Abstract
The Lower Havel in Berlin is a polymictic riverine lake, thermally stratified for some days or weeks in summer. It is characterized by a relatively high mean total phosphorus concentration (145 µg l−1) and frequent cyanobacteria mass development in summer. We quantified the potentially mobile P pool in sediments, determined P fluxes based on conventional dialysis sampler-, gel probe- and benthic chamber measurements, and combined this with column experiments and P budget calculations to evaluate whether a) a reduction of P loading would be counterbalanced by P release from sediment, and b) release of redox sensitive P would even increase with reduction of nitrogen loading. The potentially mobile P pool was relatively small (228 t) compared to mean annual external load (190 t a−1) and gross P release (1.2–36 mg m−2 day−1), and was thus of little relevance for long-term P availability. Despite 38 % of P in the sediment being redox sensitive, the seasonal course of P gross release was mainly driven by redox independent organic matter mineralization of diagenetically young surface sediment. Under anoxic conditions, P release was higher than under oxic conditions and to some extent controlled by nitrate under laboratory conditions. However, ambient nitrate availability was too low to affect P release which was more dependent on mineralization and Fe availability than on redox. Therefore, the Lower Havel would benefit from P load reduction but internal P loading would be largely unaffected from further N load reduction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lowland rivers in agriculturally used or densely inhabited catchments are often rich in nutrients. Intervening riverine lakes and impoundments may therefore become extremely productive (Kleeberg and Dudel 1997; Kleeberg and Kozerski 1997; Selig and Schlungbaum 2002). This counteracts various uses like recreation or drinking water production. The Lower Havel in Berlin is a typical example of such a system. The section we studied is a polymictic, hypertrophic riverine lake with frequent cyanobacteria (Microcystis sp.) mass developments in summer. To support various uses of this urban water body and to meet the requirements of the European Water Framework Directive, the Lower Havel could benefit from further reduction of nitrogen (N) and/or phosphorus (P) import. Both possibilities require the evaluation of ecological meaningfulness and possible side effects.
The reduction of P-import may be counterbalanced by high internal loading from a large historical P-pool in the sediment. This is known to delay recovery for 10–15 years or longer in lakes (Jeppesen et al. 2005; Sondergaard et al. 2013) and also in streams (Hamilton 2012), where intervening riverine lakes and impoundments accumulate nutrient rich sediments comparable to those in lakes (Selig and Schlungbaum 2002). Riverbed sediments are also known to act as net sources of P during phases of particular ecological sensitivity in spring and summer (House 2003; Jarvie et al. 2005). Retrospective reports on the response of pelagic nutrient concentrations and biota are quite frequent (Sas 1989; Jeppesen et al. 2005; Welch and Cooke 2005). However, this is rarely combined with sediment analyses to demonstrate the relevance of the accumulated P-pool, versus the short term effect of remineralization of very recently sedimented organic material (Lewandowski et al. 2003; Grüneberg et al. 2011) and to predict the longevity of the recovery process (Sondergaard et al. 1999; Kneis et al. 2006; Schauser et al. 2006).
The concept of a time lag in the recovery of lakes is based on the existence of a large, yet exchangeable, potentially mobile P pool (PMP) in the sediment. This pool may be estimated based on vertical distribution of P content in the sediment (Jørgensen 1976; Sondergaard et al. 2003), element ratios (Maaßen et al. 2005), sequential extraction (Reitzel et al. 2005; Wauer et al. 2005a) or laboratory experiments (Kneis et al. 2006). However, most approaches give ambiguous interpretations of the long term accumulation, and still do not allow conclusions about the short term (seasonal) relevance of P release from this pool. The PMP may be regarded relevant if it is able to support gross release rates high enough to significantly affect pelagic P availability in relation to external loading (Lewandowski et al. 2003). In consequence, the P gross release from recently settled detritus needs to be determined too, and distinguished from a background P flux from historically accumulated PMP. This has been considered in modeling (Jensen et al. 2006; Kneis et al. 2006) but less in interpretation of measured release rates as attempted in this study.
The transfer of P from the sediment pool is influenced by multiple factors (Sondergaard et al. 2003). Nitrate (NO3 −) can substitute oxygen (O2) in supporting a high iron (Fe) based P-sorption capacity and thus suppress P release rate from sediment (Wauer et al. 2005a; Petzoldt and Uhlmann 2006; Schauser et al. 2006). With ~85 % N-import into Lower Havel in nitrate form, it is thought that a load reduction may cause higher P release from sediment. Although the biochemical processes are generally known, it is difficult to assess the relevance of nitrate within the redox-controlled P dynamics for a specific water body. Clear nitrate effects were only demonstrated when nitrate loading was very high due to high single dose additions (Ripl and Lindmark 1978; Foy 1986; Wauer et al. 2005b), or in cases when nitrate was applied continuously to yield high concentrations (Sondergaard et al. 2000). Gabriel et al. (2006) found that NO3-N concentrations of 0.4 mg l−1 in river interstitial water caused a suppression of P release, but also that interstitial NO3-N concentrations up to 20 mg l−1 did not guarantee an effect if P release is forced by mineralization of organic matter. Andersen (1982) published threshold NO3-N concentrations between 0.5 mg l−1 for shallow and 1.0 mg l−1 for deep lakes, but it is not completely clear to what extent the observed seasonal P dynamics were influenced by nitrate or other factors like P loading, hydrologic conditions and redox independent release processes. This difficulty in differentiating between temperature and nitrate effects (see also Schauser et al. 2006) is a common problem in lake mass balance studies as discussed by Jensen and Andersen (1992), because low nitrate concentrations in summer (due to denitrification loss) are typically synchronized with high P concentrations and high P gross release rates related to temperature and organic matter availability (Andersen 1982; Sinke et al. 1990; Sondergaard et al. 1999). Hupfer and Lewandowski (2008) also argue that P-retention (and P-release) is often not controlled by redox conditions but by P load. In eutrophic lakes, decomposition is often the major P release mechanism, while redox insensitive P forms (Apatite, Al–P) are of minor relevance.
Therefore, to assess redox- and especially nitrate control of P release, it is necessary to consider not only nitrate concentration, but also nitrate supply (areal load, advective transport) and the relevance of mineralization (Gabriel et al. 2006), the Fe availability (Jensen et al. 1992; Maaßen 2003; Schauser et al. 2006) and sulfidic Fe immobilization (Kleeberg et al. 2013). Furthermore, inferring concrete management options on nitrogen or phosphorus load control also requires considering the seasonality of the sediment processes relevant for P mobility. As an example, the surplus of Fe mobilization in relation to P (e.g. Fe: P > 2.0) has been identified as meaningful indicator for the Fe availability as major sorption partner for P (Tessenow 1974; Kleeberg and Dudel 1997; Gunnars et al. 2002).
The Lower Havel is ideal to study nitrate influence as the sediment is Fe rich, and inflow nitrate concentration is relatively high (0.3–2.2 mg l−1) (Kneis et al. 2006) and could potentially be managed by sewage treatment technology. It has been stated that a large P excess in sediments of the Lower Havel would support high internal load for almost two decades and thus prevent the improvement of trophic state even under reduced phosphorus inputs (Kneis et al. 2006). Here, we test this assumption and suggest new interpretations on the quantity and origin of the P pool susceptible to release, and comment on the Lower Havel response to reductions of N- and/or P-loading.
Therefore, we combined sediment pool-, flux- and turnover determination with P budget calculations. By this, we investigate the role of the sediment P pool as a long term source that potentially counterbalances external P-load reduction, compared to its role as short term source influencing seasonality of P internal load. We further estimate how much of the seasonal P dynamic is redox-controlled and conditioned by nitrate.
Materials and methods
Characterization of the Lower Havel
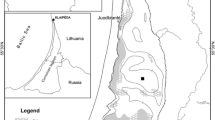
The Lower Havel is a polymictic riverine lake situated in the west of Berlin (Fig. 1). The section studied starts at Pichelsdorfer Gemünd (52.5030°, 13.2000°) about 4 km downstream of the confluence of the lowland rivers Spree and Havel, and stretches about 11 km downstream to Krughorn (52,4277°, 13,1100°) with an area of 11.75 × 106 m2 and a volume of 58.67 × 106 m3 (excluding the Wannsee). The northern lake basins are shallow (68 % <4.0 m), while the southern basins have maximum depths between 6.0 and 10.5 m (mean depth 5.1 m) and are thermally stratified for some days or weeks during calm weather and low discharge conditions in summer.
Hydromorphologic map of Lower Havel with sampling stations LH0 (Pichelsee), LH1 (Pichelsdorfer Gemünd), LH2 (station Kälberwerder near peninsular Schwanenwerder) and LH3 (Krughorn), based on data from the Berlin Senate Department for Urban Development and the Environment. Black arrows show direction of flow and approximate location of discharge measurements at stations Freybrücke and Stößenseebrücke
The average discharge during our study period (2011–2012) was 64.4 m3 s−1 which is exceptionally high compared to the longer average of about 36.0 m3 s−1 (mean 2000–2009). The hydraulic residence time thus was 11 days during our study period and 19 days in the longer average (2000–2009). Especially the summer (Jul–Sept) discharge of 56 m3 s−1 during our study period was more than twice the longer-term summer average (25 m3 s−1) (Fig. 2).
Discharge (five days running average), surface temperature, Ammonium-, Nitrate- and total phosphorus concentration for Lower Havel stations LH0 (Pichelsee = Inflow) and LH2 (Kälberwerder). The black bottom lines in the nitrate diagram marks periods with <0.5 mg l−1 NO3-N. (All data from Berlin Senate Department for Urban Development and the Environment)
Due to passage through Berlin, the Havel has been highly loaded with nutrients since the 18th century (Nützmann et al. 2011). Waste water was discharged into the river without treatment until 1875. Despite installation of sewage farms thereafter and the first waste water treatment plant in the 1920s (with mechanical treatment only), P emission into Berlin rivers constantly increased until the 1980s. Following structural changes and improved treatment technologies after the German unification in 1990, total phosphorus (TP) loading dramatically decreased from 2,780 t a−1 in 1976–1990 to 190 t a−1 thereafter. TP annual mean concentration in the Lower Havel therefore decreased from ~0.8 mg l−1 in the 1980s (Rohde 1995) to <0.3 mg l−1 in the 1990s (Kneis et al. 2006).
Annual mean TP concentration at station LH2 (Fig. 2) during the period 2005–2012 was 0.15 mg l−1. TP concentration normally peaked in late summer (September) with concentrations up to 0.5 mg l−1, which was sometimes twice the inflow (LH0) concentration. Ammonium (mean concentration 0.18 mg l−1) showed at least two peaks, one peak up to 0.57 mg l−1 in winter due to high inflow concentration, and one peak in summer during low discharge. Inflow nitrate concentration decreased from maximally 2.2 mg l−1 in late autumn (October) to 0.2–0.6 mg l−1 in summer (July), while concentration at LH2 was below the detection limit of 0.02 mg l−1 at times (Fig. 2). Mean chlorophyll-a concentration for the vegetation period (Apr–Oct) was 20 µg l−1 (not shown), but summer peaks between 34 and 97 µg l−1 were common.
Sediment sampling and analysis
The sediment (and also pore water) was sampled mainly at stations LH1 (52.499623°, 13.191571°) and LH2 (52.450269°, 13.157251°; see Fig. 1). Locations were chosen based on preliminary sediment mapping and water quality data. While LH1 (~3.5 m depth) close to the inflow represents an organic- and nutrient rich sediment of the highly loaded upper basin, LH2 (~7 m depth) sediment properties are typical for the remaining 80–85 % of the studied Lower Havel section. This partitioning is based on visual observation of sediment at 70 locations and a gradient in sediment TP content along the flow of the Lower Havel as described below.
Sediment cores were obtained in March and May 2011 at LH1 and LH2 using a gravity corer (Uwitec, Mondsee, Austria). After transport to the laboratory, sediment cores were immediately sliced, and aliquots were taken for the determination of dry weight (DW, 105 °C, 48 h), organic matter as loss on ignition (550 °C, 3 h) and sequential P-extraction according to Psenner et al. (1984), each in duplicate. Reactive iron (FeHCl) was determined by cold extraction in 0.5 N HCl (1 h) (Canfield 1989). Fe extracted with cold 0.11 M bicarbonate-dithionite solution (FeBD) was interpreted as Fe-(hydr)oxide (Raiswell et al. 1994). Dried samples were pulverized for the determination of total C, N, and S (vario Macro CHNS, Elementar Analysensysteme, Hanau, Germany). Total P (TP) and total Fe were determined photometrically after combustion (550 °C, 2 h) and extraction in hot 2 M HCl (Andersen 1976). The recovery rate was 95 % for P and 87 % for Fe using international standard reference materials (NIST 2711, 2782).
A separate core was taken in May 2012 at LH2 and sliced under N2 flushing for determination of total reduced inorganic sulfur species following Canfield (1989), Fossing and Jorgensen (1989) and Hsieh and Yang (1989). Acid volatile sulphide (=FeS) was extracted with 1 M HCl (4 h), chromium reducible sulphur (=FeS2) with a mixture of 2 M CrCl2 and 12 M HCl (4 h), followed by addition of dimethylformamide solution for 4 h at 60 °C to extract elemental sulphur (S0). Total reduced inorganic sulfur data were used to characterize reactivity of sedimentary iron phases based on Raiswell et al. (1994).
The P release potential was estimated by calculating the potentially mobile P pool (PMP) by two approaches. PMP was calculated from the sediment TP vertical profile assuming steady state conditions for burial and release (Jørgensen et al. 1975; Schauser et al. 2006). Here, PMP is defined as the P mass above the sediment depth where no change of TP content with depth occurs minus the permanent P mass. PMP was also estimated from sequential P-extraction with NH4Cl soluble P, redox sensitive P (bicarbonate-dithionite soluble) and organic P (NaOH-NRP = non-reactive NaOH soluble) interpreted as potentially mobile (Reitzel et al. 2005; Wauer et al. 2005a).
To estimate the PMP for the whole section of Lower Havel studied, we estimated the lake area covered by autochthonous (organic- and nutrient rich) sediment, and related sediment areas to the sampling points LH1 and LH2. With sediment mappings in 2011 (30 points) and 2014 (70 points) we found organic sediment only below 2.5–3.0 m water depth in the upper lake basin (0 to 2.5–3.5 km downstream of inflow), and below 3.5–4.0 m water depth in the remaining basin. Sediment mapping revealed that LH1 was representative for the upper lake basin and thus for 15–20 % of sediment bearing area (equivalent to 2.5–3.5 km distance from inflow). This is supported by visual observation of sediments and a downstream TP gradient in surface sediment based on the sampling in 2014. The TP content decreased from 6.8 mg g−1 at LH1 to 5.6 and 3.2 mg g−1 at 2 and 4 km distance from inflow, respectively. The TP content in the remaining basin represented by LH2 was lower at 3.6 ± 1.0 mg g−1 (n = 6). A range for the total PMP was calculated by considering the uncertainties in sediment coverage (below 2.0–4.0 m water depth) and sub-basin partitioning (LH1 representing 15–20 % area).
Gross sedimentation rate was determined by a set of four cylindrical sediment traps (length 30 cm, inner diameter 5.7 cm) which were installed at LH2 at a depth of 5 m (about 2 m above sediment) between April and August 2012 four times for two weeks sampling periods. The material of the four traps was pooled, most of the water was siphoned off and aliquots were taken for the determination of dry weight, organic matter, TP and TFe (all in triplicate).
Sediment pore water sampling and analysis
We used two different methods for pore water sampling, conventional dialysis samplers (peepers) with 1 cm vertical resolution according to Hesslein (1976), and DET gel probes (Diffusive Equilibrium in Thin films), (Krom et al. 1994) with 0.25 cm resolution at the sediment–water interface and 1 cm resolution below 2 cm depth. Conventional dialysis sampler chambers were filled with oxygen-free de-ionized water, covered with a polysulfone membrane (0.2 µm, Supor 200, Pall Corporation, Port Washington, USA) and exposed in situ into the sediment at stations LH1 and LH2 for 14 days using a metal frame. After retrieval, samplers were stored cool in air tight plastic bags that had previously been filled with N2. Samples were analyzed photometrically within three hours after retrieval for soluble reactive phosphorus (SRP) using the ammonium molybdate method (DIN EN ISO 6878 2004), ammonium (NH4-N) using the indophenol method (DIN 38 406 E5 1983), total dissolved Fe (TDFe) using the phenanthroline method (Canfield 1989), and total dissolved Manganese (Mn) by 1-(2-pyridylazo)-2-naphthol (Spectroquant®, Merck KGaA, Darmstadt, Germany).
DET Gel probes (DGT Research Ltd, Lancaster, UK) were deployed ex situ for three days in undisturbed sediment columns (8.6 cm inner diameter) freshly taken at LH2, at in situ temperature in the dark. Sediment overlying water was constantly bubbled with air (and thus slightly mixed) and renewed with filtered Lower Havel water using a peristaltic pump at a flow rate of 0.5 l day−1. After retrieval, the gel was cut into strips within a few minutes, back equilibrated for 24 h in 1 ml de-ionized water and centrifuged at 18,000 g for 5 min. Samples were analyzed immediately for SRP, NH4-N and NO3-N by segmented flow analysis (RFA 300, Astoria Pacific, Clackamas, USA) with a low detection limit for NO3-N at 5.2 µg l−1. High resolution (0.1 mm) dissolved oxygen profiles were measured with oxygen microsensors (PreSens, Regensburg, Germany).
Diffusive flux was calculated from concentration gradients between pore water and overlying water using Fick’s first law of diffusion as described in Lewandowski et al. (2002). Positive rates represent transport from sediment to overlying water. Turnover rates were calculated using the software Profile V 1.0 (Berg et al. 1998). In situ flux was determined at LH1 and LH2 by benthic chambers (Burger et al. 2007) made of stainless steel (circular, volume 40.6 l, surface area 0.123 m2). Chambers were sampled 4–5 times during each two day deployment period. In situ flux was calculated from the slope of linear regression of concentration versus time (Burger et al. 2007).
Nitrate influence on P release in undisturbed cores
A series of sediment column experiments were conducted by incubating 12 undisturbed sediment cores (57 mm diameter) at in situ temperature for three weeks. The cores were grouped into 4 treatments, each with 3 replicates: The control was filtered Havel water (ambient NO3-N concentration). For one treatment (+NO3), KNO3 was added to increase the NO3-N concentration in the inflow by 0.5 mg l−1. For two other treatments, NO3-N concentration was reduced by a factor of 2 (−NO3) and 4 (−NO3) by dilution with artificial water made by adding salts of the major ions to demineralised water. For the four oxic experiments, sediment overlying water was constantly bubbled with air, and for the anoxic experiment it was bubbled with a mixture of N2/CO2 (390 ppm CO2). The water was renewed with corresponding water using a peristaltic pump at a flow rate 0.5 l day−1. In the anoxic experiment, glucose was added at a rate of 5 g C m−2 day−1, which is within the range of carbon supply by gross sedimentation at station LH2 (1.4 and 6.2 g m−2 day−1) measured between April and August 2012 with sediment traps. One way ANOVA with Tukey or Games-Howell post hoc test was used to test for differences between treatments.
Phosphorus balance calculations
P inflow load (Pin) was calculated by multiplying daily discharge at the inflow gauges with daily TP concentration which was linearly interpolated from monthly to fortnightly measurements at LH0 (station Pichelsee). Total discharge was dominated by ~97 % of the discharge at station “Freybrücke” (close to LH0) and the rest being measured at the station “Stößenseebrücke” (see arrows in Fig. 1). For outflow load (Pout) calculation, SRP loss by water works extraction (Tiefenwerder) was considered. Net sedimentation was calculated as difference between Pin and Pout, corrected for the change in lake internal concentration (Plake) during that period:
where, NS net sedimentation, P lake(start) in-lake P content at the start ∆T, P lake(end) in-lake P content at the end of ∆T, P in (∆T) mean P load within ∆T, P out ∆(T) mean P outflow within ∆T, ∆T time period for which the calculation is done, usually 1 month.
Estimation of Plake at the beginning and the end of each month was possible by linearly interpolating data to daily values. This also allowed calculation of monthly mean values for Pin and Pout.
For Plake, TP concentration at stations were weighted according to the volume of their corresponding sub basins with LH2 representing 85 % of the total water volume.
Propagation of error calculations where applied to estimate the uncertainty of NS. The relative error for Plake was 9.5 % based on the analytical error for TP (Dr. A. Kleeberg, Berlin-Brandenburg State Laboratory). The error for ADCP discharge measurements was 3 % (Dr. B. Creutzfeldt, Berlin Senate Department for Urban Development and the Environment). The relative error for P load (Pin and Pout) was thus 10 % (based on Gauss error propagation). Net sedimentation values are shown together with their estimated uncertainty (with 95 % confidence level).
While the P budget is based on concentrations at stations LH0 and LH2, the whole area between LH0 and LH3 (11.75 Mio m2) was used for normalization to areal rates. This approach was chosen as most flux measurements were done at LH2.
Results
Sediment composition and P release potential
The sediment of the Lower Havel was characterized by a high organic matter (24–29 % of dry mass) and low Ca (30–80 mg g−1) and Al (11 mg g−1) content (Fig. 3). Sediments at both stations showed a distinct TP rich upper sediment layer with P contents up to 7.4 mg g−1 at LH1 and 3.9 mg g−1 at LH2. TP content decreased to a relatively constant level of 1.7–2.3 mg g−1 below ~15 cm at LH1 and below ~10 cm depth at LH2. This gradient was not as strong for any other parameter. The PMP was calculated based on these vertical profiles with TP above 2.1 mg g−1 (>20 cm depth) at LH1 and 1.8 mg g−1 (>15 cm depth) at LH2 interpreted as potentially mobile (Fig. 3). The PMP thus decreased downstream from 90 g m−2 at LH1 to 13.5 g m−2 at LH2. Based on these data, total mean PMP was 228 t, and ranged from 183 to 282 t (min − max), considering the uncertainties in sediment coverage and sediment area related to PMP at LH1 and LH2.
Sediment composition: organic matter (OM), total P (TP) with dotted lines representing the threshold for the calculation of permanent P and potentially mobile P, total iron (TFe), HCl- and Bicarbonate-Dithionite (BD; only for LH2) soluble Fe, manganese, sulfur species and total sulfur. Permanently bound P is also shown as sum of residual P, HCl-P and NaOH-SRP; and potentially mobile P as sum of NaOH-NRP, BD-P and NH4Cl-P (both from sequential extraction, only for LH2). All element contents per dry (105 °C) mass
Sequential extraction of the upper 10 cm of sediment resulted in a slightly higher value of 17 g m−2 PMP for LH2. The upper sediment was dominated by redox sensitive BD-SRP (38 %) and biogenic NaOH-NRP (22 %), both interpreted as potentially mobile. Permanent P was present as 16 % calcium bound HCl-P, 11 % NaOH-SRP normally interpreted as associated to aluminum, and 8 % refractory organic P.
Iron content was higher at LH1 (max. 60 mg g−1) than at LH2 (max. 47 mg g−1). About 60 % of total Fe was acid soluble FeHCl for both stations, while redox sensitive FeBD decreased from 45 % in the upper 2 cm to 17 % below 8 cm (only LH2). The atomic Fe:P ratio was only influenced by the trend in TP and increased from 5 to 7 in the upper sediment to 20–30 below 15 cm sediment depth. Mn was almost constant at depth below 2 cm, but showed a surface accumulation different to Fe.
Sulfur content was increasing with depth for both stations, from 15 mg g−1 in the top 2 cm to 57 and 45 mg g−1 in 30 cm depth for LH1 and LH2 respectively. In the upper 10 cm, about 36 % of total S and 55–85 % of inorganic S was bound in disulfides, and only 1.4 % was monosulfidic S. Accordingly, 13–24 % of total Fe was present in immobile sulfidic form, and another ~30 % as silicates. Only 10–20 % of Fe was found as reactive Fe-(hydr)oxide (FeBD), while the reactivity of the remaining 40–50 % acid soluble non sulfidic Fe (oxides, silicates, or vivianite) is unknown. Below 10 cm depth, up to 63 % of total Fe was found as FeSx.
Seasonal dynamics of in situ flux and diffusive flux
Diffusive flux calculated from conventional peeper data (Fig. 4) varied from 0.5 to 5.5 mg m−2 day−1 for SRP and from 2.6 to 23 mg m−2 day−1 for NH4-N, while benthic chamber and gel probe rates gave much higher values varying between 3.5 and 36 mg m−2 day−1 for SRP and between 20 and 124 mg m−2 day−1 for NH4-N. The benthic chamber gave negative rates (sorption) for SRP in April and May 2012, probably due to resuspension during exposure with P adsorbing onto precipitating Fe-hydroxides. Column experiment release rates followed the benthic chamber and gel probe trend for SPR, while the trend for NH4 was less clear. The mean in situ flux (mean of benthic chamber, gel probe and column experiment, as discussed later) and also diffusive flux (not shown) significantly correlated with bottom temperature for SPR (r2 = 0.68, p < 0.01) and NH4 (r2 = 0.73, p < 0.01) (Fig. 4), and SRP flux correlated with NH4 flux (r2 = 0.74, p < 0.01).
Left SRP and NH4-N release rates from sediment at station LH2 as determined with conventional peepers (1 cm vertical resolution), benthic chambers (~3 days exposition time), DET gel probes (0.25 cm vertical resolution) and column experiments (initial rates for NH4-N and long term rates (~days 3–20, n = 8) for SRP, error bars are standard deviation). Right mean in situ flux (mean of benthic chamber, gel probe and column exp.) 2011–2012 for LH2 versus bottom temperature, with standard deviation if more than one value per month
Seasonality of O2/NO3-consumption related to redox sensitive P sorption
Pore water data (Fig. 5, Table 1) provide an insight into the seasonality of processes controlling P sorption and release. SRP concentration in the top sediment layer increased from spring to summer indicating that SRP production and flux increased about 20-fold. This was related to a loss of redox sensitive P sorption capacity, as reflected by an almost three fold decrease in oxic and suboxic layer thickness with oxygen and nitrate as major electron acceptors, respectively. Consumption and flux followed this decreasing trend only for NO3, while they remained constant or even slightly increased for O2 (Table 1). These different trends for NO3 and O2 are caused by different supply, as O2 concentration remained constant (no stratification in 2012) while NO3 areal load decreased 2.5-fold between April and August. The suboxic layer thickness was still >1 cm in August and thus about 10 fold the oxic layer thickness.
Pore water concentration for NH4-N, NO3-N and SRP (from gel probe), O2 (from optode), total dissolved Fe and Mn (from conventional peeper) and turnover rates (2012, station LH2). The software Profile V 1.0 (Berg et al. 1998) was used for calculated concentrations and turnover rates. Note the different scales for NO3 and O2
Conversion of NO3 oxidizing capacity into O2 equivalents based on molar ratios for organic matter mineralization (one O2 equivalent = 4/5 NO3-N) allows direct comparison of the two electron acceptors. While NO3 dominated in spring, 70 % of surface sediment oxidation in summer was realized by O2 despite an oxic layer thickness of less than 1 mm.
Redox sensitive sorption of mobilized SRP in surface sediment was evidenced by an SRP peak in 1.7 cm depth in August (Fig. 5, bottom panel). Because a comparable loss mechanism is lacking for NH4, the NH4 peak was much closer to the sediment surface at 0.2 cm depth. Metal availability for P sorption was indicated by the Fe:P (and Mn:P, not shown) atomic ratio, either as surface flux or pore water concentration (Fig. 6). While a surplus of Fe existed in spring and early summer, an Fe:P ratio <2 was detected between end of June and end of October. Fe and Mn concentrations in the pore water were almost similar.
Nitrate influence on P release in undisturbed cores
With column experiments we directly tested whether the P release rate depends on NO3 supply. There was no treatment-based trend in SRP release in the oxic experiments (one way ANOVA, p > 0.44). However, under anoxic conditions, NO3 concentration significantly influenced P release (one way ANOVA, p = 0.012) (Fig. 7). Here, the P-release in the treatment with the lowest nitrate load was double that of the highest (+NO3) nitrate load and thus differed significantly according to Games-Howell post hoc comparison (p = 0.007). In this experiment, NO3 was almost completely consumed for all treatments as outflow concentration was always at the detection limit (<50 µg l−1). Thus, SRP release under laboratory anoxic conditions was related to NO3 load and consumption, but not to NO3 concentration in the column overlying water (=outflow concentration).
Phosphorus temporary storage and internal load relevance
According to mass balance calculations, the Lower Havel was a net sink for P in winter and spring (Nov–Jun) in most years, and a net source of P in summer (Jul–Oct) (Fig. 8), except for 2011 and 2012 when high discharge occurred during summer (Fig. 2). Thus, the Lower Havel became a net source of P in summer only in situations with low discharge. Due to the uncertainty of the budget calculation, NS was often not significantly different from zero in spring (April–June) and autumn (September–November) (Fig. 8). However, the main phases with net accumulation in winter and net release in summer are each supported by a cluster of data with similar estimates and thus of a high degree of certainty. Cumulative NS calculation demonstrates that there is currently no P pool depletion, but an accumulation of excess P. This accumulation accounts for 13.6 ± 2.4 g m−2 (128 ± 28 t) since 2005, which is 10.6 % of total import during that period. Most of this net accumulation (11.7 ± 1.7 g m−2) occurred during the last three years of the study period. This is plausible despite the uncertainty of NS, as the direction of NS was unchanged (positive) during almost the complete period since September 2010. During that period, the discharge, and thus the P load, was exceptionally high also in summer, while stagnant periods (with higher P release) were missing.
TP areal inflow- and outflow load, net sedimentation (NS) with error bars representing uncertainty with 95 % confidence level, and cumulative net sedimentation since 2005 (cum NS). Arrows show the “recharge” period, which is the (winter and spring) time of positive NS required to support net release the following summer
According to this P balance, winter and spring accumulation was sufficient to support summer release (negative net sedimentation) in most years without P release from historical sediment required. The period of accumulation required to feed summer net release (“recharge period”) was 1.5 months (2010), 4.5 months (2006, 2008, 2009) and 8.5 months (2007). Summer release is thus fed by constant external load and recycling plus the release from material accumulated during prior winter and spring.
The relevance of P release from sediment for the pelagic P availability is indicated by the ratio of external load versus gross internal load. With increasing gross internal load towards summer (Fig. 9), the ratio decreased from ~20 in spring to ~1.6 in summer. Increasing gross P release rates in summer caused Plake to increase faster than Pin, resulting in negative net sedimentation rates between −10 and −67 mg m−2 day−1 (Fig. 8). This is in the range or higher than our measured in situ flux (max. 3.5–36 mg m−2 day−1). Thus, our in situ rates well explain Plake peaks in summer. As an example, 66–90 % of the Plake increase during August 2012 (2.9 µg l−1 day−1) could be explained by measured in situ flux, and only 24 % by diffusive flux (interpreted as background flux from deeper sediment as discussed later).
Discussion
Lower P input reduces P release and seasonal P availability
Short term (seasonal) variation of Plake in the Lower Havel, with negative net sedimentation and peak TP-concentrations in summer, are to a large extent caused by P release from the sediment. This seasonal pattern is typical for shallow lakes, while the amplitude of the variation in Plake is known to increase with trophic state (Sondergaard et al. 2013). However, the high summer gross release rates in the Lower Havel are mainly driven by release from diagenetically young surface sediment, which is largely independent from the historical P pool but depends on constant “recharge” by new import and sedimentation. This argumentation is first supported by the calculation of a ~4.5 month winter “recharge period” required to support summer net release (Fig. 8). Second, the measured in situ P flux would exhaust the present mobile P pool within 1–5 years, which is obviously not the case. In other words, the P pool in the deeper sediment is not required to support high gross release rates necessary to explain changes in Plake during summer. High in situ (gross) release rates, but not the diffusive flux from deeper sediment, explain Plake gradients in summer. Furthermore, the PMP is small (183–282 t) compared to the mean annual external load (190 ± 56 t a−1; 2005–2012).
Based on gel probe data and turnover rate calculations, we identified the top 7–16 mm as reactive surface layer (Fig. 5) causing high in situ flux. A number of authors reported up to 10 times higher in situ fluxes (mostly measured by benthic chambers) compared to diffusive fluxes, and attributed these to macrofaunal irrigation (Callender and Hammond 1982) or preferential release from fresh surface sediment (upper few millimeters) due to rapid mineralization (Urban et al. 1997; Thamdrup et al. 1994; Clavero et al. 2000). Thus, we could show that our ~2.5 mm gel probe resolution was sufficient to detect surface processes (ammonification and P mobilization) relevant for gross release. As this flux calculated from gel probe data was almost similar to in situ flux measured with benthic chambers, molecular diffusion must have been the main transport mechanism for SRP and NH4-N. In contrast, the diffusive flux measured with conventional peepers with a real resolution up to 2 cm (due to slight tilt of samplers) originates from slower mineralization in deeper sediment and may represent a background flux, which can be interpreted as long term reaction of sediments (Urban et al. 1997).
Little relevance of sediment P pool for long term P availability and lake recovery
We postulate that the potentially mobile P pool would, in contrast to the assumptions of Rohde (1995) and Kneis et al. (2006), not significantly affect the water quality of the Lower Havel if the external P load was reduced. Our contrasting conclusions derive from the complex methodical design, which now allows us to distinguish different release processes and their relevance, and to better evaluate P pool availability. Additionally, the external P load has decreased since 1990, so that P retention (net sedimentation relative to import) may have improved since these earlier studies have been conducted.
A common approach to evaluating the long term relevance of the P pool is to relate the labile or exchangeable P (potentially mobile P pool) to the P release rate (Jørgensen et al. 1975; Lewandowski 2002). This would result in depletion times of 16–72 years, assuming 13.5 and 90 mg m−2 PMP and an annual mean background flux of 0.82 and 1.24 g m−2 a−1 for LH2 and LH1 respectively. However, this simple method has several drawbacks, as both parameters required (P pool and P flux) may vary strongly depending on the method used.
The background flux, representing P release from a historic P pool below the active surface layer, was only 1.2–5.5 mg m−2 day−1 during summer. This flux is of minor importance compared to 45 ± 33 mg m−2 day−1 external load, and would further decrease with burial. Not only the flux but also the PMP is small compared to external load. The PMP:external load ratio of 1.3 is on a similarly low level (0.2–1.8) to that for other lakes considered to have little sediment influence (Hupfer and Lewandowski 2005; Lewandowski et al. 2003). Perhaps more importantly, the potentially mobile P pool is very likely overestimated as a large portion of the P we find as surface (15–20 cm) accumulation today is permanently bound. The approach based on vertical P profiles requires long term equilibrium conditions for load, accumulation and internal processes. The Lower Havel was highly P loaded between ~1950 and 1990, but emissions dropped dramatically thereafter, by a factor of 15 (Nützmann et al. 2011). Thus, there is no equilibrium, and decreased loading and sediment TP content (surface accumulation, Fig. 3) show contradicting trends. If we assume a constant (natural) Fe import, the relative Fe availability for P sorption has increased and thus higher P contents were possible.
The alternative approach based on P sequential extraction also overestimates PMP, as it has been shown several times that the portion of P identified as redox sensitive (bicarbonate-dithionite P) is to a large extent immobile even under strongly anoxic conditions (Grüneberg and Kleeberg 2005; Lewandowski 2002). Fe related permanent P sinks could be FeS-coated Fe-hydroxide particles (Golterman 2004), P associated to metal organic complexes (Gerke 1993) and vivianite (Rothe et al. 2014). Fe content in Lower Havel sediment (40–60 mg g−1) was at the upper level or higher than the 2–39 mg g−1 normally found in the region (Kleeberg et al. 2013), and dissolved Fe was available in pore water throughout the year. These conditions are favorable for authigenic vivianite formation, and vivianite crystals were clearly visible in freeze dried LH2 sediment below 1 cm depth down to 10 cm (M. Rothe, IGB Berlin, pers. commun.). TP profiles comparable to those in the Lower Havel, with surface accumulation of TP in the upper 10–15 cm, are known from internal lake restoration projects where Fe- or Al-compounds were added to increase P sorption capacity (Lewandowski et al. 2003; Kleeberg et al. 2013). Consequently, a depletion of sediments below 1.5 mg g−1 TP as assumed by Kneis et al. (2006) is unlikely. The P surface accumulation does not denote a risk for future P release, but rather indicates favorable conditions for P sorption.
To summarize, in case of further P load reduction, a significant long term influence of background P flux on short term P dynamics from sediment PMP existing today is very unlikely, and a calculated theoretical depletion time is of no practical relevance. The Lower Havel was a net annual P sink before 1975 (Höhne 1995) and a net source thereafter (Kneis et al. 2006), and changed back into a net sink at the latest in 2008 (Fig. 8).
Redox sensitive temporary sorption delays P release in spring
Potential increase of P mobilization from redox sensitive forms is regarded a major negative side effect of reduced N import and nitrate availability (e.g. Petzoldt and Uhlmann 2006). To evaluate the relevance of this mechanism for a specific water body, it is necessary to know whether and to what extent P release is redox sensitive, and how much of that is controlled by Nitrate and other oxidizers, especially oxygen.
In the Lower Havel, negative P net sedimentation occurred only when NO3-N loading rate was below ~0.3 g m−2 day−1 in summer. NO3-N load and Plake show an opposing trend (Fig. 9). This is, however, no proof of a causal relationship. Firstly, P inflow concentration also increased in summer, so that 43 % of the seasonal variation of Plake was explained by Pin (p < 0.01). Secondly, the increasing gross P release in summer was strongly related to temperature (Fig. 4) and NH4-N flux (r2 = 0.74, p < 0.01). This indicates a large relevance of P release from organic matter decomposition, which is known to peak in late summer due to high temperatures and higher availability of fresh organic matter in the surface sediment (Andersen 1982; Sondergaard et al. 2013). Rising temperatures also indirectly cause a release of Fe-bound P with decreasing thickness of oxidized sediment (Table 1) (Jensen and Andersen 1992).
Thus, redox sensitive P sorption and release modifies to a certain extent the seasonal pattern predefined by Pin and organic matter mineralization by delaying P release in spring and early summer, as suggested by Petzoldt and Uhlmann (2006). This delay is evidenced by a large deviation of the N:P ratio in settling particles compared to the flux of dissolved constituents from the sediment (Fig. 10) which persists until August. This indicates a preferential release of N compared to P (Clavero et al. 2000) and buildup of a sediment P pool in spring.
These deviations between N- and P mobilization and sorption in surface sediment were also detected by high resolution pore water gradients (Fig. 5). For SRP, sorption in oxic (0.1–1 mm) and suboxic (max. 11–27 mm) surface sediment causes a thick (1.6–2.0 cm) layer of positive turnover (production) with peak concentration in 1.6–1.9 cm depth throughout the year. In contrast, nitrification (hence loss of NH4) in surface sediment drives the NH4 peak deeper in spring, but moves towards the sediment surface with increasing temperature and dominating ammonification. Thus, redox conditions are likely to influence the time of the lag period between P accumulation and release.
Iron availability is not sufficient to suppress P release in summer
Based on element ratios in sediment and pore water we found that iron availability limited the suppression of P release in the Lower Havel during summer. On the one hand, iron contents in LH sediments are higher than found in most lakes in the region (Kleeberg et al. 2013). Fe content was high enough not to limit desulfurization as we never found hydrogen sulfide in sediment pore water while we did find dissolved Fe and SO4. This indicates a closed iron cycle with reductive dissolution and re-precipitation (Schauser et al. 2006). Despite ~12 % of Fe being immobilized in sulfidic form, the Fe:S atomic ratios of 1.4 (LH2) and 2.2 (LH1) were slightly higher than the 0.7–1.0 assumed by Kleeberg et al. (2013) and Wolter (2004) as threshold for exhaustion of P sorption capacity. With sulfate concentrations around 140 mg l−1 (mean 2005–2012), the Lower Havel falls into the “intermediate” group according to Caraco et al. (1989) for which redox coupled sorption and release should apply. The Fe hydroxide content is relatively high (based on the FeBD:PBD ratio of 2.7) which suggests some extra oxic P sorption capacity (Schauser et al. 2006). On the other hand, atomic Fe:P ratios in sediment (5.0–6.5) and settling matter (4.0–5.4; April–August 2012) were close to or below the threshold 5.5–8.3 (Jensen et al. 1992; Sondergaard et al. 2003; Maaßen et al. 2005) so that only limited oxic suppression of P release can be expected.
The Fe availability is more precisely described by seasonal changes of pore water Fe:P ratios (Fig. 6). Complete immobilization of mobilized SRP as reoxidized Fe(III)–P requires an Fe surplus, thus Fe(II):SRP ratios in pore water >2.0–2.2 (Tessenow 1974; Gunnars et al. 2002) or even >6.5 (Lehtoranta and Heiskanen 2003). The requirement Fe:P > 2.0 was only met in spring (until ~ end of June), while ratios during summer were on a similarly low level as for lake Müggelsee, where 0.6–0.9 was too low to prevent P release into oxic and anoxic water (Kleeberg and Dudel 1997). Thus, Fe mobilization (and re-oxidation) delays P release in spring and early summer, but is too low to retain mobilized P during the later months of summer. Net sedimentation of P during winter and spring is accompanied by Fe accumulation so that sorption capacity is also recharged. However, this pool plus constant Fe sedimentation is too low to prevent summer P release.
In addition to Fe, Mn may also play an important role. Mn content in the sediment was relatively high with a typical surface accumulation, and Mn concentration in pore water was similar to Fe. Mn may either directly affect P by adsorption to Mn-oxides in surface sediment, or indirectly by reoxidation of Fe(II) in suboxic sediment (Herzsprung et al. 2010) similar to NO3.
Nitrate insufficiently substitutes oxygen oxidation capacity
Under oxic conditions, O2 supplied the majority of oxidation capacity to the sediment. Based on turnover rate calculations, the relevance of NO3 compared to O2 as an oxidation agent decreased from 61 % in April to 30 % in August 2012 (Table 1) following the trend in NO3 areal load and concentration. Up to 30 % of O2 consumption can be caused by nitrification (Jensen et al. 1994) which represents an indirect NO3 supply and may influence the sorption-active suboxic layer thickness more than direct NO3 diffusion from overlying water. With O2 present at saturation all year round (in 2011 and 2012), NO3 influence on P release was not detectable in oxic column experiments even in summer (Fig. 7), when P sorption became more limited by Fe availability than by redox (Fig. 6).
In contrast, NO3 influenced P release under anoxic conditions (Fig. 7). Anoxic P flux was higher than under oxic conditions because NO3 supply was not able to sufficiently substitute O2. Summer NO3-N concentrations (~0.22 mg l−1 in June–August) were lower than published thresholds necessary to suppress P release in lakes of between 0.5 and 1.4 mg l−1 (Andersen 1982; Hemond and Lin 2010), and up to an order of magnitude lower than in rivers for which NO3 control on P release was demonstrated (Gabriel et al. 2006).
However, using concentrations ignores the fact that a certain amount of NO3 put into a system will act as an oxidizing agent without necessarily being detectable in the water (after reduction) as demonstrated by column experiments and also discussed by Gabriel et al. (2006) and Kleeberg and Dudel (1997). The Lower Havel summer NO3-N areal load of 97 mg m−2 day−1 (mean 2005–2010) was lower than reported from case studies where P release was successfully suppressed by NO3 additions, e.g. 135 mg m−2 day−1 NO3-N in a dimictic lake (Sondergaard et al. 2000), or the even higher single dose applications of between 24 and 141 g m−2 (Ripl and Lindmark 1978; Foy 1986; Wauer et al. 2005b).
Consequently, the NO3 to P relation found in the laboratory is of little practical relevance for the Lower Havel. Firstly, in highly loaded systems NO3 cannot diminish P release if organic matter mineralization is the dominant P mobilization process (Jensen and Andersen 1992; Gabriel et al. 2006). Secondly, present NO3 supply in Lower Havel is too low. This is in line with Knösche (2006) who found that NO3-N concentrations above 1–2 mg l−1 were necessary to affect P release for Lower Havel lakes downstream of our studied section, and even then nitrate explained only 8–11 % of variability of net P release. Furthermore, under anoxic conditions, when NO3 control on P release would be valuable, NO3 supply is cut off due to stratification. In these situations, the hypolimnetic NO3-N pool would be low (~0.9 g m−2, summer mean 2005–2010) compared to 73 mg m−2 day−1 consumption (anoxic column experiments). Thus, the summer anoxic P release rate is unlikely to increase with potential future reductions in NO3 load.
Summary and consequences for the management of the Lower Havel
Processes controlling P release in the Lower Havel are summarized in Fig. 11 and combined with consequences for future management regarding the response to lower P or lower N loading.
-
(1a)
Under oxic conditions, the P mobilization from diagenetically young surface sediments drives the seasonal course of gross P in situ flux.
-
(1b)
The amplitude of the P flux is largely redox independent, and determined by the external P load, the accumulation rate during the previous months (temporary storage), and temperature driven organic matter mineralization. As the historical P pool is small compared to summer gross release and external load, the Lower Havel would respond quickly to reductions of external P import.
-
(2)
The delay between accumulation and release is based on oxic re-adsorption to Fe(III) and thus limited by Fe mobilization in relation to P. Reductions of P import would result in an increased Fe:P ratio in sediment and pore water, and thus shift this delay further into summer, assuming constant Fe import and mobilization.
-
(3)
Under anoxic conditions, P flux is higher than under oxic conditions, and controlled to some extent by NO3 areal load. However, this is currently of little practical relevance because mineralization dominates the P mobilization process, NO3 loss rates are high, and NO3 availability is low during thermal stratification periods.
In conclusion, the Lower Havel would quickly benefit from lower P import, resulting in less oxic and anoxic P release from the sediment. A reduction of NO3 loading would have no or only minor effects on P availability.
References
Andersen JM (1976) An ignition method for determination of total phosphorus in lake sediments. Water Res 10:329–331
Andersen JM (1982) Effect of nitrate concentration in lake water on phosphate release from the sediment. Water Res 16:1119–1126
Berg P, Risgaard-Petersen N, Rysgaard S (1998) Interpretation of measured concentration profiles in sediment pore water. Limnol Oceanogr 43:1500–1510
Burger D, Hamilton DP, Pilditch CA, Gibbs MM (2007) Benthic nutrient fluxes in a eutrophic, polymictic lake. Hydrobiologia 584:13–25
Callender E, Hammond DE (1982) Nutrient exchange across the sediment–water interface in the Potomac River estuary. Estuar Coast Shelf S 15:395–413
Canfield DE (1989) Reactive iron on marine sediments. Geochim Cosmochim Acta 53:619–632
Caraco NF, Cole JJ, Likens GE (1989) Evidence for sulfate-controlled phosphorus release from sediments of aquatic systems. Nature 341:316–318
Clavero V, Izquierdo JJ, Fernandez JA, Niell FX (2000) Seasonal fluxes of phosphate and ammonium across the sediment–water interface in a shallow small estuary (Palmones River, southern Spain). Mar Ecol Prog Ser 198:51–60
DIN 38 406 E5 (1983) German standard methods for the examination of water, waste water and sludge; cations (group E); determination of ammonia-nitrogen (E 5). Deutsches Institut für Normung e.V., Berlin
DIN EN ISO 6878 (2004) Water quality- determination of phosphorus- ammonium molybdate spectrometric method. European Committee for Standardization, Brussels
Fossing H, Jorgensen BB (1989) Measurement of bacterial sulfate reduction in sediments- evaluation of a single-step chromium reduction method. Biogeochemistry 8:205–222
Foy RH (1986) Suppression of phosphorus release from lake sediments by the addition of nitrate. Water Res 20:1345–1351
Gabriel O, Balla D, Kalettka T, Schilling C, Zessner M (2006) Estimating the influence of increased nitrate loads from wastewater on phosphorus mobility in river sediments. Acta Hydrochim Hydrobiol 34:333–345
Gerke J (1993) Phosphate adsorption by humic/Fe-oxide mixtures aged at pH 4 and 7 and by poorly ordered Fe-oxide. Geoderma 59:279–288
Golterman H (2004) The chemistry of phosphate and nitrogen compounds in sediments. Kluver, Dordrecht
Grüneberg B, Kleeberg A (2005) Benthic phosphorus forms and transformations during neutralization of acid mining lakes. In: Serrano L, Golterman H (eds) Phosphates in sediments. Backhuys Publishers, Leiden, pp 127–137
Grüneberg B, Rücker J, Nixdorf B, Behrendt H (2011) Dilemma of non-steady state in lakes—development and predictability of in-lake P concentration in dimictic Lake Scharmutzelsee (Germany) after abrupt load reduction. Int Rev Hydrobiol 96:599–621
Gunnars A, Blomqvist S, Johansson P, Andersson C (2002) Formation of Fe(III) oxyhydroxide colloids in freshwater and brackish seawater, with incorporation of phosphate and calcium. Geochim Cosmochim Acta 66:745–758
Hamilton SK (2012) Biogeochemical time lags may delay responses of streams to ecological restoration. Freshw Biol 57:43–57
Hemond HF, Lin K (2010) Nitrate suppresses internal phosphorus loading in an eutrophic lake. Water Res 44:3645–3650
Herzsprung P, Schultze M, Hupfer M, Boehrer B, von Tümpling W, Duffek A Jr, Van der Veen A, Friese K (2010) Flood effects on phosphorus immobilisation in a river water filled pit lake–case study Lake Goitsche (Germany). Limnologica 40:182–190
Hesslein RH (1976) An in situ sampler for close interval pore water studies. Limnol Oceanogr 21:912–914
Höhne L (1995) Entwicklung der Eutrophierung von Spree und Havel im Zeitraum von 1955–1990. Die Havel. Landesumweltamt Brandenburg, pp 33–37
House WA (2003) Geochemical cycling of phosphorus in rivers. Appl Geochem 18:739–748
Hsieh YP, Yang CH (1989) Diffusion methods for the determination of reduced inorganic sulfur species in sediments. Limnol Oceanogr 34:1126–1129
Hupfer M, Lewandowski J (2005) Retention and early diagenetic transformation of phosphorus in Lake Arendsee (Germany)—consequences for management strategies. Arch Hydrobiol 164:143–167
Hupfer M, Lewandowski J (2008) Oxygen controls the phosphorus release form lake sediments—a long-lasting paradigm in limnology. Int Rev Hydrobiol 93:415–432
Jarvie HP, Jürgens MD, Williams RJ, Neal C, Davies JJL, Barrett C, White J (2005) Role of river bed sediments as sources and sinks of phosphorus across two major eutrophic UK river basins: the Hampshire Avon and Herefordshire Wye. J Hydrol 304:51–74
Jensen HS, Andersen FO (1992) Importance of temperature, nitrate, and pH for phosphate release from aerobic sediments of four shallow, eutrophic lakes. Limnol Oceanogr 37:577–589
Jensen HS, Kristensen P, Jeppesen E, Skytthe A (1992) Iron:phosphorus ratio in surface sediment as an indicator of phosphate release from aerobic sediment in shallow lakes. Hydrobiologia 235:731–743
Jensen K, Sloth NP, Risgaardpetersen N, Rysgaard S, Revsbech NP (1994) Estimation of nitrification and denitrification from microprofiles of oxygen and nitrate in model sediment systems. Appl Environ Microbiol 60:2094–2100
Jensen JP, Pedersen AR, Jeppesen E, Sondergaard M (2006) An empirical model describing the seasonal dynamics of phosphorus in 16 shallow eutrophic lakes after external load reduction. Limnol Oceanogr 51:791–800
Jeppesen E, Sondergaard M, Jensen JP, Havens KE, Anneville O, Carvalho L, Coveney MF, Deneke R, Dokulil MT, Foy B, Gerdeaux D, Hampton SE, Hilt S, Kangur K, Kohler J, Lammens EHHR, Lauridsen TL, Manca M, Miracle MR, Moss B, Noges P, Persson G, Phillips G, Portielje R, Schelske CL, Straile D, Tatrai I, Willen E, Winder M (2005) Lake responses to reduced nutrient loading—an analysis of contemporary long-term data from 35 case studies. Freshw Biol 50:1747–1771
Jørgensen SE (1976) A eutrophication model for a lake. Ecol Model 2:147–165
Jørgensen SE, Kamp-Nielsen L, Jacobsen OS (1975) A submodel for anaerobic mud-water exchange of phosphate. Ecol Model 1:133–146
Kleeberg A, Dudel GE (1997) Changes in extent of phosphorus release in a shallow lake (Lake Großer Müggelsee, Germany, Berlin) due to climatic factors and load. Mar Geol 139:61–75
Kleeberg A, Kozerski HP (1997) Phosphorus release in lake Grosser Müggelsee and its implications for lake restoration. Hydrobiologia 342:9–26
Kleeberg A, Herzog C, Hupfer M (2013) Redox sensitivity of iron in phosphorus binding does not impede lake restoration. Water Res 47:1491–1502
Kneis D, Knoesche R, Bronstert A (2006) Analysis and simulation of nutrient retention and management for a lowland river-lake system. Hydrol Earth Syst Sci 10:575–588
Knösche R (2006) Nährstoffaushagerung von Flusssee-Sedimenten. In: Bronstert A, Itzerott S (eds) Bewirtschaftungsmöglichkeiten im Einzugsgebiet der Havel. Mathematisch-Naturwissenschaftliche Fakultät der Universität Potsdam, Potsdsam, Abschlussberichtsbericht zum BMBF-Projekt, pp 1–74
Krom MD, Davison P, Zhang H, Davison W (1994) High-resolution pore-water sampling with a gel sampler. Limnol Oceanogr 39:1967–1972
Lehtoranta J, Heiskanen AS (2003) Dissolved iron:phosphate ratio as an indicator of phosphate release to oxic water of the inner and outer coastal Baltic Sea. Hydrobiologia 492:69–84
Lewandowski J (2002) Untersuchungen zum Einfluss seeinterner Verfahren auf die Phosphor-Diagenese in Sedimenten. Dissertation, Humbold-Universität Berlin
Lewandowski J, Rüter K, Hupfer M (2002) Two dimensional small-scale variability of pore water phosphate in freshwater lakes: results from a novel dialysis sampler. Environ Sci Technol 36:2039–2047
Lewandowski J, Schauser I, Hupfer M (2003) Long term effects of phosphorus precipitations with alum in hypereutrophic Lake Süsser See (Germany). Water Res 37:3194–3204
Maaßen S (2003) Vergleichende Untersuchungen über den potentiellen Eintrag von Nährstoffen in den Wasserkörper von Talsperren durch Freisetzung aus dem Sediment. Dissertation, Fakultät Mathematik und Naturwissenschaften der Technischen Universität Dresden
Maaßen S, Uhlmann D, Röske I (2005) Sediment and pore water composition as a basis for the trophic evaluation of standing waters. Hydrobiologia 543:55–70
Nützmann G, Wolter C, Venohr M, Pusch M (2011) Historical patterns of anthropogenic impacts on freshwaters in the Berlin-Brandenburg Region. Erde 142:41–64
Petzoldt T, Uhlmann D (2006) Nitrogen emissions into freshwater ecosystems: is there a need for nitrate elimination in all wastewater treatment plants? Acta Hydrochim Hydrobiol 34:305–324
Psenner R, Pucsko R, Sager M (1984) Die Fraktionierung organischer und anorganischer Phosphorverbindungen von Sedimenten - Versuch einer Definition ökologisch wichtiger Faktoren. Arch Hydrobiol Beih 70:111-155
Raiswell R, Canfield DE, Berner RA (1994) A comparison of iron extraction methods for the determination of degree of pyritisation and the recognition of iron-limited pyrite formation. Chem Geol 111:101–110
Reitzel K, Hansen J, Andersen FO, Hansen KS, Jensen HS (2005) Lake restoration by dosing aluminum relative to mobile phosphorus in the sediment. Environ Sci Technol 39:4134–4140
Ripl W, Lindmark GK (1978) Ecosystem control by nitrogen metabolism in sediment. Vatten 34:135–144
Rohde E (1995) Zur Problematik der Phosphorfreisetzung aus den Sedimenten der Potsdamer Havelseen. Die Havel. Landesumweltamt Brandenburg, pp 57–60
Rothe M, Frederichs T, Eder M, Kleeberg A, Hupfer M (2014) Evidence for vivianite formation and its contribution to long-term phosphorus retention in a recent lake sediment: a novel analytical approach. Biogeosciences 11:5169–5180
Sas H (1989) Lake restoration by reduction of nutrient loading. Expectations, experiences, extrapolations. Academia-Verl Richarz, Sankt Augustin
Schauser I, Chorus I, Lewandowski J (2006) Effects of nitrate on phosphorus release: comparisons of two Berlin lakes. Acta Hydrochim Hydrobiol 34:325–332
Selig U, Schlungbaum G (2002) Longitudinal patterns of phosphorus and phosphorus binding in sediment of a lowland lake-river system. Hydrobiologia 472:67–76
Sinke AJC, Cornelese AA, Keizer P, van Tongeren OFR, Cappenberg ThE (1990) Mineralisation, pore water chemistry and phosphorus release from peaty sediments in the eutrophic Loosdrecht lakes, The Netherlands. Freshw Biol 23:587–599
Sondergaard M, Jensen JP, Jeppesen E (1999) Internal phosphorus loading in shallow Danish lakes. Hydrobiologia 408(409):145–152
Sondergaard M, Jeppesen E, Jensen JP (2000) Hypolimnetic Nitrate Treatment to Reduce Internal Phosphorus Loading in a Stratified Lake. Lake Reserv Manage 16:195–204
Sondergaard M, Jensen JP, Jeppesen E (2003) Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 506:135–145
Sondergaard M, Bjerring R, Jeppesen E (2013) Persistent internal phosphorus loading during summer in shallow eutrophic lakes. Hydrobiologia 710:95–107
Tessenow U (1974) Lösungs-, Diffusions- und Sorptionsprozesse in der Oberschicht von Seesedimenten IV. Reaktionsmechanismen und Gleichgewichte im System Eisen- Mangan-Phosphat. Arch Hydrobiol Suppl 47:1–79
Thamdrup B, Glud RN, Hansen JW (1994) Manganese oxidation and in situ manganese fluxes from a coastal sediment. Geochim Cosmochim Acta 58:2563–2570
Urban NR, Dinkel C, Wehrli B (1997) Solute transfer across the sediment surface of a eutrophic lake: I. Porewater profiles from dialysis samplers. Aquat Sci 59:1–25
Wauer G, Gonsiorczyk T, Casper P, Koschel R (2005a) P-immobilisation and phosphatase activities in lake sediment following treatment with nitrate and iron. Limnologica 35:102–108
Wauer G, Gonsiorczyk T, Kretschmer K, Casper P, Koschel R (2005b) Sediment treatment with a nitrate-storing compound to reduce phosphorus release. Water Res 39:494–500
Welch EB, Cooke GD (2005) Internal phosphorus loading in shallow lakes: importance and control. Lake Reserv Manage 21:209–217
Wolter KD (2004) Nachhaltige Entwicklung - Prozessbetrachtung und Konsequenzen für die Ökosystemsteuerung am Beispiel der Seerestaurierung. Technische Universiät Berlin, Institut für Ökologie, Habilitation
Acknowledgments
We sincerely thank all persons who directly or indirectly contributed to this study. Ingo Henschke, Thomas Wolburg and Dagmar Steubing helped in lake sampling. Sabine Natusch, Ute Abel and Gudrun Lippert performed chemical analysis at the BTUC, and Dagmar Steubing and Simone Troßen at the BfG. Annette Becker supplied sediment samples and basic data of the 2014 mapping. We thank Kurt Friese from the UFZ Magdeburg for providing lab facilities for sulfur analysis. Water quantity and quality data where supplied by the Berlin Senate Department for Urban Development and the Environment. We thank Thomas Petzoldt, Brigitte Nixdorf, David Kneis and Andrew Dolman for statistics support and helpful comments on the manuscript. This work was performed as part of the research project NITROLIMIT and funded by the framework program Research for Sustainable Development (Grant Number: 033L041A) of the German Federal Ministry of Education and Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: James Sickman.
Rights and permissions
About this article
Cite this article
Grüneberg, B., Dadi, T., Lindim, C. et al. Effects of nitrogen and phosphorus load reduction on benthic phosphorus release in a riverine lake. Biogeochemistry 123, 185–202 (2015). https://doi.org/10.1007/s10533-014-0062-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-014-0062-3