
Successful design of siderophore-drug conjugates contain a siderophore moiety that can be recognized and imported; a suitable linker stable to the extracellular environment but suitably labile either in the cytoplasm or periplasm; and an effective drug moiety, commonly of the β-lactam drug family (Fig. 3) (de Carvalho and Fernandes 2014). Siderophores-linked lactam antibiotics have an increased penetration through the outer membrane (Kline et al. 2000) and pathogen selectivity can be tuned with altered, conjugated siderophores. Specifically, tris-catecholate siderophore-aminopenicillin conjugates (14) inhibit Gram-negative bacteria such as Pseudomonas aeruginosa (Ji et al. 2012). The related biscatecholate-monohydroxamate siderophore-carbacephalosporin conjugates (15) are selective for pathogenic A. baumannii (Wencewicz and Miller 2013). Another hexadentate siderophore-mediated drug delivery system has been successfuly addressed with enterobactin-cargo conjugates in E. coli (and P. aeruginosa) (Zheng et al. 2012; Zheng and Nolan 2014). Enterobactin-antibiotic conjugates, including β-lactam antibiotics ampicillin (Amp) and amoxicillin (Amx) conjugates (Ent-Amp/Amx, 16), have a 1000-fold decrease in minimum inhibitory concentration (MIC) value against E. coli CFT073 relative to Amp/Amx alone (Zheng and Nolan 2014). In general, the enterobactin-mediated delivery is FepA dependent. Ent-Amp conjugate selectivity can be further constricted with salmochelin modification (GlcEnt-Amp/Amx, 17) to target specific, pathogenic E. coli with the iroN encoded salmochelin receptor, this modification also lowers mammalian cell toxicity (Chairatana et al. 2015). The lactam-family of drugs can also be delivered with relatively smaller siderophores. The bidentate siderophore-sulfactam BAL30072 (18) has promising activity against multi-resistant Gram-negative Bacilli, including strains with multiple drug resistant β-lactamases, such as meropenem-resistance A. baumannii (Page et al. 2010; Higgins et al. 2012). Additionally, the antimicrobial efficacy of BAL30072 can be further enhanced through the carbapenem combination treatments and BAL30072 was submitted to clinical trials phase-I in 2013 (Hofer et al. 2013; Butler et al. 2013). Comparably, siderophore-monocarbam conjugates MC-1 (19) possess in vitro and predicted in vivo activity against MDR pathogens as P. aeruginosa (Flanagan et al. 2011; Murphy-Benenato et al. 2015a). The MC-1/penicillin-binding protein co-complex crystal structure established the molecular basis for the recognition specificity and coupling activity (Han et al. 2010; Murphy-Benenato et al. 2015b). Similar pyridone-monobactam conjugates also possess in vitro antibacterial activity against clinically relevant MDR Gram-negative species (Brown et al. 2013). MC-1 and analogs are most likely to use the TonB-dependent outer membrane siderophore receptor (PiuA, or PirA) as the primary mean of entry (McPherson et al. 2012; Brown et al. 2013).
Besides siderophore-lactam conjugates, several lactam-independent siderophore-drug candidates have been reported. The trihydroxamate siderophore-fluoroquinolone conjugates (20) target Gram-positive S. aureus SG511 (Wencewicz et al. 2013). The lactivicin analog-phthalimide conjugates (21) utilize a wider set of TonB receptors (compare with hydroxypyridone-lactams) to target penicillin-binding proteins and compliment the in vitro Gram-negative antibiotics activity (Starr et al. 2014). Lastly the mycobactin-T analog-artemisinin conjugate (22) displays high activity against extensively drug-resistance (XDR) M. tuberculosis strains (Miller et al. 2011; Juárez-Hernández et al. 2012).
Targeting siderophore pathways
Specific targeting of siderophore pathways is another applicable therapeutic approach to address the microbial virulence. The disruption of iron recycling constrains the microbial survival and replication, and the interruption of holo-siderophore acquisition is a potential therapeutically approach (Miethke and Marahiel 2007). Most siderophore-drug conjugates do have an added effect of interfering with the native siderophore acquisition. In addition, the reported antibiotic lasso-peptide Microcin-J25 (23), and its analogs target the FepA receptor (Mathavan et al. 2014). Lasso-peptides structurally hijack FepA to disrupt ferric-siderophore acquisition and reduce the microbial growth in vitro (Pan and Link 2011). Targeting FepA is an attractive approach as several human pathogens largely acquire iron from host siderophores through FepA (Liu et al. 2014).
Stalling siderophore biosynthesis through small molecule inhibition is an approach to reduce virulence and has received much attention recently. In NRPS-based siderophore biosynthesis, acyl adenylate intermediates, includes SA- and DHB-AMP analogs interrupt the substrate recognition of the initial adenylation domain, and subsequently inhibit a broad range of microorganisms with aryl-capped siderophores production (Miethke et al. 2006). Salicyl-AMS (24) and its analogs inhibit NRPS MbtA in M. tuberculosis, and significantly reduce the pathogen growth in mouse lungs in vivo (Ferreras et al. 2005; Lun et al. 2013). Biaryl nitrile (25) and analogs target PvdQ, an essential N-terminal nucleophile hydrolase in pyoverdine biosynthesis pathway, and decrease the in vitro siderophore production, thus limit the growth of P. aeruginosa under iron-limiting conditions (Wurst et al. 2014).
Targeting the components of siderophore secretion is also a possible therapeutic approach toward pathogenic microbes. Pathogenic E. coli requires siderophores for iron acquisition during infection, while the exporters EntS and IroC have been shown to be important for the systemic virulence in a chicken infection model (Caza et al. 2011). Mutation of the outer membrane transporter, TolC, displays a morphological defect in minimal medium, that is likely caused by periplasmic enterobactin accumulation (Vega and Young 2013). Similar approaches and findings have addressed the RND efflux system of M. tuberculosis (Jones et al. 2014). Exporter-deficient ΔmmpS4/S5, M. tuberculosis has an inhibited growth rate, and the growth defect could not be rescued by supplementing external mycobactin (Jones et al. 2014). Cytoplasmic mycobactin (and carboxymycobactin) accumulation provides a possible self-poisoning inhibition mechanism. In this context, siderophore secretion is a more advantageous target, as the biosynthesis or specific acquisition defect can be overcome by using alternative ferric-carrying heme or siderophores (Jones et al. 2014).
Conclusions
The chemistry and biology of siderophore-mediated iron acquisition is a complex process and a key for step for microbial survival and virulence. Understanding the siderophore-related pathways enables the design and development of siderophore-scaffold drugs, as well as the therapeutic approaches in targeting the pathogenic iron assimilation. Recent progress in siderophores and their related applications provide a much-improved insight into the complex and important process of iron acquisition. The developing clinical endeavors to target microorganism/pathogen in “iron battle” will continue to stimulate research in the area.
References
Barry SM, Challis GL (2009) Recent advances in siderophore biosynthesis. Curr Opin Chem Biol 13:205–215. doi:10.1016/j.cbpa.2009.03.008
Bleuel C, Große C, Taudte N et al (2005) TolC Is Involved in enterobactin efflux across the outer membrane of Escherichia coli. J Bacteriol 187:6701–6707. doi:10.1128/JB.187.19.6701
Braun V (2001) Iron uptake mechanisms and their regulation in pathogenic bacteria. Int J Med Microbiol 291:67–79. doi:10.1078/1438-4221-00103
Braun V, Hantke K, Winkelmann G, Carrano CJ (1997) Transition metals in microbial metabolism. Harwood, Amsterdam
Brown MF, Mitton-Fry MJ, Arcari JT et al (2013) Pyridone-conjugated monobactam antibiotics with Gram-negative activity. J Med Chem 56:5541–5552. doi:10.1021/jm400560z
Butler MS, Blaskovich MA, Cooper MA (2013) Antibiotics in the clinical pipeline in 2013. J Antibiot 66:571–591. doi:10.1038/ja.2013.86
Caza M, Lépine F, Milot S, Dozois CM (2008) Specific roles of the iroBCDEN genes in virulence of an avian pathogenic Escherichia coli O78 strain and in production of salmochelins. Infect Immun 76:3539–3549. doi:10.1128/IAI.00455-08
Caza M, Lépine F, Dozois CM (2011) Secretion, but not overall synthesis, of catecholate siderophores contributes to virulence of extraintestinal pathogenic Escherichia coli. Mol Microbiol 80:266–282. doi:10.1111/j.1365-2958.2011.07570.x
Chairatana P, Zheng T, Nolan EM (2015) Targeting virulence: salmochelin modification tunes the antibacterial activity spectrum of b-lactams for pathogen-selective killing of Escherichia coli. Chem Sci 6:4458–4471. doi:10.1039/C5SC00962F
Chu BCH, Vogel HJ (2011) A structural and functional analysis of type III periplasmic and substrate binding proteins: their role in bacterial siderophore and heme transport. Biol Chem 392:39–52. doi:10.1515/BC.2011.012
Chu BCH, Otten R, Krewulak KD et al (2014) The solution structure, binding properties, and dynamics of the bacterial siderophore-binding protein FepB. J Biol Chem 289:29219–29234. doi:10.1074/jbc.M114.564021
Condurso HL, Bruner SD (2012) Structure and noncanonical chemistry of nonribosomal peptide biosynthetic machinery. Nat Prod Rep 29:1099–1110. doi:10.1039/c2np20023f
Crouch M-LV, Castor M, Karlinsey JE et al (2008) Biosynthesis and IroC-dependent export of the siderophore salmochelin are essential for virulence of Salmonella enterica serovar Typhimurium. Mol Microbiol 67:971–983. doi:10.1111/j.1365-2958.2007.06089.x
de Carvalho CCCR, Fernandes P (2014) Siderophores as “Trojan Horses”: tackling multidrug resistance? Front Microbiol 5:1–3. doi:10.3389/fmicb.2014.00290
Deneer HG, Healey V, Boychuk I (1995) Reduction of exogenous ferric iron by a surface-associated ferric reductase of Listeria spp. Microbiology 141:1985–1992. doi:10.1099/13500872-141-8-1985
Du D, Wang Z, James NR et al (2014) Structure of the AcrAB-TolC multidrug efflux pump. Nature 509:512–515. doi:10.1038/nature13205
Ferguson AD, Chakraborty R, Smith BS et al (2002) Structural basis of gating by the outer membrane transporter FecA. Science 295:1715–1719. doi:10.1126/science.1067313
Ferreras JA, Ryu J-S, Di Lello F et al (2005) Small-molecule inhibition of siderophore biosynthesis in Mycobacterium tuberculosis and Yersinia pestis. Nat Chem Biol 1:29–32. doi:10.1038/nchembio706
Fischbach MA, Lin H, Liu DR, Walsh CT (2005) In vitro characterization of IroB, a pathogen-associated C-glycosyltransferase. Proc Natl Acad Sci USA 102:571–576. doi:10.1073/pnas.0408463102
Flanagan ME, Brickner SJ, Lall M et al (2011) Preparation, Gram-negative antibacterial activity, and hydrolytic stability of novel siderophore-conjugated monocarbam diols. ACS Med Chem Lett 2:385–390. doi:10.1021/ml200012f
Fukushima T, Allred BE, Sia AK et al (2013) Gram-positive siderophore-shuttle with iron-exchange from Fe-siderophore to apo-siderophore by Bacillus cereus YxeB. Proc Natl Acad Sci 110:13821–13826. doi:10.1073/pnas.1304235110
Fukushima T, Allred BE, Raymond KN (2014) Direct evidence of iron uptake by the Gram-positive siderophore-shuttle mechanism without iron reduction. ACS Chem Biol 9:2092–2100. doi:10.1021/cb500319n
Furrer JL, Sanders DN, Hook-Barnard IG, McIntosh MA (2002) Export of the siderophore enterobactin in Escherichia coli: involvement of a 43 kDa membrane exporter. Mol Microbiol 44:1225–1234. doi:10.1046/j.1365-2958.2002.02885.x
Gauglitz JM, Iinishi A, Ito Y, Butler A (2014) Microbial tailoring of acyl peptidic siderophores. Biochemistry 53:2624–2631. doi:10.1021/bi500266x
Giessen TW, Franke KB, Knappe TA et al (2012) Isolation, structure elucidation, and biosynthesis of an unusual hydroxamic acid ester-containing siderophore from Actinosynnema mirum. J Nat Prod 75:905–914. doi:10.1021/np300046k
Górska A, Sloderbach A, Marszałł MP (2014) Siderophore-drug complexes: potential medicinal applications of the “Trojan horse” strategy. Trends Pharmacol Sci 35:442–449. doi:10.1016/j.tips.2014.06.007
Granger JB, Lu Z, Ferguson JB et al (2013) Cloning, expression, purification and characterization of an iron-dependent regulator protein from Thermobifida fusca. Protein Expr Purif 92:190–194. doi:10.1016/j.pep.2013.09.010
Gründlinger M, Yasmin S, Lechner BE et al (2013) Fungal siderophore biosynthesis is partially localized in peroxisomes. Mol Microbiol 88:862–875. doi:10.1111/mmi.12225
Han S, Zaniewski RP, Marr ES et al (2010) Structural basis for effectiveness of siderophore-conjugated monocarbams against clinically relevant strains of Pseudomonas aeruginosa. Proc Natl Acad Sci USA 107:22002–22007. doi:10.1073/pnas.1013092107
Hider RC, Kong X (2010) Chemistry and biology of siderophores. Nat Prod Rep 27:637–657. doi:10.1039/b906679a
Higgins PG, Stefanik D, Page MGP et al (2012) In vitro activity of the siderophore monosulfactam BAL30072 against meropenem-non-susceptible Acinetobacter baumannii. J Antimicrob Chemother 67:1167–1169. doi:10.1093/jac/dks009
Hofer B, Dantier C, Gebhardt K et al (2013) Combined effects of the siderophore monosulfactam BAL30072 and carbapenems on multidrug-resistant Gram-negative bacilli. J Antimicrob Chemother 68:1120–1129. doi:10.1093/jac/dks527
Horiyama T, Nishino K (2014) AcrB, AcrD, and MdtABC Multidrug Efflux Systems Are Involved in Enterobactin Export in Escherichia coli. PLoS One 9:e108642. doi:10.1371/journal.pone.0108642
Ji C, Miller PA, Miller MJ (2012) Iron transport-mediated drug delivery: practical syntheses and in vitro antibacterial studies of tris-catecholate siderophore-aminopenicillin conjugates reveals selectively potent antipseudomonal activity. J Am Chem Soc 134:9898–9901. doi:10.1021/ja303446w
Johnstone T, Nolan E (2015) Beyond iron: non-classical functions of bacterial siderophores. Dalt Trans 44:6320–6339. doi:10.1039/C4DT03559C
Jones CM, Wells RM, Madduri AVR et al (2014) Self-poisoning of Mycobacterium tuberculosis by interrupting siderophore recycling. Proc Natl Acad Sci USA 111:1945–1950. doi:10.1073/pnas.1311402111
Jordan LD, Zhou Y, Smallwood CR et al (2013) Energy-dependent motion of TonB in the Gram-negative bacterial inner membrane. Proc Natl Acad Sci USA 110:11553–11558. doi:10.1073/pnas.1304243110
Juárez-Hernández RE, Franzblau SG, Miller MJ (2012) Syntheses of mycobactin analogs as potent and selective inhibitors of Mycobacterium tuberculosis. Org Biomol Chem 10:7584. doi:10.1039/c2ob26077h
Kem MP, Butler A (2015) Acyl peptidic siderophores: structures, biosyntheses and post-assembly modifications. Biometals 28:445–459. doi:10.1007/s10534-015-9827-y
Kem MP, Naka H, Iinishi A et al (2015) Fatty acid hydrolysis of acyl marinobactin siderophores by marinobacter acylases. Biochemistry 54:744–752. doi:10.1021/bi5013673
Kishimoto S, Nishimura S, Kakeya H (2015) Total synthesis and structure revision of mirubactin, and its iron binding activity. Chem Lett 44:1303–1305. doi:10.1246/cl.150520
Kline T, Fromhold M, McKennon T et al (2000) Antimicrobial effects of novel siderophores linked to b-lactam antibiotics. Bioorg Med Chem 8:73–93. doi:10.1016/S0968-0896(99)00261-8
Krewulak KD, Vogel HJ (2011) TonB or not TonB: is that the question? Biochem Cell Biol 89:87–97. doi:10.1139/O10-141
Larsen NA, Lin H, Wei R et al (2006) Structural characterization of enterobactin hydrolase IroE. Biochemistry 45:10184–10190. doi:10.1021/bi060950i
Lazos O, Tosin M, Slusarczyk AL et al (2010) Biosynthesis of the putative siderophore erythrochelin requires unprecedented crosstalk between separate nonribosomal peptide gene clusters. Chem Biol 17:160–173. doi:10.1016/j.chembiol.2010.01.011
Li K, Bruner SD (2016) Structure and functional analysis of the siderophore periplasmic binding protein from the fuscachelin gene cluster of Thermobifida fusca. Proteins 84:118–128. doi:10.1002/prot.24959
Li XZ, Nikaido H, Poole K (1995) Role of MexA-MexB-OprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother 39:1948–1953. doi:10.1128/AAC.39.9.1948
Li K, Chen W-H, Bruner SD (2015) Structure and mechanism of the siderophore-interacting protein from the fuscachelin gene cluster of Thermobifida fusca. Biochemistry 54:3989–4000. doi:10.1021/acs.biochem.5b00354
Lin H, Fischbach MA, Liu DR, Walsh CT (2005) In vitro characterization of salmochelin and enterobactin trilactone hydrolases IroD, IroE, and Fes. J Am Chem Soc 127:11075–11084. doi:10.1021/ja0522027
Liu Z, Reba S, Chen W-D et al (2014) Regulation of mammalian siderophore 2,5-DHBA in the innate immune response to infection. J Exp Med 211:1197–1213. doi:10.1084/jem.20132629
Lun S, Guo H, Adamson J et al (2013) Pharmacokinetic and in vivo efficacy studies of the mycobactin biosynthesis inhibitor salicyl-AMS in mice. Antimicrob Agents Chemother 57:5138–5140. doi:10.1128/AAC.00918-13
Mathavan I, Zirah S, Mehmood S et al (2014) Structural basis for hijacking siderophore receptors by antimicrobial lasso peptides. Nat Chem Biol 10:340–342. doi:10.1038/nchembio.1499
Matzanke BF, Anemüller S, Schünemann V et al (2004) FhuF, part of a siderophore-reductase system. Biochemistry 43:1386–1392. doi:10.1021/bi0357661
McPherson CJ, Aschenbrenner LM, Lacey BM et al (2012) Clinically relevant Gram-negative resistance mechanisms have no effect on the efficacy of MC-1, a novel siderophore-conjugated monocarbam. Antimicrob Agents Chemother 56:6334–6342. doi:10.1128/AAC.01345-12
Miethke M, Marahiel MA (2007) Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71:413–451. doi:10.1128/MMBR.00012-07
Miethke M, Bisseret P, Beckering CL et al (2006) Inhibition of aryl acid adenylation domains involved in bacterial siderophore synthesis. FEBS J 273:409–419. doi:10.1111/j.1742-4658.2005.05077.x
Miethke M, Schmidt S, Marahiel MA (2008) The major facilitator superfamily-type transporter YmfE and the multidrug-efflux activator Mta mediate bacillibactin secretion in Bacillus subtilis. J Bacteriol 190:5143–5152. doi:10.1128/JB.00464-08
Miethke M, Hou J, Marahiel MA (2011a) The siderophore-interacting protein YqjH acts as a ferric reductase in different iron assimilation pathways of Escherichia coli. Biochemistry 50:10951–10964. doi:10.1021/bi201517h
Miethke M, Pierik AJ, Peuckert F et al (2011b) Identification and characterization of a novel-type ferric siderophore reductase from a Gram-positive extremophile. J Biol Chem 286:2245–2260. doi:10.1074/jbc.M110.192468
Milano A, Pasca MR, Provvedi R et al (2009) Azole resistance in Mycobacterium tuberculosis is mediated by the MmpS5–MmpL5 efflux system. Tuberculosis 89:84–90. doi:10.1016/j.tube.2008.08.003
Miller MJ, Walz AJ, Zhu H et al (2011) Design, synthesis, and study of a mycobactin-artemisinin conjugate that has selective and potent activity against tuberculosis and malaria. J Am Chem Soc 133:2076–2079. doi:10.1021/ja109665t
Murphy-Benenato KE, Bhagunde PR, Chen A et al (2015a) Discovery of efficacious Pseudomonas aeruginosa-targeted siderophore-conjugated monocarbams by application of a semi-mechanistic pharmacokinetic/pharmacodynamic model. J Med Chem 58:2195–2205. doi:10.1021/jm501506f
Murphy-Benenato KE, Dangel B, Davis HE et al (2015b) SAR and structural analysis of siderophore-conjugated monocarbam inhibitors of Pseudomonas aeruginosa PBP3. ACS Med Chem Lett 6:537–542. doi:10.1021/acsmedchemlett.5b00026
Naikare H, Butcher J, Flint A et al (2013) Campylobacter jejuni ferric–enterobactin receptor CfrA is TonB3 dependent and mediates iron acquisition from structurally different catechol siderophores. Metallomics 5:988. doi:10.1039/c3mt20254b
Nikaido H, Pagès JM (2012) Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol Rev 36:340–363. doi:10.1111/j.1574-6976.2011.00290.x
Olivieri NF, Brittenham GM, Matsui D et al (1995) Iron-chelation therapy with oral deferiprone in patients with thalassemia major. N Engl J Med 332:918–922. doi:10.1056/NEJM199504063321404
Ollis AA, Postle K (2012a) Identification of functionally important TonB-ExbD periplasmic domain interactions in vivo. J Bacteriol 194:3078–3087. doi:10.1128/JB.00018-12
Ollis AA, Postle K (2012b) ExbD mutants define initial stages in TonB energization. J Mol Biol 415:237–247. doi:10.1016/j.jmb.2011.11.005
Page MGP, Dantier C, Desarbre E (2010) In vitro properties of BAL30072, a novel siderophore sulfactam with activity against multiresistant Gram-negative bacilli. Antimicrob Agents Chemother 54:2291–2302. doi:10.1128/AAC.01525-09
Pan SJ, Link AJ (2011) Sequence diversity in the lasso peptide framework: discovery of functional microcin J25 variants with multiple amino acid substitutions. J Am Chem Soc 133:5016–5023. doi:10.1021/ja1109634
Pawelek PD, Croteau N, Ng-Thow-Hing C et al (2006) Structure of TonB in complex with FhuA. E. coli outer membrane receptor. Science 312:1399–1402. doi:10.1126/science.1128057
Pei X-Y, Hinchliffe P, Symmons MF et al (2011) Structures of sequential open states in a symmetrical opening transition of the TolC exit duct. Proc Natl Acad Sci USA 108:2112–2117. doi:10.1073/pnas.1012588108
Penwell WF, DeGrace N, Tentarelli S et al (2015) Discovery and characterization of new hydroxamate siderophores, baumannoferrin A and B, produced by Acinetobacter baumannii. ChemBioChem 16:1896–1904. doi:10.1002/cbic.201500147
Pramanik A, Stroeher UH, Krejci J et al (2007) Albomycin is an effective antibiotic, as exemplified with Yersinia enterocolitica and Streptococcus pneumoniae. Int J Med Microbiol 297:459–469. doi:10.1016/j.ijmm.2007.03.002
Radhakrishnan A, Kumar N, Wright CC et al (2014) Crystal structure of the transcriptional regulator Rv0678 of Mycobacterium tuberculosis. J Biol Chem 289:16526–16540. doi:10.1074/jbc.M113.538959
Raymond-Bouchard I, Carroll CS, Nesbitt JR et al (2012) Structural requirements for the activity of the MirB ferrisiderophore transporter of Aspergillus fumigatus. Eukaryot Cell 11:1333–1344. doi:10.1128/EC.00159-12
Reddy VS, Shlykov MA, Castillo R et al (2012) The major facilitator superfamily (MFS) revisited. FEBS J 279:2022–2035. doi:10.1111/j.1742-4658.2012.08588.x
Rees DC, Johnson E, Lewinson O (2009) ABC transporters: the power to change. Nat Rev Mol Cell Biol 10:218–227. doi:10.1038/nrm2646
Rodriguez GM, Smith I (2006) Identification of an ABC transporter required for iron acquisition and virulence in Mycobacterium tuberculosis. J Bacteriol 188:424–430. doi:10.1128/JB.188.2.424
Ryndak MB, Wang S, Smith I, Rodriguez GM (2010) The Mycobacterium tuberculosis high-affinity iron importer, IrtA, contains an FAD-binding domain. J Bacteriol 192:861–869. doi:10.1128/JB.00223-09
Saha R, Saha N, Donofrio RS, Bestervelt LL (2012) Microbial siderophores: a mini review. J Microbiol 52:1–15. doi:10.1002/jobm.201100552
Soe CZ, Codd R (2014) Unsaturated macrocyclic dihydroxamic acid siderophores produced by Shewanella putrefaciens using precursor-directed biosynthesis. ACS Chem Biol 9:945–956. doi:10.1021/cb400901j
Starr J, Brown MF, Aschenbrenner L et al (2014) Siderophore receptor-mediated uptake of lactivicin analogues in Gram-negative bacteria. J Med Chem 57:3845–3855. doi:10.1021/jm500219c
Sulochana MB, Jayachandra SY, Kumar SKA, Dayanand A (2014) Antifungal attributes of siderophore produced by the Pseudomonas aeruginosa JAS-25. J Basic Microbiol 54:418–424. doi:10.1002/jobm.201200770
Sutak R, Lesuisse E, Tachezy J, Richardson DR (2008) Crusade for iron: iron uptake in unicellular eukaryotes and its significance for virulence. Trends Microbiol 16:261–268. doi:10.1016/j.tim.2008.03.005
Sverzhinsky A, Fabre L, Cottreau AL et al (2014) Coordinated rearrangements between cytoplasmic and periplasmic domains of the membrane protein complex ExbB-ExbD of Escherichia coli. Structure 22:791–797. doi:10.1016/j.str.2014.02.010
Tong Y, Guo M (2009) Bacterial heme-transport proteins and their heme-coordination modes. Arch Biochem Biophys 481:1–15. doi:10.1016/j.abb.2008.10.013
Vega DE, Young KD (2013) Accumulation of periplasmic enterobactin impairs the growth and morphology of Escherichia coli tolC mutants. Mol Microbiol 91:508–521. doi:10.1111/mmi.12473
Wang S, Wu Y, Outten FW (2011) Fur and the novel regulator YqjI control transcription of the ferric reductase gene yqjH in Escherichia coli. J Bacteriol 193:563–574. doi:10.1128/JB.01062-10
Wang S, Blahut M, Wu Y et al (2014) Communication between binding sites is required for Yqji regulation of target promoters within the yqjH-yqjI intergenic region. J Bacteriol 196:3199–3207. doi:10.1128/JB.01835-14
Weinberg ED (2009) Iron availability and infection. Biochim Biophys Acta Gen Subj 1790:600–605. doi:10.1016/j.bbagen.2008.07.002
Wells RM, Jones CM, Xi Z et al (2013) Discovery of a siderophore export system essential for virulence of Mycobacterium tuberculosis. PLoS Pathog 1:e1003120. doi:10.1371/journal.ppat.1003120
Wencewicz TA, Miller MJ (2013) Biscatecholate–monohydroxamate mixed ligand siderophore–carbacephalosporin conjugates are selective sideromycin antibiotics that target Acinetobacter baumannii. J Med Chem 56:4044–4052. doi:10.1021/jm400265k
Wencewicz TA, Möllmann U, Long TE, Miller MJ (2009) Is drug release necessary for antimicrobial activity of siderophore-drug conjugates? Syntheses and biological studies of the naturally occurring salmycin “Trojan Horse” antibiotics and synthetic desferridanoxamine-antibiotic conjugates. Biometals 22:633–648. doi:10.1007/s10534-009-9218-3
Wencewicz TA, Long TE, Möllmann U, Miller MJ (2013) Trihydroxamate siderophore-fluoroquinolone conjugates are selective sideromycin antibiotics that target Staphylococcus aureus. Bioconjug Chem 24:473–486. doi:10.1021/bc300610f
Woo J-S, Zeltina A, Goetz BA, Locher KP (2012) X-ray structure of the Yersinia pestis heme transporter HmuUV. Nat Struct Mol Biol 19:1310–1315. doi:10.1038/nsmb.2417
Wurst JM, Drake EJ, Theriault JR et al (2014) Identification of inhibitors of PvdQ, an enzyme involved in the synthesis of the siderophore pyoverdine. ACS Chem Biol 9:1536–1544. doi:10.1021/cb5001586
Yan N (2015) Structural biology of the major facilitator superfamily transporters. Annu Rev Biophys 44:257–283. doi:10.1146/annurev-biophys-060414-033901
Zane HK, Naka H, Rosconi F et al (2014) Biosynthesis of amphi-enterobactin siderophores by Vibrio harveyi BAA-1116: identification of a bifunctional nonribosomal peptide synthetase condensation domain. J Am Chem Soc 136:5615–5618. doi:10.1021/ja5019942
Zeng X, Mo Y, Xu F, Lin J (2013) Identification and characterization of a periplasmic trilactone esterase, Cee, revealed unique features of ferric enterobactin acquisition in Campylobacter. Mol Microbiol 87:594–608. doi:10.1111/mmi.12118
Zheng T, Nolan EM (2014) Enterobactin-mediated delivery of b-lactam antibiotics enhances antibacterial activity against pathogenic Escherichia coli. J Am Chem Soc 136:9677–9691. doi:10.1021/ja503911p
Zheng T, Bullock JL, Nolan EM (2012) Siderophore-mediated cargo delivery to the cytoplasm of Escherichia coli and Pseudomonas aeruginosa: syntheses of monofunctionalized enterobactin scaffolds and evaluation of enterobactin-cargo conjugate uptake. J Am Chem Soc 134:18388–18400. doi:10.1021/ja3077268
Acknowledgments
We thank the National Science Foundation for supporting siderophore biosynthesis research in the Bruner lab (NSF-1411991, S.D.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Li, K., Chen, WH. & Bruner, S.D. Microbial siderophore-based iron assimilation and therapeutic applications. Biometals 29, 377–388 (2016). https://doi.org/10.1007/s10534-016-9935-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-016-9935-3