Abstract
As important carbon sinks, alpine wetlands on the Tibetan Plateau are undergoing severe degradation. To reveal warming-induced ecological shifts in alpine environments, this study determined soil nutrient contents, enzyme activities, absorption and fluorescence spectra and quadrupole time-of-flight mass spectra (metabolomes) of dissolved organic matter (DOM) and metagenomes based on short-term incubation (0 °C, 10 °C and 20 °C) of topsoil from alpine wetlands and meadows (degraded wetlands). Compared with meadows, wetlands had higher contents of soil DOM (dissolved organic carbon, dissolved organic nitrogen and dissolved phosphorous) and greater activities of hydrolases (β-glucosidase, cellobiohydrolase, β-N-acetylglucosaminidase and acid phosphatase), with those parameters all being highest at 20 °C in meadows and showing various dynamics in wetlands. Soil DOM in wetlands presented the lowest values of specific ultraviolet absorbances (SUVA254 and SUVA260) at 0 °C and the highest values at 10 °C, whereas the opposite was true in the meadows. Wetland soils had greater diversities of DOM molecular compositions and microbial communities, with warming gradually increasing the number of identified DOM compounds in meadows and decreasing the number of microbial species in both soils. Wetland soils had more Proteobacteria (44.2%) and Acidobacteria (21.1%) and fewer Actinobacteria (18.0%) than meadow soils and contained many temperature-sensitive archaea (which were abundant at 0 °C). Distance-based redundancy analysis and Procrustes analysis indicated the greater complexity of ecological responses in alpine wetlands, which may be attributed to the higher adaptive capacity of soil microbial communities. Our results suggest that both degradation and warming decrease soil DOM content and microbial activities in alpine wetlands, providing important references for alpine wetland conservation under current climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global climate change and its ecological consequences have received much attention over the past several decades (Parmesan and Yohe 2003; Trisos et al. 2020; Walther et al. 2002; Yuan et al. 2021a). As the third pole, the Tibetan Plateau is currently experiencing significantly greater warming than the global average (Ran et al. 2018). Many studies have shown that climate warming has a significant impact on alpine ecosystems on the Tibetan Plateau (Chen et al. 2013, 2014; Gao et al. 2016; Li et al. 2014; Wang et al. 2014; Zhang et al. 2016). The carbon dioxide (CO2) and methane (CH4) in alpine permafrost will gradually be released as global warming progresses, and refractory organic matter will decompose and then be released (Ran et al. 2018; Schuur et al. 2015; Wu et al. 2018). Warming directly accelerates all biogeochemical reactions and increases both the input and output of alpine ecosystems; however, owing to the complex interactions between temperature and other environmental factors (including moisture, soil physiochemical properties, substrate availability and microbial activities), the feedback of warming in alpine regions varies with latitude, altitude, topography and continentality (Donhauser and Frey 2018). Long-term warming will alter surface morphology, hydrothermal conditions and nutrient levels, leading to succession towards wetter or drier ecosystems on the Tibetan Plateau (Jin et al. 2020; Liu et al. 2017; Zheng et al. 2020).
The ecological responses of alpine ecosystems to warming are diverse due to the range and duration of warming and the diversity of ecosystems (Wang et al. 2014). Previous studies have shown that warming increases soil respiration and microbial activities and promotes the decomposition of organic matter (Romero-Olivares et al. 2017; Steinweg et al. 2013); however, when the temperature increases above the maximum activity, it leads to a decrease in enzyme activity and cell damage because of the strong selection pressure on the microbial community (Barcenas-Moreno et al. 2009; van Gestel et al. 2013). As high temperatures induce lysis of sensitive taxa and provide nutrients to survivors, the dynamics of nutrient responses to warming cannot be predicted from the kinetic properties of microbial activity and substrate consumption (Mooshammer et al. 2017). The proposed mechanisms underlying warming include the depletion of labile soil organic matter (SOM), the enrichment of plant-derived residues and the stabilization of microbial necromass (Lehmann and Kleber 2015). In addition, Wang et al. (2014) reported that experimental warming led to a decrease in soil moisture in alpine meadows on the Tibetan Plateau, which had a more profound effect on alpine ecosystems.
Alpine wetlands and meadows have much higher SOM densities (69.5 and 59.3 kg C/m2, respectively) than the global average (10.40 kg C/m2) (Luan et al. 2014; Ma et al. 2016). The slow decomposition rate of biological residues at low temperatures leads to the accumulation of SOM in alpine wetlands. However, the alpine wetlands on the Tibetan Plateau have experienced severe degradation in recent years (Gu et al. 2019; Iqbal et al. 2019; Kang et al. 2016), with the total area decreasing by 28.97% from 2000 to 2014 (Wu et al. 2016) and the SOM content decreasing by 15% from 1966 to 2016 (Li et al. 2021). Generally, the successional sequence during alpine wetland degradation is from swamp to meadow, steppe and desert (Gu et al. 2018; Li et al. 2022). Numerous studies focusing on alpine wetlands have shown that plant biomass, soil organic C and organic N decrease after alpine wetland degradation, which may weaken the soil C sink effect and enhance the effect of climate warming (Gao et al. 2016; Huo et al. 2013). Li et al. (2019) reported that the loss of soil nutrients in alpine meadows under short-term warming was reduced by the responses of soil microbial communities. However, insufficient attention has been given to microbial community function and specific response mechanisms in alpine ecosystems.
Recently developed omics methods have enabled more in-depth exploration of the specific mechanisms underlying environmental nutrient migration and transformation in various alpine ecosystems (Overy et al. 2021). Yun et al. (2021) investigated methane oxidation in alpine wetlands via DNA stable isotope probing (DNA-SIP) metagenomics and methanotroph metagenome-assembled genomes (MAGs). Yuan et al. (2021b) compared soil carbon dynamics and related plant‒microbe regulations to warming in two alpine swamp meadows on the basis of solid-state 13C NMR spectroscopy and high-throughput sequencing. D'Alò et al. (2023) investigated the responses of soil archaea to long-term warming in alpine grasslands and snow-beds via combining metagenomics and metatranscriptomics. Using metabolomics and metagenomics, Zhang et al. (2022) reported that an increase in the alpine elevation gradient decreased the molecular diversity of soil dissolved organic matter (DOM) and the abundance and diversity of soil bacterial and fungal communities, and Zhou et al. (2023) simulated the warming effect on the depth-dependent responses of soil DOM molecular composition and microbial composition in alpine meadows. However, insufficient attention has been given to the application of omics methods to compare warming responses in alpine wetlands and meadows, and efficiently integrating multi-omics data in environmental studies remains challenging.
The objective of this study was to reveal and explain the ecological responses of alpine soils on the Tibetan Plateau to wetland degradation and short-term warming. In this study, we collected the topsoil of alpine wetlands and meadows (degraded wetlands) for short-term laboratory incubation at different temperatures (0 °C, 10 °C and 20 °C) and then analyzed the contents of soil nutrients, the activities of soil enzymes, the absorption and fluorescence spectra of soil DOM, the quadrupole time-of-flight mass spectra (metabolomes) of soil DOM, and the structure and function of soil microbial communities (metagenomes), as well as their relationships and similar or diverse responses in alpine wetlands and meadows. Our study aimed to reveal the potential threats of environmental changes to alpine wetlands (such as declines or increases in soil nutrients and microbial activities) based on the dynamics of those biochemical indicators and to provide important references for the conservation of alpine ecosystems as well as the application of multi-omics techniques in environmental research.
Materials and methods
Site description and sampling
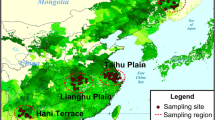
The study area is located between the southeastern Tibetan Plateau and the Sichuan Basin (30° 30′–33° 30′ N, 100° 30′–103° 30′ E; Fig. 1a). This area has a continental plateau climate with an average altitude of 4000 m, an average annual temperature of 0–2 °C, and 800 mm of precipitation. The flat terrain and meandering river channels formed large swamp wetlands, and after a long evolutionary process, typical alpine habitats of wetlands, peat, and meadow soil formed. In August 2021, soil samples were collected from alpine wetlands and meadows in Longriba Prairie, located in Hongyuan County, Aba Prefecture, Sichuan Province, China (32° 32′44.13′′ N, 102° 21′34.49′′ E, 3520 m, Fig. 1b). Wetland samples were collected from peat mounds next to flooded soil (Fig. 1c), and meadow samples were collected from arid soils adjacent (within 200 m) to the wetland samples (Fig. 1d). Topsoil samples (0–20 cm for wetlands and 0–15 cm for meadows) were collected using the five-point sampling method with a hoe and sealed in Ziploc bags, and then transported at 4 °C to the laboratory for further analysis and incubation.
Incubation experiment
After the coarse grass roots were removed, the soil samples were backfilled into 100 × 63.7 mm (500 cm3) cutting rings for short-term (60 days) laboratory incubation. The monthly temperature in the study area is lowest in January (− 10 °C) and highest in July and August (10 °C), with a mean annual temperature of 0–2 °C. Accordingly, we set three temperature gradients: a mean annual temperature of 0 °C (L), a maximum monthly temperature of 10 °C (M), and a simulated warming scenario of 20 °C (H), as the maximum ambient temperature in the study area exceeds 20 °C every year (reaching 22–26 °C). Shading and air circulation were maintained during incubation, and the soil moisture was adjusted to 70% of the saturated water content (SWC, mass ratio; 565% for wetland and 52% for meadow). There were 6 experimental conditions in total (W-L, W-M, W-H, M-L, M-M and M-H), and each treatment included 3 replicates.
Soil physicochemical properties
The soil pH was measured via a PHS-25 (Shanghai Leici Sensor Technology, China). Before incubation, the total carbon (TC), total nitrogen (TN) and total phosphorus (TP) contents of the soil samples were measured after drying at 105 °C. TC and TN were measured using the combustion method via a Vario DOC analyzer (Elementar, Germany) in solid sample measurement mode, and TP was measured by inductively coupled plasma–atomic emission spectroscopy (ICP‒AES, Thermo Jarrell Ash, USA) after the soil was digested with H2SO4 and HClO4.
After incubation, the soil DOM was extracted according to Jones and Willett (2006). The mixtures of fresh soil (10 g) and water (100 mL) were shaken at 200 r/min for 1 h, and then the suspensions were centrifuged at 8000 × g for 10 min and filtered through precombusted (450 °C) 0.22 μm glass fiber filters (Shanghai Xingya Purification Material Factory, China). The DOM solutions were stored at 4 °C for further analyses (completed within 5 days). Dissolved organic carbon (DOC) and dissolved organic nitrogen (DON) were measured via a Vario DOC analyzer (Elementar, Germany) in liquid sample measurement mode, and dissolved phosphorus (DP) was measured via an ICP‒AES (Thermo Jarrell Ash, USA).
Soil enzyme activities
The determination of enzyme activities was conducted within 2 days after the whole incubation period, with the soils stored under previous incubation conditions before the assays. According to DeForest (2009), the activities of β-glucosidase (βG, EC 3.2.1.21), cellobiohydrolase (CBH, EC 3.2.1.91), β-N-acetylglucosaminidase (NAG, EC 3.2.1.14) and acid phosphatase (AP, EC 3.1.3.2) were measured fluorometrically after soil samples were incubated in black 96-well microplates (Greiner, Germany) with 4-methylumbelliferon (MUB)-linked substrates (Table S1), and the activity of peroxidase (Perox, EC 1.11.1.7) was measured spectroscopically after soil samples were incubated in clear 96-well microplates (Greiner, Germany) with L-dihydroxyphenylalanine (L-DOPA) as the substrate. Before measurement, acetate buffer (50 mM) was newly made and adjusted to the mean pH value (6.0) of two soils with HCl, and soil slurry (1.0 g of fresh soil mixed with 125 mL of buffer) was homogenized using a magnetic stir bar for 1 h before being dispensed.
The dispensing procedures for the four hydrolases (βG, CBH, NAG and AP) were 250 μL of buffer (blank), 200 μL of buffer and 50 μL of 10-μM MUB solution (reference standard) or 50 μL of 200-μM MUB-linked substrate (negative control), 50 μL of buffer and 200 μL of soil slurry (sample control), 200 μL of soil slurry and 50 μL of 10-μM MUB solution (quench standard) or 50 μL of 200-μM MUB-linked substrate (sample assay). The dispensing procedures for Perox were 250 μL of buffer (blank), 200 μL of buffer and 50 μL of 5-mM L-DOPA solution (negative control), 200 μL of soil slurry and 50 μL of buffer (sample control) or 50 μL of 5-mM L-DOPA solution (sample assay), and 10 μL of 0.3% H2O2 solution (all wells). There were 8 replicate wells for each condition. All reactions were stopped by adding 10 μL of newly-made 1.0 M NaOH solution after incubation in the dark for 4 h (four hydrolases) or 18 h (Perox), and then immediately (within 1 min) measuring fluorescence (excitation wavelength = 365 nm, emission wavelength = 450 nm) or absorbance (wavelength = 450 nm) via a SpectraMax iD3 microplate reader (Molecular Devices, USA).
Spectra of soil DOM
The chromophoric DOM (CDOM) and fluorescent DOM (FDOM) of alpine soils were characterized via UV–Vis absorption spectroscopy (UV–Vis ABS) and three-dimensional excitation-emission matrix fluorescence spectroscopy (3D-EEM), respectively. Spectral determination was conducted via an Aqualog® spectrometer (Horiba Scientific, USA) using Millipore ultrapure water as a blank. The scanning range for UV–Vis ABS was 240–550 nm, with an interval of 5 nm and an optical path of 10 mm. The excitation (Ex) and emission (Em) wavelengths for 3D-EEM were 240–550 nm and 280–620 nm, respectively, with an interval of 3 nm and a slit width of 2.5 nm, and Raman and Rayleigh scattering were removed in the measurement system. Representative spectral parameters of UV–Vis ABS (a355, SR, SUVA254 and SUVA260) and 3D-EEM (fluorescence intensity (FI), biological index (BIX), Fn355 and β:α) were used to characterize soil DOM (Table S2). The fluorescence region integration (FRI) was used to quantify the fluorescence intensity of five fractions of 3D-EEM (Table S3), representing aromatic protein I (tyrosine-like) (peak I), aromatic protein II (tryptophan-like) (peak II), fulvic acid-like (peak III), soluble microbial byproduct-like (peak IV), and humic acid-like (peak V).
Metabolomes of soil DOM
The soil DOM was enriched via solid-phase extraction (SPE) before metabolomic analysis. First, the DOM solution was adjusted to pH = 2 using 0.1 mol/mL HCl. Second, a Bond Elut PPL cartridge (100 mg, 3 mg; Agilent Technologies) was activated with 3 mL of methanol and 3 mL of acidic water (pH = 2), and 100 mL of acidified solution was injected into the cartridge for DOM extraction. Then, 6 mL of acidic water was added to remove impurities, and the cartridge was dried with a vacuum pump. Finally, 3 mL of methanol was added to elute the DOM at a rate lower than 2 mL/min. The eluent was kept in chromatography bottles and stored at − 20 °C until analysis.
Non-targeted metabolomics was performed using high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (HPLC-Q-TOF-MS/MS; Agilent 1290–6545, USA) with an RRHD Eclipse Plus C18 column (Agilent, 1.8 μm × 2.1 mm × 50 mm, USA). Determination was performed in both positive (ESI +) and negative electrospray ionization (ESI −) modes, with a column temperature of 40 °C, an injection volume of 5 μL, an acquisition range of 50–1000 m/z, and a mobile phase flow rate of 0.4 mL·min−1. The elution gradient for the ESI + mode (phase A: 0.1% formic acid, phase B: 100% acetonitrile) was as follows: 0–15 min 3–90% B, 15–20 min 90% B, 20–21 min 90–3% B, 21–25 min 3% B. The elution gradient for the ESI − mode (phase A: 10-mM ammonium acetate, phase B: 100% acetonitrile) was as follows: 0–15 min 3–90% B, 15–20 min 90% B, 20–21 min 90–3% B, 21–25 min 3% B.
The raw data were pre-processed before analysis, which included the removal of low-quality features, the filling of missing values, and data normalization. Molecular feature extraction and alignment were performed using Profinder v10.0 software, and then, Mass Profiler Professional v15.1 software was used for metabolomic analysis. The components were identified via the Metabolite Link (METLIN) database (https://metlin.scripps.edu). The van Krevelen diagrams and bar graphs were used to show the characteristics and classification (Zhang et al. 2022) of the identified compounds. Venn diagrams and ternary plots showing the distributions of the compounds at different temperatures. Principal component analysis (PCA) and significance tests between different soils (unpaired t test) and different temperatures (one-way analysis of variance) were conducted in Mass Profiler Professional v15.1 software.
Metagenomic sequencing and analysis
The soils were frozen with liquid nitrogen immediately after sampling and then stored at − 80 °C until analysis. DNA was extracted using a FastDNA® Spin Kit for Soil (MP Biomedicals, USA); then, the purity and concentration were detected by a NanoDrop2000 and TBS-380, and the integrity was determined by 1% agarose gel electrophoresis. After the DNA was fragmented to 400 bp by a Covaris M220 sonicator (Gene Company Limited, China), a paired-end (PE) library was constructed using an EXTFLEX® Rapid DNA-Seq Kit (Bioo Scientific, USA). After bridge PCR amplification, metagenomic determination was performed using the Illumina NovaSeq sequencing platform following the platform instructions.
The raw data were qualified using Fastq v0.20.0 (Chen et al. 2018) and assembled using MEGAHIT v1.1.2 (Li et al. 2015) to obtain long contigs (≥ 300 bp). Prodigal was used to predict open reading frames (ORFs) for long contigs and to select genes (≥ 100 bp) for translation into amino acid sequences (Hyatt et al. 2010). The sequences were subsequently clustered using CD-HIT v4.6 (Fu et al. 2012; Zhang et al. 2015). Representative sequences were selected to construct a nonredundant gene catalogue, and high-quality sequences in the samples were aligned with the nonredundant gene set and counted using SOA Paligner v2.21 (Li et al. 2008). Using Diamond v0.8.35 (Buchfink et al. 2015), the nonredundant gene catalogue sequences were aligned with the NR database (BLASTP; E-value ≤ 1e-5) and counted at each taxonomic level (from domain to species); with the evolutionary genealogy of genes: Non-supervised Orthologous Groups (eggNOG) database (BLASTP; E-value ≤ 1e-5) and counted at different functional categories and Cluster of Orthologous Groups of proteins (COGs); with the Kyoto Encyclopedia of Genes and Genomes (KEGG) GENES database (BLASTP; E-value ≤ 1e-5) and then further annotated with different KEGG pathways (from level 1 to level 3) using the KEGG Orthology Based Annotation System (KOBAS) v2.0.
After processing and annotating the raw sequence data, metagenomic analyses were performed using the Majorbio Cloud Platform (www.majorbio.com). Venn diagrams and bar graphs were generated to show the composition of the soil microbial communities. Statistical analysis of metagenomic profiles (STAMP) (Parks et al. 2014) and linear discriminant analysis effect size (LEfSe) (Chang et al. 2022) were used for statistical tests of differences between groups (p < 0.05 indicates significant differences). Ternary plots were generated to show the distributions of significantly changed species at different temperatures. Principal coordinate analysis (PcoA) and distance-based redundancy analysis (db-RDA) based on the Bray–Curtis distance algorithm were used to show the distributions of soil microbial communities in different samples and the effects of soil nutrient contents and enzyme activities on them, respectively. Procrustes analysis was used for consistency analysis between the metagenomics and metabolomics data.
Statistical analyses
The schematic diagram of the study area was drawn in ArcMap 10.8. The 3D-EEM spectra, box diagrams, ternary plots, van Krevelen diagrams and bar graphs were drawn in Origin 9. Statistical tests and calculations for the Pearson correlation coefficient of soil nutrients, enzyme activities and spectral parameters were performed in SPSS 21, with p < 0.05 indicating significant differences between two soils (unpaired t test) or different incubation temperatures (one-way analysis of variance). Data statistics were organized in Excel. Other statistical analyses of the soil metabolomes and metagenomes were performed using Mass Profiler Professional v15.1 or the Majorbio Cloud Platform (www.majorbio.com), as described in Sects. "Spectra of soil DOM" and "Metabolomes of soil DOM".
Results
Contents of soil nutrients and activities of soil enzymes
The dynamics of soil nutrients and enzyme activities revealed distinct responses of alpine wetlands and meadows to short-term incubation at different temperatures. In this study, the average pH was 6.3 ± 0.1 in the wetland soils and 5.6 ± 0.1 in the meadow soils. The wetland soils had higher TC (69.4 ± 2.0 g/kg) and TN (4.59 ± 0.10 g/kg) contents than did meadow soils (TC = 32.3 ± 0.8 g/kg, TN = 2.77 ± 0.09 g/kg), whereas the soil TP content in the wetlands (0.21 ± 0.01 g/kg) was lower than that in the meadows (0.64 ± 0.02 g/kg). The soil DOC, DON and DP contents were all significantly (p < 0.05) greater in the wetlands than in the meadows (Table 1). The DOC content decreased with incubation temperature in the wetlands but increased with incubation temperature in the meadows, although statistical tests showed that the differences among the three groups were not significant (p > 0.05). The DON content significantly (p < 0.05) increased with incubation temperature in the wetlands, and it was significantly (p < 0.05) highest at 20 ℃, moderate at 0 ℃ and lowest at 10 ℃ in the meadows. In addition, the DP content was significantly (p < 0.05) lowest at 0 °C in both the wetlands and meadows.
The soil enzyme activities at different incubation temperatures are shown in Table 1. Statistical tests revealed that the activities of hydrolases (βG, CBH, NAG and AP) were significantly (p < 0.05) higher in the wetlands than in the meadows, whereas Perox activity did not significantly differ between the wetlands and meadows (p > 0.05). Temperature changes significantly (p < 0.05) influenced the activities of all the enzymes, and the responses of these enzymes varied between the wetlands and meadows. The activities of βG, CBH, NAG and AP were significantly (p < 0.05) highest at 20 °C in the meadows, whereas they exhibited different patterns in the wetlands, with βG and NAG activities being highest at 0 °C, CBH activity being highest at 10 °C, and AP activity being highest at 0 °C and 20 °C and lowest at 10 °C. In addition, Perox activity was significantly (p < 0.05) highest at 0 °C in both wetlands and meadows, and it was significantly (p < 0.05) lowest at 20 °C in the meadows.
In conclusion, alpine wetlands presented higher contents of most soil nutrients (TC, TN, DOC, DON and DP), greater activities of hydrolases (βG, CBH, NAG and AP) and greater variability in dissolved nutrients in response to short-term warming (DOC, DON and DP being highest at different temperatures) than did alpine meadows. The wetland soils had the highest DOC content and βG and NAG activities at 0 °C, whereas the meadow soils had the highest DOC, DON and DP contents and βG, CBH, NAG and AP activities at 20 °C after incubation.
Spectral and molecular characteristics of soil DOM
The spectral parameters of soil DOM are shown in Table 2. The results showed that both alpine soils had high terrestrial inputs (FI < 1.4 and BIX < 0.5). Statistical tests revealed that wetland soils had significantly (p < 0.05) higher relative abundances of CDOM (a355) and humus-like components (Fn355), whereas soil DOM in the meadows had significantly (p < 0.05) higher proportions of newly generated components (β:α) as well as higher aromaticity (SUVA254), hydrophobicity (SUVA260) and molecular weight (SR). The 3D-EEM spectra of alpine wetlands (Fig. 2a) and meadows (Fig. 2b) show the fluorescent characteristics of soil DOM. The intensities of peaks II–V in alpine wetlands were significantly (p < 0.05) higher than those in alpine meadows were, and wetland soils had a higher percentage of peak II and a lower percentage of peak V than meadow soils (Fig. 2c). After short-term incubation, wetland soils had the significantly (p < 0.05) highest SUVA254, SUVA260 and the intensity and percentage of peak II at the middle temperature of 10 ℃, whereas meadow soils had the significantly (p < 0.05) highest a355, SUVA254, SUVA260, Fn355, intensities of peaks III–V and percentage of peak IV at the lowest temperature of 0 ℃.
Three-dimensional excitation-emission matrix (3D-EEM) fluorescence spectra of soil DOM in alpine a wetlands and b meadows and c the percentages of five peaks in the fluorescence region integration (FRI) of 3D-EEM. Peak I: aromatic protein I (tyrosine-like), Peak II: aromatic protein II (tryptophan-like), Peak III: fulvic acid-like component, Peak IV: soluble microbial byproduct-like component, Peak V: humic acid-like component
The molecular dynamics of soil DOM in alpine wetlands and meadows were revealed by HPLC-Q-TOF-MS/MS. A total of 1511 compounds were identified (Table S4), with 92.6% shared by wetlands and meadows, 5.8% unique to wetlands and 1.6% unique to meadows. Statistical analysis revealed that 322 compounds had significantly (p < 0.05) higher abundances in the wetlands, whereas 284 compounds had significantly (p < 0.05) higher abundances in the meadows. The van Krevelen diagram (Fig. S1a) and classification (Fig. S1b) showed the molecular characteristics of these compounds, and PCA (Fig. S2) indicated the separate clustering of wetlands and meadows and weaker clustering at each temperature. Venn diagrams (Fig. 3a, b) and ternary plots (Fig. 3c, d) show the shared and unique compounds as well as the distributions of their relative abundances at different temperatures. Alpine wetlands had the highest number of identified compounds at 10 °C and few unique compounds at all temperatures, whereas the numbers of identified and unique compounds both increased with increasing incubation temperature in alpine meadows. Statistical tests revealed that 33 compounds in the wetlands varied significantly (p < 0.05), among which 20 had the highest abundance at 20 °C, whereas 54 compounds in the meadows varied significantly (p < 0.05), among which 33 had the highest abundance at 0 °C.
a, b Venn diagrams and c, d ternary plots of identified soil DOM compounds at different temperatures in alpine wetlands and meadows. W-L, W-M and W-H represent wetland soils incubated at 0 °C, 10 °C and 20 °C, respectively. M-L, M-M and M-H represent meadow soils incubated at 0 °C, 10 °C and 20 °C, respectively (the same below)
In conclusion, soil DOM in alpine wetlands and meadows presented distinct spectral and molecular characteristics, with warming gradually increasing the identified DOM compounds only in alpine meadows. The aromaticity and hydrophobic components (SUVA254 and SUVA260) of soil DOM were significantly higher in alpine meadows, and they were significantly highest at 10 ℃ in the wetland soils but at 0 ℃ in the meadow soils after short-term incubation.
Composition and function of soil microbial communities
The composition and function of soil microbial communities linked the responses of alpine nutrient dynamics to environmental changes via microbial activities. A total of 28,329 and 23,078 microbial species were found in the wetlands (containing 99.2% bacteria, 0.6% archaea and 0.1% eukaryotes) and meadows (containing 99.6% bacteria, 0.2% archaea and 0.1% eukaryotes), respectively. Wetlands and meadows shared 78.7% of those species, with the remaining 21.3% unique to wetlands and 4.0% unique to meadows. The α-diversity (Shannon-index = 5.72 ± 0.03, Simpson-index = 0.036 ± 0.002) and evenness (Pielou-index = 0.572 ± 0.003) of soil microbial communities in alpine wetlands were significantly (p < 0.05) higher than those in the meadows (Shannon-index = 5.19 ± 0.02, Simpson-index = 0.045 ± 0.001, Pielou-index = 0.533 ± 0.002). At the phylum level, Proteobacteria and Acidobacteria were significantly (p < 0.05) more abundant in the wetlands (accounting for 44.2% and 21.1%, respectively) (Fig. 4), whereas Actinobacteria were significantly more abundant in the meadows (accounting for 29.8%). The functional composition based on the eggNOG (Fig. S3) and KEGG pathway (Fig. S4) databases revealed that wetlands had greater abundances in the “T: signal transduction mechanisms” function, “M: cell wall/membrane/envelope biogenesis” function, “signal transduction” pathway (at level 2, the same below) and “cell motility” pathway, whereas meadows had greater abundances in the “L: replication, recombination and repair” function, “K: transcription” function, “carbohydrate metabolism” pathway and “cellular community-prokaryotes” pathway.
Short-term incubation changed the composition of the microbial communities in alpine soils. The α-diversity and evenness of the microbial communities were lowest at the middle temperature of 10 °C in both the wetlands and meadows and highest at 20 °C in the wetlands and 0 °C in the meadows (Table S5), although these differences were not significant (p > 0.05). Venn diagrams revealed that 81.1% of soil microbial species in the wetlands (Fig. 5a) and 77.9% of those in the meadows (Fig. 5b) were shared at all temperatures, with the highest number of species found at 0 °C (followed by 10 °C and finally 20 °C) in both soils. The ternary plots of significantly (p < 0.05) changed species showed that wetland soils (Fig. 5c) had more Proteobacteria at 0 °C and Actinobacteria and Thaumarchaeota at 20 °C, whereas meadow soils (Fig. 5d) had more Actinobacteria at 0 °C and Bacteroidetes at 20 °C, with many species of Firmicutes changing with temperature in both wetlands and meadows. LEfSe visualized the significantly changed (p < 0.05) bacteria and archaea in the wetlands (Fig. S5a, b) and meadows (Fig. S5c, d) from phylum to species level, indicated that wetland soils contained many temperature-sensitive archaea, with many species of Euryarchaeota (Methanomicrobia at the class level) presenting the highest abundance at 0 °C and many species of Thaumarchaeota presenting the highest abundance at 20 °C.
a, b Venn diagrams of all microbial species and c, d ternary plots of significantly (p < 0.05) changed microbial species at different temperatures in alpine wetlands and meadows. Statistical tests are performed using Kruskal‒Wallis test, with different colours in ternary plots indicating the phyla that those species belong to
Temperature changes also influence the functions of soil microbial communities in the alpine wetlands and meadows. Statistical tests showed that two functions and four pathways (at level 2, the same below) in the wetlands (Fig. 6a–f) changed significantly (p < 0.05), many of which increased in abundance with increasing incubation temperature, except for the “environmental adaptation” pathway, which showed the opposite trend. Four functions and one pathway in the meadows (Fig. 6g–k) varied significantly (p < 0.05) at the different temperatures, with the functions “T: signal transduction”, “H: coenzyme transport and metabolism” and “F: nucleotide transport and metabolism” having the lowest abundance at 0 °C and the “L: replication, recombination and repair” function and “signal transduction” pathway having the highest abundance at 0 °C. In addition, although most of the pathways (at level 3, the same below) were found at all three temperatures, 7 pathways in the wetlands and 13 pathways in the meadows were found only at specific temperatures (Table S6), including the “phototransduction” pathway (KEGG ID: ko04744) in the wetlands and two pathways in “glycan biosynthesis and metabolism” (KEGG ID: ko00601 and ko00563) in the meadows, which were absent at 0 °C.
Functions and pathways that significantly (p < 0.05) changed at different incubation temperatures in alpine a–f wetlands and g–k meadows. Functions and pathways (at level 2) are annotated based on the evolutionary genealogy of genes: Nonsupervised Orthologous Groups (eggNOG) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway databases, respectively
In conclusion, soil microbial communities in alpine wetlands presented higher α-diversity than those in alpine meadows did, and their composition and function in the two alpine soils responded to short-term warming at different scales. Warming gradually decreased the number of microbial species in both alpine soils, significantly decreasing the number of many species in Proteobacteria but increasing many species in Thaumarchaeota in alpine wetlands.
Integrated analysis of soil nutrients, enzyme activities and microbial communities
The relationships between soil nutrients, enzyme activities and spectral parameters in all alpine soils (Table S7), wetland soils (Table S8) and meadow soils (Table S9) were indicated by the Pearson correlation coefficient. The results showed that some parameters were always significantly (p < 0.05) positively (a355 and Fn355, SUVA254 and SUVA260, BIX and β:α) or negatively (a355 and SR) correlated with each other. After incubation, DP content and βG activity in alpine wetlands (r = − 0.728) and DON content and CBH activity in alpine meadows (r = 0.875) were significantly correlated. SUVA254 and SUVA260 were significantly positively (r > 0.68) correlated with DP content, BIX, a355 and Fn355 but negatively (r < − 0.85) correlated with βG activity and SR in alpine wetlands, whereas they were significantly negatively (r < − 0.82) correlated with DP content but positively (r > 0.75) correlated with a355 and Fn355 in alpine meadows. In wetland soils, βG and AP activities positively (r > 0.71) influenced SR and negatively (r < − 0.62) influenced a355, Fn355 and intensities of fluorescence peaks, whereas BIX and β:α were significantly positively (r > 0.68) correlated with DON and DP contents and negatively (r < − 0.67) correlated with DOC content. In meadow soils, Fn355 was significantly negatively (r < − 0.71) correlated with DOC and DP contents and CBH activity, and the intensities of fluorescence peaks were generally negatively correlated with hydrolase activities, whereas the intensity of peak IV was significantly positively (r = 0.674) correlated with peroxidase activity.
Soil nutrient and enzyme dynamics are closely related to soil microbial communities. The db-RDA (Fig. 7a) revealed differences in soil microbial communities between alpine wetlands and meadows (separate clustering of wetland and meadow samples, same as in the PCoA, Fig. S6) and indicated that soil nutrients (DOC, DON and DP) were the most important parameters influencing the distribution of soil microbial communities (R2 = 0.49–0.73, p < 0.01; Table S10). After short-term incubation, soil samples at different temperatures clustered separately, with samples from the W-M (Fig. 7b) and M-H (Fig. 7c) treatments showing greater variability. The influence of the DP content in the wetlands (R2 = 0.606, p = 0.053; Table S11) and DON content in the meadows (R2 = 0.503, p = 0.078; Table S12) was greater than that of other environmental parameters. The db-RDA also indicated the relationships between soil nutrients and enzyme activities, with Perox activity strongly negatively correlated with soil DP content in both alpine wetlands (Fig. 7b) and meadows (Fig. 7c), which was consistent with the results of the Pearson correlation coefficient (Table S8–S9).
Distance-based redundancy analysis between soil microbial communities and environmental parameters in a two alpine soils and different groups in alpine b wetlands and c meadows. Procrustes analysis between metagenomes and metabolomes in d two alpine soils and different groups in alpine e wetlands and f meadows, with a lower M2 value representing a higher consistency between the two datasets
There were also close relationships between the molecular composition of soil DOM and soil microbial communities. Procrustes analysis (Fig. 7d–f) revealed consistency between the soil metagenomes and metabolomes in the wetlands and meadows, with a larger M2 value representing lower consistency between the two datasets. Similar to the db-RDA results, the wetland and meadow samples were distinguished separately (Fig. 7d: M2 = 0.474, p < 0.001), indicating different response mechanisms between soil DOM and microbial communities in alpine wetlands and meadows. After short-term incubation at different temperatures, the consistency of the metagenomes and metabolomes in the wetlands (Fig. 7e: M2 = 0.602, p = 0.631) was lower than that in the meadows (Fig. 7f: M2 = 0.575, p = 0.063), with the separate clustering of soil samples at different temperatures found only in the meadows. The results indicated that, on the one hand, warming had significant impacts on alpine meadows where changes in soil DOM compositions were closely related to soil microbial communities; on the other hand, alpine wetlands were more adaptive to environmental changes, which may be attributed to the high soil nutrient contents and enzyme activities as well as complex compositions of soil DOM and microbial communities in alpine wetlands, as described earlier in this study.
Discussion
Different dynamics of soil nutrient contents and enzyme activities in alpine wetlands and meadows under short-term warming
Alpine wetlands play an important role in the global ecosystem and are currently undergoing severe degradation (Zhao et al. 2015). Previous studies have shown that wetland degradation leads to changes in soil physicochemical properties (increases in soil porosity and bulk density and a decrease in water-holding capacity) (Huo et al. 2013; Wang et al. 2007), as well as changes in nutrient contents, enzyme activities and microbial communities (Gu et al. 2017, 2019; Wu et al. 2016, 2017). In this study, most soil nutrients (TC, TN, DOC, DON and DP) and enzyme activities (βG, CBH, NAG and AP), as well as the diversities of soil DOM compounds and microbial communities, decreased after wetland degradation. These results were consistent with those of previous studies (Huo et al. 2013; Luan et al. 2014; Ma et al. 2016; Yang et al. 2022), indicating that alpine wetland degradation depressed the transformation of soil nutrients and microbial activities. However, the soil TP in alpine meadows was greater than that in alpine wetlands, similar to the findings of previous studies showing that degraded alpine meadows have higher TP (Wu et al. 2020), which was attributed to the lower soil phosphorus uptake required for root growth in degraded meadows with lower plant biomass.
Temperature is a key environmental factor in alpine ecosystems, and our results showed that the responses of soil nutrients and enzyme activities to short-term warming were diverse in alpine wetlands. In this study, warming from 0 °C to 20 °C continuously decreased soil DOC but increased DON in alpine wetlands, with soil DP being lowest at 0 °C and higher at both 10 °C and 20 °C. Soil enzyme activities implied the complex mechanisms between soil nutrients and microbes, with the highest activities of soil enzymes found at different incubation temperatures (βG, NAG and Perox at 0 ℃; CBH at 10 °C; AP at 0 °C and 20 °C). The results indicated that warming within ambient temperature (10 ℃) promoted the initial decomposition of cellulose (CBH activity) but impeded its later decomposition (βG activity), whereas the opposite was true under a further simulated warming scenario (20 °C), resulting in a continuous decrease in soil DOC; the hydrolyses of chitin (NAG activity) and phosphate (AP activity) were active at the lowest incubation temperature of 0 °C, along with the lowest contents of soil DON and DP. Our results were partly consistent with those of previous studies, which showed that three years of experimental warming significantly decreased the contents of soil DOC, MBC and amino nitrogen while decreasing βG activity and increasing AP activity in boreal peatlands (Song et al. 2019), indicating that warming led to the depletion of soil organic carbon and the emission of greenhouse gases in alpine wetlands (Cui et al. 2015).
Compared with alpine wetlands, alpine meadows presented simpler responses in terms of soil nutrients and enzyme activities to short-term warming, with most of those parameters being greatest at the highest incubation temperature of 20 °C. In alpine meadows, warming gradually promoted the hydrolysis of cellulose (revealed by βG and CBH activities) and resulted in a constant increase in soil DOC, even when the incubation temperature exceeded the natural ambient temperature; the decomposition of chitin (NAG activity) and phosphate esters (AP activity) was relatively slow at both 0 °C and 10 °C and significantly faster at the simulated warming condition of 20 °C, with soil DON being lowest at 10 °C and highest at 20 °C and soil DP being highest at both 10 °C and 20 °C, implying that warming increased soil N and P availability at different scales. Our results were similar to those of a four-year warming study in an alpine swamp meadow (Yuan et al. 2021c), which showed that warming increased plant and microbial biomass, hydrolytic enzyme activities and bacterial functional genes. Unlike the activities of hydrolases, the activity of peroxidase significantly decreased with increasing temperature in alpine meadows. Peroxidase is involved in lignin depolymerization and is less stable compared with extracellular hydrolases, and its activity generally increases with soil pH and lignin content but is not correlated with SOM on a global scale (Sinsabaugh 2010).
Warming gradually decreased the number of microbial species in alpine soils and altered soil microbial communities in alpine wetlands and meadows in different ways
Soil microorganisms participate in the decomposition of soil organic matter and mediate soil biogeochemical processes, and competition between different functional microorganisms determines the dynamics of soil nutrients under environmental changes (Liang et al. 2015; Romero-Olivares et al. 2017; Supramaniam et al. 2016). Warming increases the energy provided for soil microbial activities and leads to shifts in microbial growth peaks (to higher temperatures) and bacterial community structure (towards the dominance of heat-resistant taxa) (Birgander et al. 2013; Donhauser et al. 2020; Gobiet et al. 2014). Previous studies have shown that soil microbial community assembly is based on deterministic and stochastic processes and varies greatly among different environments (Jiao et al. 2022; Nemergut et al. 2005). Wang et al. (2022) reported the primary ecological processes influencing the vertical distributions of bacterial (niche-based processes) and fungal communities (niche and dispersal processes) in alpine peatlands during warming and noted that the bacterial communities in the lower soil layer of alpine peatlands became more similar after warming.
The structure and function of soil microbial communities in alpine wetlands greatly changed after degradation. Wu et al. (2022) reported that vegetation biomass, soil nutrients and microbial adaptation declined while microbial beta diversity (dominated by species replacement) increased from alpine swamps to meadows and steppes, with vegetation biomass being the main factor influencing microbial distribution and community assembly. In this study, the diversity and evenness of soil microbial communities in alpine wetlands were significantly higher than those in alpine meadows, with greater abundances of Proteobacteria and Acidobacteria found in wetlands and greater abundance of Actinobacteria found in alpine meadows, which were similar to the microbial compositions reported in previous studies (Kang et al. 2022; Qi et al. 2021; Wang et al. 2020; Zhou et al. 2019). Our study also suggested that wetland soils were significantly more dominant in the functions of “signal transduction mechanisms” and “cell wall/membrane/envelope biogenesis”, whereas meadow soils were superior in the functions of “replication, recombination and repair” and “transcription”, further indicating that degradation from alpine wetlands to alpine meadows has resulted in shifts in both the structure and function of soil microorganisms in order to adapt to new habitats.
The resistance and adaptation of soil microbes and related ecological processes to warming vary in ecosystems, as shifts in vegetation composition and consequent changes in substrate quality and microclimate alter nutrient competition between soil organisms (Bardgett et al. 2008). In this study, both alpine wetlands and meadows presented the highest number of soil microbial species at 0 °C, followed by those at 10 °C and 20 °C, indicating that warming gradually decreased the number of microbial species in alpine soils. Compared with meadow soils, wetland soils had more temperature-sensitive microbial species, many of which were Methanomicrobia in archaea, which is consistent with the findings of previous studies that revealed the important role of soil archaea in natural alpine wetlands (D'Alò et al. 2023; Shi et al. 2016). Alpine wetlands had many Proteobacteria that preferred 0 °C and Thaumarchaeota that favored 20 °C, whereas meadow soils had more Actinobacteria at 0 °C and more Bacteroidetes at 20 °C. Furthermore, although our results showed that warming increased many functions of soil microbial communities, “environmental adaptation” (KEGG pathway at level 2) in alpine wetlands and “signal transduction” in alpine meadows significantly decreased with increasing temperature, suggesting a potential threat of climate change to important ecological functions of alpine soils.
Correlations between soil DOM and microbes revealed differential biological responses to short-term warming in alpine wetlands and meadows
As the most active and mobile organic matter, soil DOM influences the dynamics and interactions of soil nutrients and microbes in natural environments, serving as an important indicator of biogeochemical processes (Ding et al. 2020). Our results revealed that warming gradually decreased soil DOC content and microbial species in alpine wetlands, with diverse ecological responses found in different temperature ranges. Soil DOM in alpine wetlands had low microbial inputs (BIX) and aromatic and hydrophobic components (SUVA254 and SUVA260) at the lowest temperature of 0 ℃, whereas the active decomposition of micromolecular substrates (high enzyme activities) increased the DOM molecular weight (SR) and DOC content, with DON and DP contents remaining low due to the cold environment. Warming to the maximum monthly ambient temperature of 10 ℃ greatly increased soil microbial inputs (BIX and tryptophan-like peak II) and hydrolysis of macromolecular cellulose (CBH activity), leading to increases in the aromaticity and hydrophobicity of DOM (SUVA254 and SUVA260) and the contents of DON and DP (especially DP), whereas the decreased decomposition of other substrates (βG, AP, NAG and Perox activities) resulted in a decrease in soil DOC content. The simulated warming scenario to 20 ℃ did not further increase microbial inputs but promoted the decomposition of some substrates (βG and AP activities), which slightly increased the DOM molecular weight (SR) and DON content but further decreased DOC content in wetland soils.
Soil microbial species in alpine meadows also gradually decreased with warming, whereas soil DOC content in alpine meadows showed an opposite increasing trend compared with that in alpine wetlands. Alpine meadow soils had inactive nutrient decomposition (low enzyme activities) and the lowest DOC and DP contents at the lowest temperature of 0 ℃, even though soil microbial inputs (BIX and soluble microbial byproduct-like peak IV) were relatively high due to the presence of low-temperature dominant microbial species, which increased the aromaticity and hydrophobicity of DOM (SUVA254 and SUVA260) and the content of DON. Warming to 10 ℃ increased cellulose hydrolysis (CBH and βG activities) and the contents of DOC and DP in meadow soils, whereas the declining microbial inputs (BIX and peak IV) resulted in the decreases in DOM aromaticity and hydrophobicity and DON content. Further warming scenario to 20 ℃ further increased cellulose hydrolysis (CBH and βG activities) and triggered the active hydrolyses of chitin and phosphates (NAG and AP activities) and therefore greatly increased soil DOC and DON contents, whereas the increases in aromatic and hydrophobic components of soil DOM (SUVA254 and SUVA260) might imped the further increase in soil DP content. In addition, peroxidase activity was significantly correlated (r = 0.674, p < 0.05) with the intensity of soluble microbial byproduct-like components (peak IV) and was significantly highest at 0 ℃ in alpine meadows, indicating the strong detoxification of peroxidase (Sinsabaugh 2010) found at low temperatures in alpine soils.
The molecular composition of soil DOM further revealed the biochemical dynamics in alpine wetlands and meadows under warming. In this study, alpine wetlands had fewer temperature-sensitive (significantly different among three incubation groups) compounds than did alpine meadows, and rising incubation temperature increased the identified compounds only in alpine meadows. This trend was generally consistent with the dynamics of soil DOC, DON and DP contents, which presented diverse trends in alpine wetlands but were all highest at 20 ℃ in alpine meadows. This may be due to the fact that soil microbial communities in alpine wetlands are more resistant and adaptive to environmental changes than those in alpine meadows are, thereby attenuating changes in the content and molecular composition of soil DOM. Our study identified higher percentages of degradable compounds (i.e., lipids and proteins) and lower percentages of stable compounds (i.e., lignin and tannins) in alpine soils than did previous studies (Zhang et al. 2022; Zhou et al. 2023), which may be due to differences in soil samples or experimental manipulation and instrumentation, given that the application of soil metabolomes is still new and lacks consistent standards.
Although warming is supposed to increase net carbon losses in many terrestrial environments due to the higher temperature sensitivity of soil respiration than of plant primary productivity, there is much uncertainty caused by the diversity and complexity of SOM as well as the involvement of microbial activities (Bardgett et al. 2008). Multi-omics methods, such as metabolomes of soil DOM and metagenomes of soil microbial communities in this study, provide a more comprehensive picture of the ecological responses of alpine environments. Our results showed that alpine wetlands and meadows shared 92.6% of soil DOM compounds and 78.7% of soil microbial species, and the proportions of unique compounds and species in the wetlands (5.8% and 21.3%, respectively) were higher than those in the meadows (1.6% and 4.0%, respectively), indicating the greater complexity of soil DOM and microbial compositions in alpine wetlands. In addition, the lower consistency between the two datasets (metabolomes and metagenomes) in alpine wetlands also indicated more complicated ecological responses to short-term warming, which may be attributed to their higher soil nutrient contents and enzyme activities as well as a wider variety of biochemical processes directly or indirectly involved and regulated by soil microorganisms.
In summary, this study revealed the similarities and differences in a variety of soil biochemical indicators in alpine wetlands and meadows as well as their relationships and responses to short-term experimental warming. On the one hand, alpine wetlands presented higher contents of most soil nutrients and enzyme activities as well as greater diversities of soil DOM and microbial communities than did alpine meadows, indicating greater overall variability in those parameters under short-term warming. On the other hand, warming gradually decreased soil DOC content in alpine wetlands (with most enzyme activities being highest at 0 ℃ and lowest at 10 ℃), whereas it increased that in alpine meadows (with most enzyme activities being highest at 20 ℃ and lowest at 0 ℃), with soil microbial species gradually decreasing with warming in both alpine soils. Future research should focus on long-term field experiments across the whole soil profile in larger regions and incorporate more environmental and biological parameters to validate the proposed presumptions and deepen our understanding of alpine ecology.
Conclusions
Our research aimed to study the effects of short-term warming and wetland degradation on ecological processes in alpine ecosystems on the Tibetan Plateau and to fill the research gap in the integration of multi-omics approaches in environmental studies. By implementing short-term incubation at 0 °C, 10 °C and 20 °C, we obtained insights into the different soil biological responses in alpine wetlands and meadows. The results revealed that alpine wetland degradation decreased soil nutrient contents (TC, TN, DOC, DON and DP) and hydrolase activities (βG, CBH, NAG and AP), as well as the complexities of soil DOM and microbial communities. Warming gradually decreased the number of soil microbial species in both alpine wetlands and meadows, whereas the responses of the above indicators were more diverse in the wetlands, with soil DOC content and most enzyme activities being highest at 0 ℃ in the wetlands but highest at 20 ℃ in the meadows. Our study identified temperature-sensitive soil ecological processes in alpine ecosystems and indicated that both wetland degradation and warming simplified the adaptive capacity of alpine wetlands to environmental changes, which is highly important for alpine ecosystem conservation in the context of climate change.
Data availability
The data that support the findings of this study are available at the NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra) under accession number PRJNA895485.
References
Barcenas-Moreno G, Gomez-Brandon M, Rousk J, Baath E (2009) Adaptation of soil microbial communities to temperature: comparison of fungi and bacteria in a laboratory experiment. Glob Change Biol 15:2950–2957. https://doi.org/10.1111/j.1365-2486.2009.01882.x
Bardgett RD, Freeman C, Ostle NJ (2008) Microbial contributions to climate change through carbon cycle feedbacks. ISME J 2:805–814. https://doi.org/10.1038/ismej.2008.58
Birgander J, Reischke S, Jones DL, Rousk J (2013) Temperature adaptation of bacterial growth and C-14-glucose mineralisation in a laboratory study. Soil Biol Biochem 65:294–303. https://doi.org/10.1016/j.soilbio.2013.06.006
Buchfink B, Xie C, Huson DH (2015) Fast and sensitive protein alignment using DIAMOND. Nat Methods 12:59–60. https://doi.org/10.1038/nmeth.3176
Chang F, He S, Dang C (2022) Assisted selection of biomarkers by Linear Discriminant Analysis Effect Size (LEfSe) in microbiome data. Jove-J vis Exp. https://doi.org/10.3791/61715
Chen H, Zhu Q, Peng C, Wu N, Wang Y et al (2013) The impacts of climate change and human activities on biogeochemical cycles on the Qinghai–Tibetan Plateau. Glob Change Biol 19:2940–2955. https://doi.org/10.1111/gcb.12277
Chen B, Zhang X, Tao J, Wu J, Wang J et al (2014) The impact of climate change and anthropogenic activities on alpine grassland over the Qinghai–Tibet Plateau. Agric Meteorol 189:11–18. https://doi.org/10.1016/j.agrformet.2014.01.002
Chen S, Zhou Y, Chen Y, Gu J (2018) Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34:884–890. https://doi.org/10.1093/bioinformatics/bty560
Cui M, Ma A, Qi H, Zhuang X, Zhuang G et al (2015) Warmer temperature accelerates methane emissions from the Zoige wetland on the Tibetan Plateau without changing methanogenic community composition. Sci Rep. https://doi.org/10.1038/srep11616
D’Alò F, Zucconi L, Onofri S, Canini F, Cannone N et al (2023) Effects of 5-year experimental warming in the Alpine belt on soil archaea: multi-omics approaches and prospects. Environ Microbiol Rep 15:291–297. https://doi.org/10.1111/1758-2229.13152
Ding Y, Shi ZQ, Ye QT, Liang YZ, Liu MQ et al (2020) Chemodiversity of soil dissolved organic matter. Environ Sci Technol 54:6174–6184. https://doi.org/10.1021/acs.est.0c01136
Donhauser J, Frey B (2018) Alpine soil microbial ecology in a changing world. Fems Microbiol Ecol. https://doi.org/10.1093/femsec/fiy099
Donhauser J, Niklaus PA, Rousk J, Larose C, Frey B (2020) Temperatures beyond the community optimum promote the dominance of heat-adapted, fast growing and stress resistant bacteria in alpine soils. Soil Biol Biochem. https://doi.org/10.1016/j.soilbio.2020.107873
Fu LM, Niu BF, Zhu ZW, Wu ST, Li WZ (2012) CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28:3150–3152. https://doi.org/10.1093/bioinformatics/bts565
Gao Q, Guo Y, Xu H, Ganjurjav H, Li Y et al (2016) Climate change and its impacts on vegetation distribution and net primary productivity of the alpine ecosystem in the Qinghai–Tibetan Plateau. Sci Total Environ 554:34–41. https://doi.org/10.1016/j.scitotenv.2016.02.131
Gobiet A, Kotlarski S, Beniston M, Heinrich G, Rajczak J et al (2014) 21st century climate change in the European Alps-a review. Sci Total Environ 493:1138–1151. https://doi.org/10.1016/j.scitotenv.2013.07.050
Gu Y, Wang Y, Xiang Q, Yu X, Zhao K et al (2017) Implications of wetland degradation for the potential denitrifying activity and bacterial populations with nirS genes as found in a succession in Qinghai Tibet Plateau, China. Eur J Soil Biol 80:19–26. https://doi.org/10.1016/j.ejsobi.2017.03.005
Gu Y, Bai Y, Xiang Q, Yu X, Zhao K et al (2018) Degradation shaped bacterial and archaeal communities with predictable taxa and their association patterns in Zoige wetland at Tibet plateau. Sci Rep. https://doi.org/10.1038/s41598-018-21874-0
Gu YF, Liu T, Bai Y, Xiang QJ, Zhang XP et al (2019) Pyrosequencing of nirS gene revealed spatial variation of denitrifying bacterial assemblages in response to wetland desertification at Tibet Plateau. J Mt Sci 16:1121–1132. https://doi.org/10.1007/s11629-018-5147-3
Huo L, Chen Z, Zou Y, Lu X, Guo J et al (2013) Effect of Zoige alpine wetland degradation on the density and fractions of soil organic carbon. Ecol Eng 51:287–295. https://doi.org/10.1016/j.ecoleng.2012.12.020
Hyatt D, Chen GL, LoCascio PF, Land ML, Larimer FW et al (2010) Prodigal: prokaryotic gene recognition and translation initiation site identification. Bmc Bioinform. https://doi.org/10.1186/1471-2105-11-119
Iqbal A, Shang ZH, Rehman MLU, Ju MT, Rehman MMU et al (2019) Pattern of microbial community composition and functional gene repertoire associated with methane emission from Zoige wetlands, China-A review. Sci Total Environ 694:12. https://doi.org/10.1016/j.scitotenv.2019.133675
Jiao S, Chen W, Wei G (2022) Core microbiota drive functional stability of soil microbiome in reforestation ecosystems. Glob Change Biol 28:1038–1047. https://doi.org/10.1111/gcb.16024
Jin X, Jin H, Wu X, Luo D, Yu S et al (2020) Permafrost degradation leads to biomass and species richness decreases on the Northeastern Qinghai–Tibet Plateau. Plants-Basel. https://doi.org/10.3390/plants9111453
Jones DL, Willett VB (2006) Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol Biochem 38:991–999. https://doi.org/10.1016/j.soilbio.2005.08.012
Kang XM, Hao YB, Cui XY, Chen H, Huang SX et al (2016) Variability and changes in climate, phenology, and gross primary production of an Alpine Wetland ecosystem. Remote Sens. https://doi.org/10.3390/rs8050391
Kang E, Li Y, Zhang X, Yan Z, Zhang W et al (2022) Extreme drought decreases soil heterotrophic respiration but not methane flux by modifying the abundance of soil microbial functional groups in alpine peatland. CATENA. https://doi.org/10.1016/j.catena.2022.106043
Lehmann J, Kleber M (2015) The contentious nature of soil organic matter. Nature 528:60–68. https://doi.org/10.1038/nature16069
Li RQ, Li YR, Kristiansen K, Wang J (2008) SOAP: short oligonucleotide alignment program. Bioinformatics 24:713–714. https://doi.org/10.1093/bioinformatics/btn025
Li BQ, Yu ZB, Liang ZM, Song KC, Li HX et al (2014) Effects of climate variations and human activities on runoff in the Zoige Alpine Wetland in the Eastern edge of the Tibetan Plateau. J Hydrol Eng 19:1026–1035. https://doi.org/10.1061/(asce)he.1943-5584.0000868
Li DH, Liu CM, Luo RB, Sadakane K, Lam TW (2015) MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31:1674–1676. https://doi.org/10.1093/bioinformatics/btv033
Li Y, Lv W, Jiang L, Zhang L, Wang S et al (2019) Microbial community responses reduce soil carbon loss in Tibetan alpine grasslands under short-term warming. Glob Change Biol 25:3438–3449. https://doi.org/10.1111/gcb.14734
Li H, Li T, Sun W, Zhang W, Zhang Q et al (2021) Degradation of wetlands on the Qinghai–Tibetan Plateau causing a loss in soil organic carbon in 1966–2016. Plant Soil 467:253–265. https://doi.org/10.1007/s11104-021-05086-6
Li M, Zhang KR, Yan ZQ, Liu L, Kang EZ et al (2022) Soil Water content shapes microbial community along gradients of Wetland degradation on the Tibetan Plateau. Front Microbiol. https://doi.org/10.3389/fmicb.2022.824267
Liang Y, Jiang Y, Wang F, Wen C, Deng Y et al (2015) Long-term soil transplant simulating climate change with latitude significantly alters microbial temporal turnover. ISME J 9:2561–2572. https://doi.org/10.1038/ismej.2015.78
Liu GD, Sun JF, Tian K, Xiao DR, Yuan XZ (2017) Long-term responses of leaf litter decomposition to temperature, litter quality and litter mixing in Plateau Wetlands. Freshw Biol 62:178–190. https://doi.org/10.1111/fwb.12860
Luan JW, Cui LJ, Xiang CH, Wu JH, Song HT et al (2014) Soil carbon stocks and quality across intact and degraded alpine wetlands in Zoige, east Qinghai–Tibet Plateau. Wetlands Ecol Manage 22:427–438. https://doi.org/10.1007/s11273-014-9344-8
Ma K, Zhang Y, Tang SX, Liu JG (2016) Spatial distribution of soil organic carbon in the Zoige alpine wetland, Northeastern Qinghai–Tibet Plateau. CATENA 144:102–108. https://doi.org/10.1016/j.catena.2016.05.014
Mooshammer M, Hofhansl F, Frank AH, Wanek W, Haemmerle I et al (2017) Decoupling of microbial carbon, nitrogen, and phosphorus cycling in response to extreme temperature events. Sci Adv. https://doi.org/10.1126/sciadv.1602781
Nemergut DR, Costello EK, Meyer AF, Pescador MY, Weintraub MN et al (2005) Structure and function of alpine and arctic soil microbial communities. Res Microbiol 156:775–784. https://doi.org/10.1016/j.resmic.2005.03.004
Overy DP, Bell MA, Habtewold J, Helgason BL, Gregorich EG (2021) “Omics” technologies for the study of soil carbon stabilization: a review. Front Environ Sci. https://doi.org/10.3389/fenvs.2021.617952
Parks DH, Tyson GW, Hugenholtz P, Beiko RG (2014) STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30:3123–3124. https://doi.org/10.1093/bioinformatics/btu494
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42. https://doi.org/10.1038/nature01286
Qi X-E, Wang C, He T, Ding F, Zhang X et al (2021) Bacterial community changes and their responses to nitrogen addition among different alpine grassland types at the eastern edge of Qinghai–Tibetan Plateau. Arch Microbiol 203:5963–5974. https://doi.org/10.1007/s00203-021-02535-9
Ran Y, Li X, Cheng G (2018) Climate warming over the past half century has led to thermal degradation of permafrost on the Qinghai–Tibet Plateau. Cryosphere 12:595–608. https://doi.org/10.5194/tc-12-595-2018
Romero-Olivares AL, Allison SD, Treseder KK (2017) Soil microbes and their response to experimental warming over time: a meta-analysis of field studies. Soil Biol Biochem 107:32–40. https://doi.org/10.1016/j.soilbio.2016.12.026
Schuur EAG, McGuire AD, Schaedel C, Grosse G, Harden JW et al (2015) Climate change and the permafrost carbon feedback. Nature 520:171–179. https://doi.org/10.1038/nature14338
Shi Y, Adams JM, Ni Y, Yang T, Jing X et al (2016) The biogeography of soil archaeal communities on the Eastern Tibetan Plateau. Sci Rep. https://doi.org/10.1038/srep38893
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42:391–404. https://doi.org/10.1016/j.soilbio.2009.10.014
Song Y, Song C, Ren J, Ma X, Tan W et al (2019) Short-Term response of the soil microbial abundances and enzyme activities to experimental warming in a boreal peatland in Northeast China. Sustainability. https://doi.org/10.3390/su11030590
Steinweg JM, Dukes JS, Paul EA, Wallenstein MD (2013) Microbial responses to multi-factor climate change: effects on soil enzymes. Front Microbiol 4:11. https://doi.org/10.3389/fmicb.2013.00146
Supramaniam Y, Chong C-W, Silvaraj S, Tan IK-P (2016) Effect of short term variation in temperature and water content on the bacterial community in a tropical soil. Appl Soil Ecol 107:279–289. https://doi.org/10.1016/j.apsoil.2016.07.003
Trisos CH, Merow C, Pigot AL (2020) The projected timing of abrupt ecological disruption from climate change. Nature 580(7804):496–501. https://doi.org/10.1038/s41586-020-2189-9
van Gestel NC, Reischke S, Baath E (2013) Temperature sensitivity of bacterial growth in a hot desert soil with large temperature fluctuations. Soil Biol Biochem 65:180–185. https://doi.org/10.1016/j.soilbio.2013.05.016
Walther GR, Post E, Convey P, Menzel A, Parmesan C et al (2002) Ecological responses to recent climate change. Nature 416:389–395. https://doi.org/10.1038/416389a
Wang G, Wang Y, Li Y, Cheng H (2007) Influences of alpine ecosystem responses to climatic change on soil properties on the Qinghai–Tibet Plateau, China. CATENA 70:506–514. https://doi.org/10.1016/j.catena.2007.01.001
Wang X, Dong S, Gao Q, Zhou H, Liu S et al (2014) Effects of short-term and long-term warming on soil nutrients, microbial biomass and enzyme activities in an alpine meadow on the Qinghai–Tibet Plateau of China. Soil Biol Biochem 76:140–142. https://doi.org/10.1016/j.soilbio.2014.05.014
Wang X, Zhang Z, Yu Z, Shen G, Cheng H et al (2020) Composition and diversity of soil microbial communities in the alpine wetland and alpine forest ecosystems on the Tibetan Plateau. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.141358
Wang X, Li Y, Yan Z, Hao Y, Kang E et al (2022) The divergent vertical pattern and assembly of soil bacterial and fungal communities in response to short-term warming in an alpine peatland. Front Plant Sci. https://doi.org/10.3389/fpls.2022.986034
Wu L-S, Nie Y-Y, Yang Z-R, Zhang J (2016) Responses of soil inhabiting nitrogen-cycling microbial communities to wetland degradation on the Zoige Plateau, China. J Mt Sci 13:2192–2204. https://doi.org/10.1007/s11629-016-4004-5
Wu P, Zhang H, Cui L, Wickings K, Fu S et al (2017) Impacts of alpine wetland degradation on the composition, diversity and trophic structure of soil nematodes on the Qinghai–Tibetan Plateau. Sci Rep. https://doi.org/10.1038/s41598-017-00805-5
Wu XD, Zhao L, Hu GJ, Liu GM, Li WP et al (2018) Permafrost and land cover as controlling factors for light fraction organic matter on the southern Qinghai–Tibetan Plateau. Sci Total Environ 613:1165–1174. https://doi.org/10.1016/j.scitotenv.2017.09.052
Wu J, Wang H, Li G, Ma W, Wu J et al (2020) Vegetation degradation impacts soil nutrients and enzyme activities in wet meadow on the Qinghai–Tibet Plateau. Sci Rep. https://doi.org/10.1038/s41598-020-78182-9
Wu M-H, Xue K, Wei P-J, Jia Y-L, Zhang Y et al (2022) Soil microbial distribution and assembly are related to vegetation biomass in the alpine permafrost regions of the Qinghai–Tibet Plateau. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2022.155259
Yang A, Kang X, Li Y, Zhang X, Zhang K et al (2022) Alpine wetland degradation reduces carbon sequestration in the Zoige Plateau China. Front Ecol Evol. https://doi.org/10.3389/fevo.2022.980441
Yuan MM, Guo X, Wu L, Zhang Y, Xiao N et al (2021a) Climate warming enhances microbial network complexity and stability. Nat Clim Chang 11:343–100. https://doi.org/10.1038/s41558-021-00989-9
Yuan X, Chen Y, Qin W, Xu T, Mao Y et al (2021b) Plant and microbial regulations of soil carbon dynamics under warming in two alpine swamp meadow ecosystems on the Tibetan Plateau. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.1480725
Yuan X, Qin WK, Chen Y, Xu TL, Chen KL et al (2021c) Plateau pika offsets the positive effects of warming on soil organic carbon in an alpine swamp meadow on the Tibetan Plateau. CATENA. https://doi.org/10.1016/j.catena.2021.105417
Yun J, Crombie AT, Ul Haque MF, Cai Y, Zheng X et al (2021) Revealing the community and metabolic potential of active methanotrophs by targeted metagenomics in the Zoige wetland of the Tibetan Plateau. Environ Microbiol 23:6520–6535. https://doi.org/10.1111/1462-2920.15697
Zhang XY, Dong WY, Dai XQ, Schaeffer S, Yang FT et al (2015) Responses of absolute and specific soil enzyme activities to long term additions of organic and mineral fertilizer. Sci Total Environ 536:59–67. https://doi.org/10.1016/j.scitotenv.2015.07.043
Zhang Y, Dong S, Gao Q, Liu S, Zhou H et al (2016) Climate change and human activities altered the diversity and composition of soil microbial community in alpine grasslands of the Qinghai–Tibetan Plateau. Sci Total Environ 562:353–363. https://doi.org/10.1016/j.scitotenv.2016.03.221
Zhang Y, Heal KV, Shi M, Chen W, Zhou C (2022) Decreasing molecular diversity of soil dissolved organic matter related to microbial community along an alpine elevation gradient. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.151823
Zhao ZL, Zhang YL, Liu LS, Liu FG, Zhang HF (2015) Recent changes in wetlands on the Tibetan Plateau: a review. J Geog Sci 25:879–896. https://doi.org/10.1007/s11442-015-1208-5
Zheng HF, Liu Y, Chen YM, Zhang J, Li HJ et al (2020) Short-term warming shifts microbial nutrient limitation without changing the bacterial community structure in an alpine timberline of the eastern Tibetan Plateau. Geoderma 360:10. https://doi.org/10.1016/j.geoderma.2019.113985
Zhou H, Zhang DG, Jiang ZH, Sun P, Xiao HL et al (2019) Changes in the soil microbial communities of alpine steppe at Qinghai–Tibetan Plateau under different degradation levels. Sci Total Environ 651:2281–2291. https://doi.org/10.1016/j.scitotenv.2018.09.336
Zhou X, Ma A, Chen X, Zhang Q, Guo X et al (2023) Climate Warming-Driven Changes in the molecular composition of soil dissolved organic matter across depth: a case study on the Tibetan Plateau. Environ Sci Technol 57:16884–16894. https://doi.org/10.1021/acs.est.3c04899
Funding
This study is supported by the Fundamental Research Funds for the Central Universities of P. R. China and the National Natural Science Foundation of China (Grant No. 41271094).
Author information
Authors and Affiliations
Contributions
Shiyu Fan: writing—original draft preparation, formal analysis, investigation, methodology. Jihong Qin: writing—review & editing, software, methodology. Hui Sun: Writing—review & editing, funding acquisition, methodology, conceptualization. Zhenchu Dan: data curation, investigation, visualization. Wenqing Chen: writing—review & editing, supervision, validation. Jiyuan Yang: data curation, formal analysis, investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fan, S., Qin, J., Sun, H. et al. Short-term warming decreased soil DOM content and microbial species in alpine wetlands but increased soil DOM content and hydrolase activity in alpine meadows on the Tibetan Plateau. Biogeochemistry (2024). https://doi.org/10.1007/s10533-024-01171-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10533-024-01171-x