Abstract
Manganese (Mn), iron (Fe), zinc (Zn), and copper (Cu) are essential transitions metals that are required in trace amounts, however chronic exposure to high concentrations can cause severe and irreversible neurotoxicity. Since prolonged exposure to Mn leads to manganism, a disorder exhibiting a diverse array of neurological impairments progressing to a debilitating and irreversible extrapyramidal condition symptomatically similar to Parkinson’s disease, we measured the concentration of Mn as well as Fe, Zn and Cu in three region of the brain (globus pallidus, striatum and inferior colliculus) and three regions in the cochlea (stria vascularis, basilar membrane and modiolus) under normal conditions or after 30 or 60 days of oral administration of Mn (10 mg/ml ad libitum). Under normal conditions, Mn, Zn and Fe were typically higher in the cochlea than in the three brain regions whereas Cu was equal to or lower. Oral treatment with Mn for 30 or 60 days resulted in 20–75 % increases in Mn concentrations in both cochlea and brain samples, but had little effect on Cu and Fe levels. In contrast, Zn levels decreased (20–80 %) with Mn exposure. Our results show for the first time how prolonged oral Mn-ingestion affects the concentration of Mn, Cu, Zn and Fe, in the three regions of the cochlea, the inferior colliculus in auditory midbrain and the striatum and globus pallidus, two regions implicated in Parkinson’s disorder. The Mn-induced changes in the concentration of Mn, Cu, Zn and Fe may provide new insights relevant to the neurotoxicity of Mn and the transport and accumulation of these metals in cochlea and brain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Manganese (Mn) as well as iron (Fe), zinc (Zn) and copper (Cu) are essential transition metals that play critical roles in maintaining proper cell function. Mn in particular is used as a cofactor for a variety of enzymes, aids in maintaining mitochondrial function and integrity, promotes the regeneration of connective tissue and bone and assists in the metabolism of carbohydrates and lipids (Schroeder et al. 1966; Hurley 1981; Keen et al. 1999; Erikson et al. 2002; Jenkitkasemwong et al. 2012; Roth et al. 2013). These metals are normally required in trace amounts, however, chronic exposure to abnormally high concentrations can provoke severe and irreversible neurotoxicity (Aschner 1997; Erikson et al. 2002; Reaney et al. 2006). Prolonged exposure to Mn leads to a disorder called manganism characterized by a diverse array of neurological impairments (Mena et al. 1969; Huang et al. 1993; Olanow et al. 1996; Mergler and Baldwin 1997; Pal et al. 1999). Initial neurological symptoms include reduced response speed, irritability, intellectual deficits, mood changes, and compulsive behaviors, some of which are irreversible. Prolonged exposure to Mn leads to a progressively debilitating and irreversible extrapyramidal conditions symptomatically similar to Parkinson’s disease.
Mn toxicity often occurs as a result of excess exposure to environmental and occupational sources including mining, welding, ceramics, and steel production. Concerns over the toxic effects of Mn are also elevated because of the potential health risks associated with the anticipated increases in atmospheric levels due to the impending use of the gas additive methylcyclopentadienyl manganese tricarbonyl as well as other sources (Joly et al. 2011; Moreno et al. 2011). Besides the adverse effects observed on the central nervous system upon chronic exposure to Mn, studies have also demonstrated that Mn can influence structural and functional integrity of other systems as well. For example, several studies have reported that miners and welders who have been exposed to chronic high levels of Mn exhibit hearing deficits (Khalkova and Kostadinova 1986; Korczynski 2000; Josephs et al. 2005; da Silva et al. 2007; Bouchard et al. 2008). The potential adverse effects of Mn on hearing is supported by a study reporting that acute, high-dose Mn treatment promotes its accumulation in the rat inner ear (Ma et al. 2008). More importantly, micromolar concentration of Mn provoked significant damage to the sensory hair cells, peripheral auditory nerve fibers, and spiral ganglion neurons (SGN) in cochlear organotypic cultures in postnatal day three rats (Ding et al. 2011). Importantly, Mn was more toxic to SGN than to the sensory hair cells (Ding et al. 2011).
Because of the potential in vivo ototoxicity of Mn, it would be highly informative to determine which of the three main regions of the cochlea shows the greatest accumulation of Mn since the amount of uptake likely determines its ototoxic potential. Since high doses of Mn can induce Parkinson’s-like motor symptoms, it would be informative to compare the uptake of Mn in the striatum and globus pallidus with those in the cochlea and parts of the auditory components of the brain. In addition, Mn ingestion could affect the uptake, distribution and accumulation of other essential transition metals in both the cochlea and brain. Accordingly, we measured Mn, Fe, Zn and Cu levels in several different areas of the cochlea and brain in rats chronically exposed to high levels of Mn. To accomplish this, we used a sensitive analytical method for the detection of these trace metals by inductively coupled plasma mass spectrometry (ICP-MS), which we previously used to establish endogenous levels of these metals in rat ear tissue and several areas of the brain (Wegst-Uhrich et al. 2015).
Experimental procedures
Animals
Female SASCO® Sprague–Dawley Rats (63–67 days old) ranging in weight from 275 to 300 g were used for these experiments. Experiments were performed according to the rules and regulations of the Institutional Animal Care and Use Committee of the State University of New York at Buffalo and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Manganese treatment
Rats were provided drinking water ad libitum that contained 10 mg/ml MnCl2 for 30 and 60 days. The water was supplemented with Kool-Aid™ and 0.1–0.2 % saccharin to increase palatability and nullify the influence of caloric intake between the experimental groups. Water intake was measured every 2–3 days using a calibrated water bottle over the 30 or 60 day delivery period. The daily water intake of the adult rats was approximately 20 ml/day for a 300 g rat. Based on these values, it was estimated that total Mn intake was approximately 6.6 and 13.2 g for 30 and 60 day treatments, respectively. Control rats were provided drinking water containing only Kool-Aid™ and saccharin for 30 or 60 days.
Tissue harvesting
Rats were anesthetized with ketamine (40 mg/kg, i.p.) and xylazine (6 mg/kg, i.p.), then decapitated with a small guillotine. The bulla was rapidly removed under a dissection microscope and subsequently placed in phosphate buffered saline. The bony wall of the cochlea was opened. The basilar membrane (BM) containing the organ of Corti and hair cells, the spiral ligament including the stria vascularis (SV), and the soft tissue in the cochlear modiolus (M) containing the SGN, were carefully dissected out. Brains were rapidly removed from the skull and sliced in the coronal plane at 1 mm intervals using a rat brain slicer (Plastics One Inc.) on ice. Regions of interest including the inferior colliculus (IC), located in the auditory midbrain, the striatum (S) and globus pallidus (GP) were identified using Paxinos & Watson rat brain atlas, and separated using a biopsy punch (Glowinski and Iversen 1966). To minimize metal contamination, each component of the cochlea and brain was placed in a separate metal-free polypropylene centrifuge tube.
Solid sample digestion and metal analysis
Samples were digested and prepared for metal analysis by ICP-MS using a method previously developed and validated in our lab (Wegst-Uhrich et al. 2015). Tissue specific detection limits were calculated by multipoint standard addition using pooled tissue digests spiked at 4 levels (0, 2.5, 10 and 50 ng/ml). The levels were injected 7 times to produce a calibration curve and concentrations were corrected using the obtained endogenous levels. Extraction recoveries were evaluated in homogenized brain at three spiking levels: 10, 20 and 100 μg/g with n = 3 samples per level. As an analyte-free matrix could not be obtained, spiking values were in addition to the endogenous concentrations. Recoveries were corrected by subtracting the background endogenous levels. Values are reported as averages and standard errors for the recovery levels used. Mn and Cu values are reported for all three recovery levels, Fe values are reported at the 20 and 100 µg/g levels, and Zn recoveries are reported only for the 100 µg/g level. Detection limits and recoveries are summarized in Table 1.
Statistical analysis
The size of the samples from the cochlea were extremely small (mg) and therefore were potentially subject to more assay variability. Accordingly, outlying data points were removed using a Q-test at the 95 % confidence level. Statistical analysis was performed using SigmaPlot 12. Data were analyzed using a two-way analysis of variance (ANOVA) and Tukey post hoc test with significance at p < 0.05. Error values are reported as the standard error of the mean (SEM).
Results
Analysis of metals in Kool-Aid™
Initial studies were performed to evaluate the concentrations of Mn, Fe, Cu, and Zn present in the unspiked Kool-Aid™ samples and the data are presented in Table 2. As indicated, Mn, Fe, Cu, and Zn were all found to be naturally present in unspiked Kool-Aid™, though the levels were very low and are unlikely to interfere with the final concentrations in the tissues examined. Any potential interference from tap water on our relative measurements is negated by the fact that both the control and Mn-treated animals had access to the same water for all experiments.
Manganese accumulation in the cochlea and central nervous system (CNS)
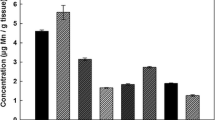
Mn concentrations were determined in three sections of the cochlea, the M, BM, and SV, and three regions of the brain, the S, GP, and IC, for control rats as well as rats exposed to Mn for 30 and 60 days. As indicated by the data in Fig. 1, the concentration of Mn (µg/g tissue dry weight) in the treated rats was greater in all cochlea specimens (M, BM, SV) in comparison to concentrations in the brain (S, GP, IC). The highest percent accumulation occurred in the BM followed by the SV and M, where the Mn concentration significantly increased by 73, 62, and 27 %, respectively, in the first 30 days of exposure. In the brain, significant accumulation was apparent in the S and IC, with concentration increases of 75 and 63 %, respectively, after 60 days of Mn exposure. Between 30 and 60 days of exposure, Mn further increased in the M by 20 %. Interestingly, a decrease in Mn concentration in the BM and SV was observed between the 30 and 60 day exposure. With the exception of the GP, there was a significant increase in Mn levels in all brain areas in animals treated with Mn for both the 30 and 60 days.
Mn concentrations (μg/g) present in the cochlea: M, BM, SV and brain: S, GP, IC in rats exposed to MnCl2 orally for 0 (control), 30 or 60 days. Error bars are reported as standard error (n ≥ 8). Asterisk indicates that the exposure is statistically different from the control. (†) indicates that the 60 day exposure is statistically different from the 30 day exposure
Iron in the cochlea and CNS
The concentrations of Fe (μg/g tissue dry weight) in the cochlea and brain tissue samples are presented in Fig. 2. Similar to Mn, the levels of Fe were higher in the cochlea than in the brain specimens though none of the increases were statistically significant. Unlike Mn however, the only statistically significant change observed over the course of the experiment was a decrease in Fe concentrations in the BM after Mn exposure. The rats subjected to a 60 day exposure had Fe concentrations 24 % lower than in the 30 day exposure; this was not statistically different from the control value.
Zinc in the cochlea and CNS
The concentrations of Zn in the analyzed tissue samples are shown in Fig. 3. Zn concentrations were found to be highest in the BM and SV; in these tissues there were significant decreases from the controls after the 30 and 60 day Mn treatments. Over the course of 60 days, the Zn concentration in the BM and SV decreased significantly by 86 and 83 %, respectively, compared to control values. There were no statistical significant changes in Zn levels in the M or any of the brain sections measured. Similar to Mn and Fe, Zn concentrations in the cochlear specimens were greater than in the brain samples analyzed.
Copper in the cochlea and CNS
Concentrations of Cu (μg/g dry weight tissue) in the cochlea and brain samples are presented in Fig. 4. There were no statistically significant differences in Cu levels between controls and Mn treated animals at any exposure time for the M, BM, S, GP, or IC. Cu levels were below detection limits for the 30 day BM samples but were measurable in the other tissue specimens assayed. However, it is notable that the levels of Cu in the SV for the 60 day Mn exposure were 59 % lower than that of the controls. Unlike the other metals, Cu levels in the brain specimens were higher than in the cochlear samples assayed.
Cu concentrations (μg/g) present in the cochlea: M, BM, SV and brain: S, GP, IC in rats exposed to MnCl2 orally for 0 (control), 30 or 60 days. Error bars are reported as standard error (n ≥ 4). Thirty day BM tissue concentrations were less than the limit of quantification, and therefore no data bar is present. (*) indicates that the exposure is statistically different from the control. (†) indicates that the 60 day exposure is statistically different from the 30 day exposure
Discussion
The net accumulation of metals in tissues is regulated by their relative rates of cellular uptake and export, as well as their overall capacity to sequester the metals within specific cellular components limiting their release into the surrounding interstitial space. Similar to other organs, metal uptake in the inner ear is dependent on a variety of membrane transporters present in the blood-cochlea barrier that subsequently facilitates their selective accumulation into the separate cellular components within the cochlea. Net uptake into the different cellular components of the cochlea is, accordingly, regulated by the distribution, quantity and affinity of the transporters for different metals. Since prior studies have demonstrated that the quantity of several metal transporters differ within the three distinct regions of the cochlea, it is important to evaluate how this partitioning affects their selective distribution in cochlear tissues (Ding et al. 2014) since this likely accounts for differential toxicity of different cellular structures within the cochlea by different metals.
Since many ototoxic metals are transported by the same membrane carrier, it is important to examine how an individual metal can influence the transport and distribution of other metals. In this paper, we examined how exposure to high levels of Mn affected the accumulation and distribution of Fe, Cu, and Zn as well as Mn itself. Common transporters for these metals include divalent metal transporter 1 (DMT1), ZIP8, and ZIP14 (Garrick et al. 2006; Jenkitkasemwong et al. 2012; Roth et al. 2013). Divalent Cu is transported by CRT1 though there is evidence that monovalent Cu is transported by DMT1 as well (Zheng et al. 2012; Lin et al. 2015). Prior studies have demonstrated that the levels of these metal carriers reside in distinct populations of cells within the inner ear. In regard to export proteins, ferroportin is a common export protein for Mn, Fe, and Zn, while Cu utilizes ATP7A and B (Gupta 2014; Kaler 2014). Prior studies have also revealed the selective distribution of both ATP7A and B in cells of the cochlea. Mn and Zn are also selectively exported by SLC30A10; gene mutations in this exporter can induce Mn neurotoxicity and results in symptoms similar to the dystonic movements associated with Parkinson’s disease (Quadri et al. 2012; Stamelou et al. 2012). The reason or need for the overlap in transporter activity is not immediately obvious, but most likely is necessary to maintain homeostatic metal levels in tissues possibly under various pathological conditions.
Consistent with the observation of the uneven distribution of these metal transporters within the different cellular components of the inner ear are recent findings indicating that endogenous levels of several metals are also uniquely distributed within the cochlea (Wegst-Uhrich et al. 2015). To determine how exposure to excess Mn would affect the levels of these metals in both the cochlea and brain, we treated rats orally with Mn in drinking water. Uptake of Mn in the intestines is expected to be, at best, around 5 % of that ingested dose (Davidsson et al. 1989; Johnson et al. 1991; Canonne-Hergaux et al. 1999). Transport of Mn across the intestinal microvilli into blood requires DMT1 for uptake and ferroportin for export.
For the current paper, accumulation of Mn, Zn, Fe and Cu was assessed in the inner ear which included the M where the SGN reside, the BM containing the sensory hair cells, and the SV which contains support cells responsible for the import and export of key ions and molecules into the endolymphatic fluid space. The metal concentrations were also assessed in the IC, located in the midbrain, as well as the S and GP in the CNS, the latter two areas being associated with the extrapyramidal effects of Mn. As anticipated, Mn treatment caused an increase in Mn levels in all of the measured cochlear tissues over the 60 day treatment with the greatest accumulation in the BM. The one exception was a small, but statistically significant, decrease in Mn between 30 and 60 days in the BM. The reason for this is not known but it is interesting to note that the 60 day BM samples also have decreased Fe levels. This is unlikely an artifact of the sampling technique because the levels of Zn and Cu did not display a similar loss of metal content considering that one sample preparation was used to measure all metals. This suggests that the uptake for Mn and Fe may be linked to similar transport carriers in the cochlea.
Mn treatment also affected the levels of Zn, Cu and Fe. For example, the levels of Zn in the BM and SV decreased over both 30 and 60 days of Mn treatment as compared to control values. The reason for the decrease in Zn is unclear, but is unlikely related to DMT1 which does not transport Zn. Since Mn is transported along with Zn by the two ZIP proteins, this suggests that Mn may effectively compete and inhibit Zn uptake for these transport carriers. Prior studies have actually suggested that both ZIP proteins may be the major transport protein in hair cells of the cochlea (Ding et al. 2014). Similar to Zn, Cu also decreased in the SV after 60 days of Mn treatment. Most interestingly, Cu levels in the BM decreased to below our detection limit (<28.0 μg/g) at 30 days of Mn treatment, but were measurable in both controls and at 60 days of Mn exposure. The only other anomaly was that Fe levels in the BM significantly decreased after 60 days of Mn treatment while all other Fe measurements appeared to be unaffected by Mn treatment for reasons that are unclear.
The effect of Mn exposure on Mn, Fe, Cu and Zn concentrations in the cochlea were compared to tissues in the CNS associated with the auditory pathway and extrapyramidal structures associated with Mn toxicity. The most significant finding in this study is that the concentrations of Mn, Fe, and Zn were higher in all ear sections relative to the brain sections, whereas Cu concentrations were lower. Levels of the four metals in the brain samples are distributed between several different cell populations including neurons, astrocytes, oligodendroglia and a small subset of cells within the intestinal space. Therefore, it was not possible to determine the actual levels of these metals in any given cell population. The brain contains all the machinery for uptake and export of all four metals as well as a selective distribution of intracellular proteins that can sequester any of the metals. For example, glutamine synthetase is an enzyme involved in synthesis of the excitatory neurotransmitter, glutamic acid, and is selectively located in astrocytes. A major portion of Mn sequestered in brain is bound to this enzyme which may contain up to eight Mn per octameric species It has been suggested to contain approximately 80 % of the Mn in brain (Sarkar et al. 1972; Wedler and Denman 1984). In regard to its localization in the inner ear, prior studies indicate that glutamine synthetase is localized in satellite glial cells surrounding primary auditory neurons in the spiral ganglion but was not detected in the cochlear nerve within the organ of Corti (Eybalin et al. 1996).
In summary, this study compares, for the first time, the levels of Mn, Fe, Zn and Cu in rats treated for 30 or 60 days with Mn; measurements were obtained for three tissue compartments in the cochlea, the IC in the auditory midbrain, the S and GP, portions of extrapyramidal motor pathways implicated with Mn toxicity. With the exception of Cu, all other metals were higher in the cochlea compared to the brain areas examined whereas Cu was the only metal that was measurably lower in the cochlear samples compared to brain areas examined. Exposure to high levels of Mn, increased Mn levels in both the cochlea and brain samples, but had little effect on the Cu and Fe levels in all tissues studied. In contrast, Mn treatment was associated with a decrease of Zn concentrations in the inner ear, but had little effect on Zn levels in brain, which is consistent with results from a prior study (Garcia et al. 2006). Results of this study will aid in clarifying the effect that exposure to elevated levels of Mn has on the accumulation and distribution patterns of the other essential metals in the cochlea and brain. Knowledge on how trace metal concentrations interact and alter the concentration within the cochlea and brain may provide new insights on the mechanisms of ototoxicity and neurotoxicity resulting from exogenous sources.
References
Aschner M (1997) Manganese neurotoxicity and oxidative damage. In: Connor JR (ed) Metals and oxidative damage in neurological disorders. Plenum, New York, pp 77–93
Bouchard M, Mergler D, Baldwin ME, Panisset M (2008) Manganese cumulative exposure and symptoms: a follow-up study of alloy workers. Neurotoxicology 29:577–583
Canonne-Hergaux F, Gruenheid S, Ponka P, Gros P (1999) Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood 93:4406–4417
da Silva CJ, da Rocha AJ, Jeronymo S et al (2007) A preliminary study revealing a new association in patients undergoing maintenance hemodialysis: manganism symptoms and T1 hyperintense changes in the basal ganglia. Am J Neuroradiol 28:1474–1479
Davidsson L, Cederblad A, Lonnerdal B, Sandstrom B (1989) Manganese retention in man: a method for estimating manganese absorption in man. Am J Clin Nutr 49:170–179
Ding D, Roth J, Salvi R (2011) Manganese is toxic to spiral ganglion neurons and hair cells in vitro. Neurotoxicology 32:233–241
Ding D, Roth J, Salvi R (2014) Cellular localization and developmental changes of Zip8, Zip14 and transferrin receptor 1 in the inner ear of rats. Biometals 27:731–744
Erikson KA, Shihabi ZK, Aschner JL, Aschner M (2002) Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biol Trace Elem Res 87:143–156
Eybalin M, Norenberg MD, Renard N (1996) Glutamine synthetase and glutamate metabolism in the guinea pig cochlea. Hear Res 101:93–101
Garcia SJ, Gellein K, Syversen T, Aschner M (2006) A manganese-enhanced diet alters brain metals and transporters in the developing rat. Toxicol Sci 92:516–525
Garrick MD, Singleton ST, Vargas F et al (2006) DMT1: which metals does it transport? Biol Res 39:79–85
Glowinski J, Iversen LL (1966) Regional studies of catecholamines in the rat brain-I. J Neurochem 13:655–669
Gupta S (2014) Cell therapy to remove excess copper in Wilson’s disease. Ann NY Acad Sci 1315:70–80
Huang CC, Lu CS, Chu NS, Hochberg F, Lillienfeld D, Olanow W, Calne DB (1993) Progression after chronic manganese exposure. Neurology 43:1479–1483
Hurley LS (1981) Teratogenic aspects of manganese, zinc, and copper nutrition. Physiol Rev 61:249–295
Jenkitkasemwong S, Wang CY, Mackenzie B, Knutson MD (2012) Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals 25:643–655
Johnson PE, Lykken GI, Korynta ED (1991) Absorption and biological half-life in humans of intrinsic and extrinsic 54Mn tracers from foods of plant origin. J Nutr 121:711–717
Joly A, Lambert J, Gagnon C, Kennedy G, Mergler D, Adam-Poupart A (2011) Reduced atmospheric manganese in Montreal following removal of methylcyclopentadienyl manganese tricarbonyl (MMT). Water Air Soil Pollut 219:263–270
Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, Cowl CT (2005) Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology 64:2033–2039
Kaler SG (2014) Translational research investigations on ATP7A: an important human copper ATPase. Ann NY Acad Sci 1314:64–68
Keen CL, Ensunsa JL, Watson MH, Baly DL, Donovan SM, Monaco MH, Clegg MS (1999) Nutritional aspects of manganese from experimental studies. Neurotoxicology 20:213–223
Khalkova Z, Kostadinova G (1986) Auditory-vestibular changes in workers in ferrous metallurgy manufacture. Probl Khig 11:134–138
Korczynski RE (2000) Occupational health concerns in the welding industry. Appl Occup Environ Hyg 15:936–945
Lin C, Zhang Z, Wang T, Chen C, James Kang Y (2015) Copper uptake by DMT1: a compensatory mechanism for CTR1 deficiency in human umbilical vein endothelial cells. Metallomics 7:1285–1289
Ma C, Schneider SN, Miller M et al (2008) Manganese accumulation in the mouse ear following systemic exposure. J Biochem Mol Toxicol 22:305–310
Mena I, Horiuchi K, Burke K, Cotzias GC (1969) Chronic manganese poisoning. Individual susceptibility and absorption of iron. Neurology 19:1000–1006
Mergler D, Baldwin M (1997) Early manifestations of manganese neurotoxicity in humans: an update. Environ Res 73:92–100
Moreno T, Pandolfi M, Querol X, Lavin J, Alastuey A, Viana M, Gibbons Q (2011) Manganese in the urban atmosphere: identifying anomalous concentrations and sources. Environ Sci Pollut Res 18:173–183
Olanow CW, Good PF, Shinotoh H et al (1996) Manganese intoxication in the rhesus monkey: a clinical, imaging, pathologic, and biochemical study. Neurology 46:492–498
Pal PK, Samii A, Calne DB (1999) Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology 20:227–238
Quadri M, Federico A, Zhao T et al (2012) Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am J Hum Genet 90:467–477
Reaney SH, Bench G, Smith DR (2006) Brain accumulation and toxicity of Mn(II) and Mn(III) exposures. Toxicol Sci 93:114–124
Roth J, Ponzoni S, Aschner M (2013) Manganese homeostasis and transport. Met Ions Life Sci 12:169–201
Sarkar P, Fischman D, Goldwasser E, Moscona A (1972) Isolation and characterization of glutamine synthetasefrom chicken neural retina. J Biol Chem 247:7743–7749
Schroeder HA, Balassa JJ, Tipton IH (1966) Essential trace metals in man: manganese. A study in homeostasis. J Chron Dis 19:545–571
Stamelou M, Tuschl K, Chong WK, Burroughs AK, Mills PB, Bhatia KP, Clayton PT (2012) Dystonia with brain manganese accumulation resulting from SLC30A10 mutations: a new treatable disorder. Movement Disord 27:1317–1322
Wedler F, Denman R (1984) Glutamine synthetase: the major Mn(II) enzyme in mammalian brain. Curr Top Cell Regul 24:153–169
Wegst-Uhrich SR, Mullin EJ, Ding D, Manohar S, Salvi R, Aga DS, Roth J (2015) Endogenous concentrations of biologically relevant metals in rat brain and cochlea determined by inductively coupled plasma mass spectrometry. Biometals 28:187–196
Zheng G, Chen J, Zheng W (2012) Relative contribution of CTR1 and DMT1 in copper transport by the blood-CSF barrier: implication in manganese-induced neurotoxicity. Toxicol Appl Pharmacol 260:285–293
Acknowledgments
Research supported by National Institute for Occupational Safety and Health (NIOSH) award #R010HH010311-01. We acknowledge the National Science Foundation Major Research Instrumentation Program CHE0959565 for the ICP-MS. We are grateful to the Colón lab for the use of the microbalance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors state that they have no conflicts of interest that affect the objectivity of this publication.
Rights and permissions
About this article
Cite this article
Mullin, E.J., Wegst-Uhrich, S.R., Ding, D. et al. Effect of manganese treatment on the accumulation on biologically relevant metals in rat cochlea and brain by inductively coupled plasma mass spectrometry. Biometals 28, 1009–1016 (2015). https://doi.org/10.1007/s10534-015-9885-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-015-9885-1